Introduction
Osteoarthritis (OA) is the most prevalent
degenerative joint disease worldwide, which typically causes pain,
joint dysfunction, stiffness, swelling, limited motion and
long-term disability (1). The onset
of OA is associated with a variety of factors, such as age
(2), obesity (3) and inflammation (4). OA is characterized by thickening of
subchondral bone, degradation of articular cartilage and formation
of osteophytes (5). Increasing
morbidity rates of OA make this condition a major healthcare
problem and, therefore, a serious health burden to society
(6,7). However, there remains no effective
prevention and treatment for OA. Traditional therapies only
temporarily alleviate the clinical symptoms, but do not effectively
inhibit the pathological process (8,9).
Therefore, the probe of key molecules in the pathogenesis is
important for improving the prevention, mitigation and treatment of
OA.
Autophagy is a self-protection mechanism for cells,
which serves a vital role in the removal of dysfunctional and
damaged organelles and macromolecules, and also in cellular
homeostasis and metabolism (10,11). At
the cellular level, the dysfunction of autophagy can lead to
increased expression of abnormal genes, production of reactive
oxygen species, and cell death (12). Consequences of autophagy failure at
the organismal and tissue level include abnormal skeletal
development, cardiomyopathy, neurodegeneration and premature
mortality (13–15). Previous studies have demonstrated
that the downregulation of autophagy is closely associated with the
pathology of OA (16–19). Mammalian target of rapamycin (mTOR)
is a crucial suppressor of autophagy, involving a number of
autophagy-related proteins (Atg) (20–22).
Rapamycin, a specific mTOR inhibitor, has been used as an
immunosuppressive drug in solid organ transplantation and has been
demonstrated to induce autophagy in a variety of cell types,
including malignant glioma U87-MG cells and chondrocytes (23,24). It
has previously been reported that rapamycin can inhibit
neurodegenerative diseases, inflammation, infection, spinal cord
injury, kidney damage and other diseases via activation of
autophagy (25–28). In addition, it has been demonstrated
that rapamycin is able to reduce the severity of experimental OA,
at least in part, via autophagy activation (29).
It was recently observed that tetrahydrohyperforin
(IDN5706) is able to induce Atg5-dependent autophagy and thus exert
its neuroprotective effects (30).
IDN5706 is a tetrahydro derivative of hyperforin, which is one of
the main active components mediating the antidepressant activity of
Hypericum perforatum L. extracts with many pharmacological
uses, including anti-depression, anti-inflammation and anti-tumor
properties (31–33). Together, these findings suggest that
the pharmacological activation of autophagy may be an effective
treatment for OA (29). Therefore,
in the present study, it was investigated whether IDN5706
ameliorated the degeneration of articular cartilage and affected
autophagy in OA.
Materials and methods
Establishment of experimental OA
model
A total of 30 male Sprague-Dawley rats (weight,
200±10 g; age, 8–10 weeks) were obtained from The Laboratory Animal
Unit, Zhengzhou University (Zhengzhou, China). The rats were housed
at the Animal Center of Anhui Medical University (Hefei, China).
All experimental procedures were performed in strict accordance
with the guidelines for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (NIH Publication
No. 85-23, revised 1996) (34). All
animals were housed in a well-ventilated holding room at a
controlled temperature (24°C) and humidity (55%) with a 12 h
light/dark cycle and ad libitum access to water and food.
All animal experiments were approved by the Institutional Animal
Care and Use Committee at The First Affiliated Hospital of Anhui
Medical University (Hefei, China). Following 1 week of acclimation,
rats were weight-matched and randomly assigned into three groups
(n=10 each). Rats were anesthetized and received a 0.5 ml
intra-articular injection of collagenase solution (Clostridium
histolyticum, type II; 456 U/mg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in the right knee joint of the rat hindleg.
Rats in the normal control group were administered with an equal
volume of saline as a control. The administration was performed
twice, at days 1 and 4, in accordance with the previously detailed
method of papain injection (35).
