Introduction
Dermatomyositis (DM), polymyositis (PM) and
inclusion body myositis are the major categories of idiopathic
inflammatory myopathy (1). PM/DM may
affect any striated muscle of the body. The associated pathological
changes are mainly associated with inflammatory or degenerative
processes of muscle fibers and adjacent connective tissue (2). The diagnostic criteria are based on the
standards set by Bohan and Peter (3)
in 1975. The changes in laboratory parameters feature elevation of
serum muscle enzymes, including creatine kinase (CK), aspartate
aminotransferase (AST) and lactate dehydrogenase (LDH), which are
major non-invasive diagnostic measures of PM/DM (3). However, since laboratory indicators do
not accurately distinguish between inflammatory and
non-inflammatory myopathies, results of muscle biopsy and
electromyography are still important for PM/DM diagnosis (2). A limitation of biopsy is that the
results do not always reflect the disease activity and severity in
patients with PM/DM, as the distribution of muscle lesions is
frequently patchy. In addition, muscle biopsy frequently causes
trauma in patients (4,5). [18F]fluorodeoxyglucose
positron emission tomography/computed tomography
([18F]FDG-PET/CT) is a non-invasive hybrid imaging
modality that combines metabolic evaluation and morphological
correlation of pathophysiological processes. The most commonly used
radiotracer, FDG, is an analogue of glucose, the uptake of which is
proportional to cellular metabolic activity. It is widely used in
cancer imaging due to its sensitivity in detecting most malignant
cells (6). The uptake of FDG is
similar between malignant cells and inflammatory cells. In recent
years, PET/CT has also been used to investigate inflammatory
diseases, including inflammatory and infectious vascular disease,
and inflammatory bowel disease (7,8). The
common histopathological presentation of PM/DM includes
infiltration of inflammatory cells, and degeneration and
regeneration of muscle fibers, with the major muscles involved
being the limb girdles, neck and pharynx, although the dominant
inflammatory cell types and the typical site of infiltration within
the muscle are different between PM and DM (9,10).
It has been suggested that PET/CT imaging has
limited value for the evaluation of myositis in patients with PM/DM
due to its low sensitivity, although it may be useful for detection
of occult malignancies, including bronchogenic carcinoma and thymic
carcinoma in PM/DM patients (11–13).
However, muscle biopsy may cause pain and other complications.
According to this previous study, the application and benefits of
18F-FDG PET/CT for detecting PM/DM remain controversial.
The present study aimed to investigate the diagnostic value of
18F-FDG PET/CT in DM and determined its ability to
distinguish PM/DM from non-myopathy. In addition, the location of
FDG uptake in PM/DM and its ability to indicate muscle biopsy sites
were determined.
Materials and methods
Patients
A total of 22 patients (16 females, 6 males)
diagnosed with definite or probable PM/DM (the PM/DM group) who
underwent 18F-FDG PET/CT examinations prior to receiving
an initial corticosteroid treatment at Tianjin Medical University
General Hospital from October 2013 to August 2016 were included in
the present study. The same number of age- and sex-matched
individuals were included in the non-myopathy control group, who
did not exhibit myasthenia, movement disorders, skin rashes,
myalgia or elevated levels of serum muscle enzymes. PM/DM was
diagnosed according to the criteria published by Bohan and Peter
(3) in 1975. The Ethics Committee of
Tianjin Medical University General Hospital (Tianjin, China)
approved this retrospective study.
PET/CT imaging
PET/CT imaging was performed with a combination
PET/CT scanner (Discovery LS; GE Healthcare, Little Chalfont, UK).