Following two weeks, 6 g/kg IDN5706 (Indena S.p.A., Milan, Italy;
IDN5706 group) or an equal volume of saline (OA group) was
intragastrically administered once weekly for 6 weeks. The normal
control group also received intragastrical administration of equal
volume of saline once for 6 weeks.
Histological evaluation
Following 6 weeks of drug treatment, rats were
decapitated under intraperitoneal anesthesia with 1,000 mg/kg
urethane, and articular cartilage was separated from the knee joint
in rats (N=5/group). Knee cartilage tissue was fixed with 4%
formaldehyde at 20°C for 30 min, embedded in paraffin and cut to
obtain serial 5-µm thick sections. Subsequently, these sections
were stained with hematoxylin and eosin (H&E) at room
temperature for 10 min, and safranin O solution (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at room temperature for 5 min.
Safranin O solution (prepared in water) was added at 0.1–0.5 mg/ml
as a counterstain at room temperature for 5 min. Samples were
observed under a light microscope (Nikon Corporation, Tokyo, Japan;
magnification, ×200) and the histological changes of articular
cartilage were evaluated using the Mankin scoring system (36).
Autophagy observed via transmission
electron microscopy
Knee cartilage tissue from the other rats
(n=5/group) was fixed in 2.5% glutaraldehyde at room temperature
for 4 h, washed with 0.1 mol/l PBS (pH 7.2) for 2 h and post-fixed
in 1% osmium tetroxide at room temperature for 1 h. Following
dehydration in ethyl alcohol, the tissue was embedded in Epon
(Electron Microscopy Sciences, Hatfield, PA, USA). Tissue blocks
were cut serially into ultrathin (0.07 mm) sections, which were
stained with uranyl acetate at 4°C for 2 h and lead citrate at 4°C
for 20 min. Sections were subsequently observed under transmission
electron microscopy (magnification, ×12,000).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA levels of matrix metalloproteinase-13
(MMP-13) and vascular endothelial growth factor (VEGF) were
detected via RT-qPCR according to previously detailed methods
(37). The total RNA was extracted
from rat knee cartilage (n=10/group) via TRIzol reagent (Thermo
Fisher Scientific, Inc.). RNA samples were subjected to reverse
transcription using a GoScript Reverse Transcription system (Qiagen
GmbH, Hilden, Germany) according to the manufacturer's protocol.
RT-qPCR was performed using the SYBR® Premix Ex
Taq™ kit (Takara Bio, Inc., Otsu, Japan), according to
the manufacturer's instructions.
The sequences of the PCR primers used were as
follows: VEGF, forward 5′-CACCCACCCACATACATACA-3′ and reverse
5′-CTCAAGTCCACAGCAGTCAA-3′; MMP-13, forward
5′-TGACTATGCGTGGCTGGAA-3′ and reverse 5′-AAGCTGAAATCTTGCCTTGGA3-3′;
and GAPDH, forward 5′-CGGAGTCAACGGATTTGGTCGTATTGG-3′ and reverse
5′-GCTCCTGGAAGATGGTGATGGGATTTCC-3′. qPCR analysis was performed
using ABI 7900 PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Quantitative analysis of the relative expression
of RNA was calculated using the 2−ΔΔCq method (38). GAPDH was used as an internal
reference.
Western blotting
The protein expression of mTOR, Atg5, Beclin-1,
microtubule-associated protein 1 light chain 3 (LC3)-I, LC3-II and
phosphorylated (p-)mTOR was detected via western blotting. Rat knee
cartilage (n=10/group) were lysed in 200 µl
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) containing 50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 0.1% SDS, 1% sodium deoxycholate, 1 mM EDTA, 1%
Triton X-100 and protease inhibitors. The protein concentrations
were quantified using a Bio-Rad Bradford assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proteins (20 µg/lane) were
then separated by 10% SDS-PAGE prior to being transferred onto
nitrocellulose membranes (GE Healthcare, Chicago, IL, USA).