Case and control groups were examined after fasting for 6 h, and
blood glucose was controlled at ≤11.1 mmol/l. PET/CT scans from the
skull to the proximal thigh were performed at 60 min after the
intravenous administration of 5.5 MBq/kg of FDG. During the 60-min
uptake period, the patients were advised to take a rest and remain
calm to minimize any non-specific FDG uptake in muscles. PET images
were reconstructed with the use of CT data for attenuation
correction. Image fusion was performed after transverse, coronal
and sagittal section image display. Local delayed imaging was
performed at 3 h after radiopharmaceutical uptake on suspicious
lesions. One of the experienced nuclear physicians and a
radiologist reviewed the images separately. To assess the presence
of abnormal 18F-FDG uptake, the highest level of
radioactive concentration was selected to map the
region-of-interest and the maximum standardized uptake value
(SUVmax) was automatically measured. SUVmax
represented the FDG uptake of seven muscle groups, including the
cervical, thoracic, lumbar, upper arm, shoulder, pelvic and thigh
regions (14). Areas where the FDG
uptake was increased in other anatomical structures were not
included. The SUVmax was calculated with the following
formula: SUV (g/ml) = regional radioactive concentration
(Bq/ml)/[injection dose (Bq)/body weight (g)]. The average
SUVmax of the bilateral muscles was calculated to
represent the average FDG uptake of the proximal muscle of the
individual patient, and it was referred to as the
SUVaverage.
Clinical data
Clinical data from all patients, including sex, age,
limb muscle strength, laboratory results, muscle SUVmax
and SUVaverage were recorded. Myodynamia (MD) was
measured using Manual Muscle Testing (MMT), and evaluated with
Daniels and Worthingham's Muscle Testing (0–5 scale) (15). In order to locate the muscles that
were often involved in PM/DM, the SUVmax was measured in
the seven aforementioned muscle groups.
Statistical analysis
Statistical analysis was performed using SPSS
(version 20; IBM Corp., Armonk, NY, USA) and Graphpad Prism
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) software.
Data fitting a normal distribution are expressed as the mean ±
standard deviation and independent-samples t-tests were applied for
statistical comparisons. Data that did not follow a normal
distribution were expressed as the median and interquartile range
(IQR), and the differences between groups were compared using the
non-parametric Mann-Whitney U test. Correlation analysis was
performed by determining Spearman's correlation coefficients.
P<0.05 was considered to indicate a statistically significant
difference. Receiver operating characteristic (ROC) curve analysis
for the SUVaverage of the proximal muscle was performed
to discriminate between PM/DM and control groups.
Results
Patient characteristics
Of the 22 patients, dermatomyositis was associated
with interstitial lung disease in 16, no lung damage in 3 and lung
cancer in 1 patient, and for 2 patients, data were not available.
Of these patients, 14 cases were subjected to muscle biopsy and 17
received electromyography (EMG). Among the patients with muscle
biopsy, myositis-associated changes were detected in 13 and for 1
patient, data were not available. In the patients assessed by EMG,
myogenic injury (n=11), mixed-source injury (n=5) and neurogenic
injury (n=1) were detected (Table
I).
 | Table I.Diagnostic data of the patients with
polymyositis/dermatomyositis. |
Table I.
Diagnostic data of the patients with
polymyositis/dermatomyositis.
| Parameter | n (%) |
|---|
| Lung manifestations
(n=22) |
|
| ILD | 16 (72.73) |
| Lca | 1 (4.55) |
| NR | 3 (13.63) |
| IA | 2 (9.09) |
| Muscle biopsy
(n=14) |
|
| MYO | 13 (92.86) |
| IA | 1 (7.14) |
| Electromyography
(n=17) |
|
| MI | 11 (64.71) |
| MSI | 5 (29.41) |
| NI | 1 (5.88) |
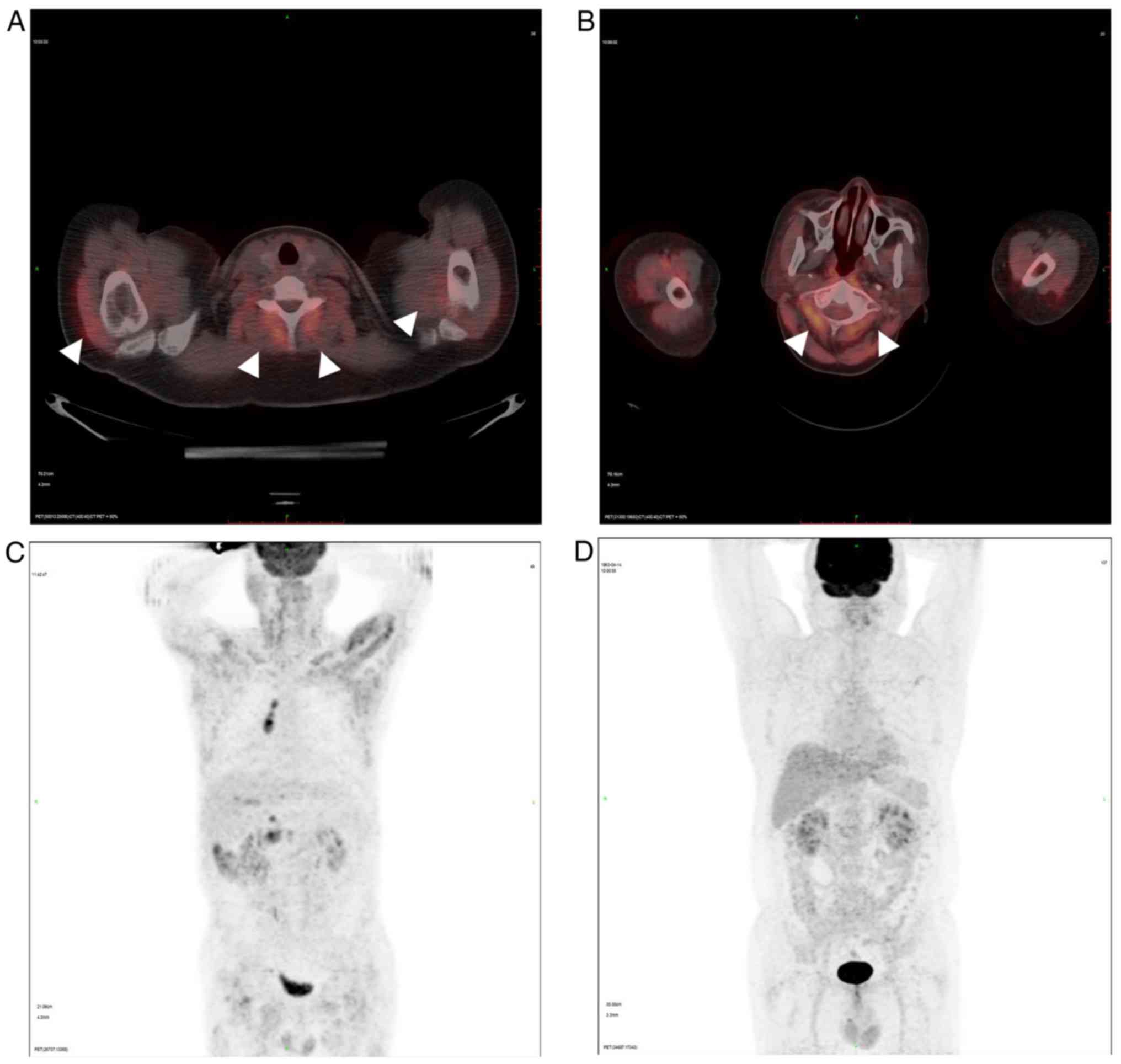
Representative PET/CT images of patients with DM and
non-muscular disease are displayed in Figs. 1 and 2
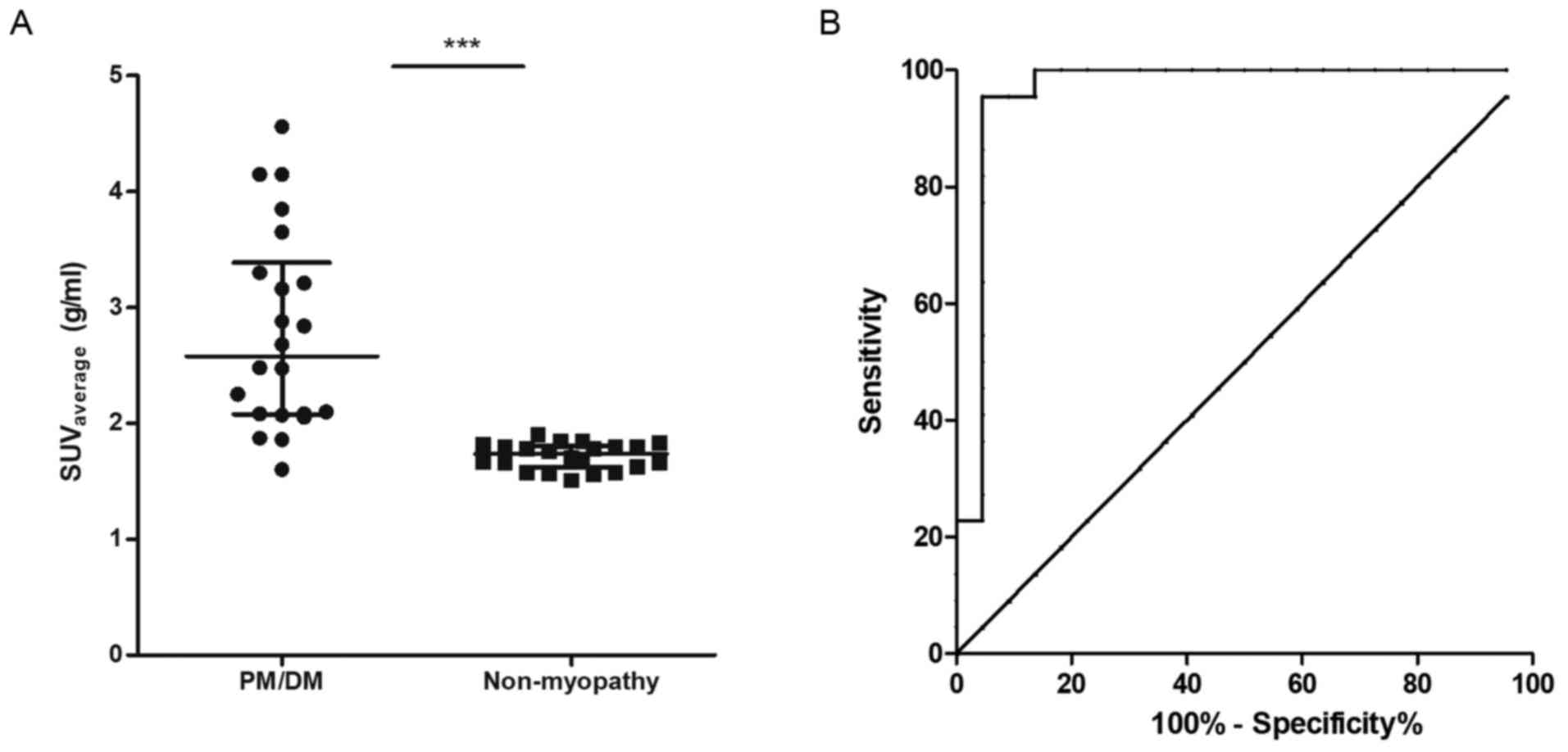
presents the distribution of SUVaverage in the proximal
muscles in the case group and the control group.
SUVaverage in the proximal muscle was significantly
(P<0.001; Table II) greater in
PM/DM patients (median, 2.58 g/ml; IQR, 2.08–3.39 g/ml) compared
with that in patients with non-muscular diseases (median, 1.74
g/ml; IQR, 1.62–1.81 g/ml). ROC analysis for the
SUVaverage to discriminate between PM/DM and
non-myopathy conditions revealed an area under the curve of 0.96
(95% confidence interval, 0.89–1.03). The optimal cut-point of
SUVmax was at 1.86 g/ml, giving the sensitivity of 95.5%
and the specificity of 95.5%. The Youden Index was 0.910. The
detailed clinical data for the PM/DM and non-myopathy groups are
summarized in Table II.
 | Table II.Clinical data of the PM/DM patients
and non-myopathy group. |
Table II.
Clinical data of the PM/DM patients
and non-myopathy group.
| Parameter | PM/DM (n=22) | Non-myopathy
(n=22) | Normal range | P-value |
|---|
| Age (years) | 52.8±13.1 | 50.1±11.1 |
| 0.46 |
| Sex
(male/female) | 6/16 | 6/16 |
| 1.00 |
| CK (U/l) | 211.00 (IQR
1588.75) | 60.50 (IQR
36.75) | 30–170 | <0.001 |
| CK-MB (U/l) | 16.50 (IQR
34.00) | 15.50 (IQR 9.25) | 0–24 | 0.072 |
| LDH (U/l) | 313.50 (IQR
229.25) | 141.00 (IQR
81.00) | 94–250 | <0.001 |
| AST (U/l) | 40.50 (IQR
70.25) | 32.50 (IQR
19.25) | 15–46 | 0.011 |
| SUVaverage
(g/ml) | 2.58 (IQR 1.31) | 1.74 (IQR 0.19) |
| <0.001 |
Correlation analysis
Next the correlations between FDG uptake values
(SUVmax and SUVaverage), serum muscle enzyme
levels and MD were investigated. The SUVmax in the
cervical, thoracic and lumbar regions all correlated with CK levels
in the serum (P<0.05). Similarly, the SUVmax in the
upper arm, thoracic and lumbar regions was significantly correlated
with serum CK-MB levels (P<0.05). However, the
SUVaverage did not correlated with the levels of any
serum enzymes (P>0.05). The CK levels in the serum were
negatively correlated with muscle strength, which was presented as
MD (r=−0.493; P=0.038). However for the other muscle enzymes, no
correlation was identified. In addition, the SUVaverage
was significantly negatively correlated with muscle strength
(r=−0.641; P=0.004). SUVmax in the cervical, thoracic,
lumbar, shoulder and pelvic regions was negatively correlated with
MD, but that in the upper arm and thigh regions was not (Table III).
 | Table III.Correlation analysis of the
SUVaverage and MD vs. serum enzyme. |
Table III.
Correlation analysis of the
SUVaverage and MD vs. serum enzyme.
| Value | CK | CK-MB | LDH | AST | MD |
|---|
|
SUVaverage | r=0.264 | r=0.175 | r=0.074 | r=0.157 | r=−0.641 |
|
| P=0.235 | P=0.437 | P=0.757 | P=0.509 |
P=0.004a |
| CK | / | / | / | / | r=−0.493 |
|
|
|
|
|
|
P=0.038b |
| CK-MB | / | / | / | / | r=−0.056 |
|
|
|
|
|
| P=0.825 |
| LDH | / | / | / | / | r=−0.026 |
|
|
|
|
|
| P=0.918 |
| AST | / | / | / | / | r=−0.126 |
|
|
|
|
|
| P=0.618 |
| CR | r=0.489 | r=0.253 | r=0.131 | r=0.110 | r=−0.566 |
|
|
P=0.021b | P=0.257 | P=0.562 | P=0.625 |
P=0.014b |
| TR | r=0.457 | r=0.424 | r=0.094 | r=0.086 | r=−0.622 |
|
|
P=0.032b |
P=0.049b | P=0.679 | P=0.704 |
P=0.006a |
| LR | r=0.503 | r=0.442 | r=0.212 | r=0.416 | r=−0.580 |
|
|
P=0.017b |
P=0.039b | P=0.344 | P=0.054 |
P=0.012b |
| UAR | r=0.329 | r=0.488 | r=0.299 | r=0.278 | r=−0.341 |
|
| P=0.135 |
P=0.021b | P=0.176 | P=0.210 | P=0.166 |
| SR | r=0.305 | r=0.185 | r=0.060 | r=0.119 | r=−0.619 |
|
| P=0.167 | P=0.410 | P=0.789 | P=0.597 |
P=0.006a |
| PR | r=0.166 | r=0.125 | r=0.089 | r=0.280 | r=−0.482 |
|
| P=0.460 | P=0.580 | P=0.695 | P=0.207 |
P=0.043b |
| Thigh region | r=0.357 | r=0.282 | r=0.080 | r=0.355 | r=−0.466 |
|
| P=0.103 | P=0.204 | P=0.722 | P=0.105 | P=0.051 |
Discussion
PM and DM are a group of systemic, non-suppurative
inflammatory diseases of the striated muscle. Clinical
manifestations include symmetrical limb proximal MD with tenderness
(5), increased serum CK and
erythrocyte sedimentation rate, and myogenic damage on EMG;
furthermore, a good response to glucocorticoid treatment is
observed. In the present study, the SUVmax of the
proximal limb muscles was measured. At the same time, in order to
evaluate the overall muscle condition, SUVaverage on
both sides of the seven muscle groups was determined. The present
study indicated that the FDG uptake value of PM/DM patients was
significantly higher than that of the non-myopathy patients, which
is in accordance with the study by Tanaka et al (5). However, in the aforementioned study,
mean proximal muscle SUVs were significantly correlated with serum
levels of CK (P=0.015). The serum levels of CK and CK-MB were not
correlated with the mean SUVmax of the proximal muscle
in the present results. In the present study, the inflammatory
response in each muscular site of the human body was identified to
be different; similarly the clinical manifestations in PM/DM
tissues were different. The SUVaverage is not
representative of the activity and severity of muscles specifically
affected by myositis in patients with PM/DM. The serum CK levels
and SUVaverage were all negatively correlated with MD,
which is similar to the results of the study by Tanaka et al
(5). It may be inferred that,
compared with AST and LDH levels, the serum CK levels and FDG
uptake value are more representative and effective to reflect the
severity of myositis in patients with PM/DM.
Regarding the comparison between the local muscle
groups, the present study indicated that in the PM/DM group, the
disease severity was not identical. In addition, SUVmax
in the cervical, thoracic and lumbar regions was correlated with
the CK levels in the serum (P<0.05). This was consistent with
the study by Streib et al (16), which concluded that, for any patient
suspected of having a myopathy, EMG examination should include the
paraspinal muscles. A recent study has revealed that anti-M2
antibodies appear to be biomarker associated with the distribution
of affected muscles (17). The
histological features were compared between myositis patients
positive and negative for anti-M2 antibodies (17). It was revealed that inflammation
appeared to be more intense in the trunk and to a lesser degree in
weak limb muscles (17). The present
study is a retrospective analysis, which cannot be further
verified, since the experimental data remains incomplete. Pathology
is the bridge of clinical work and basic research. In the diagnosis
of muscle disease, muscle biopsy is the major method to determine
the nature of the disease, and it is also irreplaceable. However,
FDG uptake reflects metabolically active sites, and muscle biopsy
may not provide results that are representative of the most
affected lesions. The most important advantage of PET/CT is that
the entire body may be imaged in one scan. It may directly assess
the scope and patterns of muscle lesions, including structures that
are not routinely screened in pathological analysis. In the present
study, active regions were identified in the paraspinal muscles.
Although PET/CT cannot replace muscle biopsy, it may provide novel
and more suitable locations for muscle biopsy.
In the present study, serum CK-MB levels were
associated with FDG uptake in the muscles of the upper arm, as well
as thoracic and lumbar regions, which was inconsistent with the
results of Pei et al (18).
CK-MB is one of the isoforms of CK and the MB type is mainly
present in cardiomyocytes, accounting for >5% of total serum CK.
However, there are still 2% CK-MB in skeletal muscle, and total CK
is >6% in the case of myocardial damage and <6% in the case
of skeletal muscle damage. The increase in CK also leads to an
increase in the corresponding proportion of CK-MB. The correlation
between CK-MB, CK and SUVmax was consistent in two of
three regions. One of the positions was inconsistent with regard to
the examination of the measurement error of SUVmax. In
one case of the case group, CK-MB was significantly higher than
normal (501 U/l), but total CK was <6% (8,973 U/l), and no
complains of palpitation, precordial discomfort or chest pain were
reported. Therefore, it was inferred that PM/DM is rarely involves
the myocardium.
DM patients frequently have a high risk of occult
malignant tumors (10). The present
study reviewed PM/DM patients who had been subjected to PET/CT
examination to exclude tumors. Of the 22 patients, one had been
diagnosed with lung cancer. In this patient, it was observed that
the SUVmax at each site was significantly higher than
that for other myositis patients. This result is similar to that of
Selva-O'Callaghan et al (19). This may provide a predictive means
for patients with myositis combined with tumors, or for patients
with myositis who gradually develop tumors (20). However, the sample size in the
present study was limited and it is necessary to perform further
studies with larger samples in order to provide statistically
significant results.
One limitation of the present study was the small
number of patients included. This is due to PET/CT not being a
common method to diagnose myositis, high cost of examination and
shortcomings including low specificity. Magnetic resonance imaging
(MRI), may be a suitable method, which may detect muscle edema in
diseased muscle, including inflammatory myopathies, with sufficient
sensitivity (21). MRI has its
limitations, including low specificity and local imaging.
Furthermore, certain patients are not suitable for MRI examination,
including those with pacemakers. The distribution patterns of the
abnormal signals of MRI and PET/CT are different. MRI detects
inflammatory edema and PET/CT detects FDG uptake by active
inflammatory cells. PET/CT directly assesses the scope and patterns
of muscle lesions. As indicated above, PET/CT allows for scanning
large areas, non-invasive examination and relatively high-contrast
imaging for evaluation of the muscle status in the whole body.
These advantages may indicate its potential clinical application
for improving the diagnostic accuracy in DM/PM.
In conclusion, [18F]FDG-PET/CT has a
diagnostic value for distinguishing PM/DM from non-myopathy cases,
and it may be used for examination to assess the severity of
myositis. Furthermore, it may indicate novel and more suitable
locations for muscle biopsy in patients with myositis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
WW and QC designed the study. LS drafted the
manuscript. NZ performed the data analysis and interpretation. YD,
QC and LS acquired the data. LS, QC, XL and WW revised and reviewed
the manuscript. LS, YD, NZ, XL, QC and WW researched the
literature. XL also contributed in the analysis of the results. The
final version of the manuscript has been read and approved by all
authors, and each author believes that the manuscript represents
honest work.
Ethical approval and consent to
participate
The Ethics Committee of Tianjin Medical University
General Hospital (Tianjin, China) approved this retrospective
study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimachkie MM, Barohn RJ and Amato AA:
Idiopathic inflammatory myopathies. Neurol Clin. 32(595–628):
vii2014.
|
|
2
|
Pearson CM: Rheumatic manifestations of
polymyositis and dermatomyositis. Arthritis Rheum. 2:127–143. 1959.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (second of two parts). N Engl J Med. 292:403–407.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalakas MC: Polymyositis, dermatomyositis
and inclusion-body myositis. N Engl J Med. 325:1487–1498. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka S, Ikeda K, Uchiyama K, Iwamoto T,
Sanayama Y, Okubo A, Nakagomi D, Takahashi K, Yokota M, Suto A, et
al: [18F]FDG uptake in proximal muscles assessed by PET/CT reflects
both global and local muscular inflammation and provides useful
information in the management of patients with
polymyositis/dermatomyositis. Rheumatology (Oxford). 52:1271–1278.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basu S and Alavi A: Unparalleled
contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med.
49(17N–21N): 37N2008.
|
|
7
|
Chrapko BE, Chrapko M, Nocun A, Stefaniak
B, Zubilewicz T and Drop A: Role of 18F-FDG PET/CT in the diagnosis
of inflammatory and infectious vascular disease. Nucl Med Rev Cent
East Eur. 19:28–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perlman SB, Hall BS and Reichelderfer M:
PET/CT imaging of inflammatory bowel disease. Semin Nucl Med.
43:420–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dalakas MC: Mechanisms of disease:
Signaling pathways and immunobiology of inflammatory myopathies.
Nat Clin Pract Rheumatol. 2:219–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lazarou IN and Guerne PA: Classification,
diagnosis, and management of idiopathic inflammatory myopathies. J
Rheumatol. 40:550–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Owada T, Maezawa R, Kurasawa K, Okada H,
Arai S and Fukuda T: Detection of inflammatory lesions by f-18
fluorodeoxyglucose positron emission tomography in patients with
polymyositis and dermatomyositis. J Rheumatol. 39:1659–1665. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herder M GJ, Welling A, De Winter GV,
Comans EF and Hoekstra OS: Accessory findings on F-18 FDG positron
emission tomography in bronchogenic carcinoma. Clin Nucl Med.
28:58–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue Y, True LD and Martins RG: Thymic
carcinoma associated with paraneoplastic polymyositis. J Clin
Oncol. 27:e33–e34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tateyama M, Fujihara K, Misu T, Arai A,
Kaneta T and Aoki M: Clinical values of FDG PET in polymyositis and
dermatomyositis syndromes: Imaging of skeletal muscle inflammation.
BMJ Open. 5:e0067632015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hislop H, Avers D and Brown M: Daniels and
Worthingham's muscle Testing-E-Book: Techniques of manual
examination and performance testing. Elsevier Health Sciences.
2013.
|
|
16
|
Streib EW, Wilbourn AJ and Mitsumoto H:
Spontaneous electrical muscle fiber activity in polymyositis and
dermatomyositis. Muscle Nerve. 2:14–18. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uenaka T, Kowa H, Ohtsuka Y, Seki T,
Sekiguchi K, Kanda F and Toda T: Less limb muscle involvement in
myositis patients with Anti-mitochondrial antibodies. Eur Neurol.
78:290–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pei L, Guan ZW, Ji XJ, Wen QF, Sun F, Zhao
Z, Jin JY, Zhu J, Zhang JL and Huang F: The application of (18)F
fluorodeoxyglucose-positron emission tomography/computed tomography
in the diagnosis and treatment of dermatomyositis. Zhonghua Nei Ke
Za Zhi. 55:525–530. 2016.(In Chinese). PubMed/NCBI
|
|
19
|
Selva-O'Callaghan A, Grau JM,
Gámez-Cenzano C, Vidaller-Palacín A, Martínez-Gómez X,
Trallero-Araguás E, Andía-Navarro E and Vilardell-Tarrés M:
Conventional cancer screening versus PET/CT in
dermatomyositis/polymyositis. Am J Med. 123:558–562. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liau N, Ooi C, Reid C, Kirkwood ID and
Bartholomeusz D: F-18 FDG PET/CT detection of mediastinal
malignancy in a patient with dermatomyositis. Clin Nucl Med.
32:304–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Degardin A, Morillon D, Lacour A, Cotten
A, Vermersch P and Stojkovic T: Morphologic imaging in muscular
dystrophies and inflammatory myopathies. Skeletal Radiol.
39:1219–1227. 2010. View Article : Google Scholar : PubMed/NCBI
|