Following blocking with 5% skimmed milk (Merck KGaA) overnight at
4°C, membranes were incubated with the following primary
antibodies; anti-mTOR (cat. no. SAB2701842; 1:500), Atg5 (cat. no.
A0731; 1:500), Beclin-1 (cat. no. SAB1306484; 1:1,000), LC3-I and
LC3-II (both from Anti-LC3B antibodies; cat. no. SAB2700738;
1:1,000), p-mTOR (cat. no. SAB4504476; 1:500), and anti-GAPDH (cat.
no. SAB2100894; 1:500)(Sigma-Aldrich; Merck KGaA) overnight at 4°C.
Subsequently, protein bands were detected by incubation with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(cat. no. A50-106P; 1:1,000; Origene Technologies, Inc, Beijing,
China) at room temperature for 1 h. The chemiluminescent signal was
visualized using an ECL Detection Reagents (GE Healthcare). GAPDH
was used as a loading control. Each protein sample was examined in
triplicate.
Cell culture
Chondrocytes were isolated from the knee joint of OA
model rats (n=5/group). In brief, the articular cartilage tissue
from the right knee joint of the rat hindleg of OA rats was cut
into small pieces (<1 mm3) and incubated with 0.2%
trypsin at 37°C for 30 min. Following removal of the trypsin
solution, the tissue was treated with 0.2% Type II collagenase at
37°C for 2 h. Released cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal calf serum (Invitrogen; Thermo Fisher
Scientific, Inc.), L-glutamine, and antibiotics including
penicillin (100 u/ml) and streptomycin (100 g/ml),). The medium was
replaced every 3 days. Following the growth of cells to 80–90%
confluence, third passage chondrocytes were used for all further
experiments. Then, the chondrocytes were cultured with IDN5706 (50,
100, 150 and 200 µmol/l) in DMEM, supplemented with 10%
heat-inactivated fetal bovine serum (Life Technologies), and
penicillin/streptomycin (Thermo Fisher Scientific, Inc.), in a 5%
CO2 atmosphere at 37°C for 16 h. Untreated chondrocytes
were used as a control.
Cell counting kit-8 (CCK-8) assay
Cell viability was assessed using a CCK-8 assay kit
according to the manufacturer's protocol (MedChem Express, Monmouth
Junction, NJ, USA). In brief, cells were seeded in a 96-well plate
at a density of 1×105 cells/well in 100 µl culture
medium in a CO2 incubator at 37°C for 24 h. Afterwards,
cells were cultured with cisplatin (10 µg/ml; Hospira Australia
Pty, Ltd., Melbourne, Australia) for 72 h. Subsequently, 10 µl
CCK-8 solution was added to each well followed by incubation at
37°C for 4 h. Absorbance was measured at 450 nm using a microplate
reader (Thermo Fisher Scientific, Inc.). All experiments were
performed in triplicate.
ELISA
Chondrocytes were isolated from the knee joint of OA
model rats (n=5/group). ELISA was used for the quantitative
detection of MMP-13 and interleukin (IL)-6. The levels of MMP-13
and IL-6 were determined using a Matrix Metalloproteinase 13 ELISA
kit (cat. no. DL-MMP13-Ra; DLdevelop; Wuxi Donglin Sci & Tech
Development Co., Ltd., Wuxi, China) and an IL-6 Mouse ELISA kit
(cat. no. BMS603HS; Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. All samples were
measured in duplicate.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA) and expressed as the mean + standard deviation.
Student's t-test was used to analyze differences between two
groups. One-way analysis of variance followed by a Dunnett's
post-hoc test was used to perform multiple group analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
IDN5706 reduces the pathological
injury of OA
Initially, the effect of intragastric administration
of IDN5706 on OA was evaluated by determining pathological changes
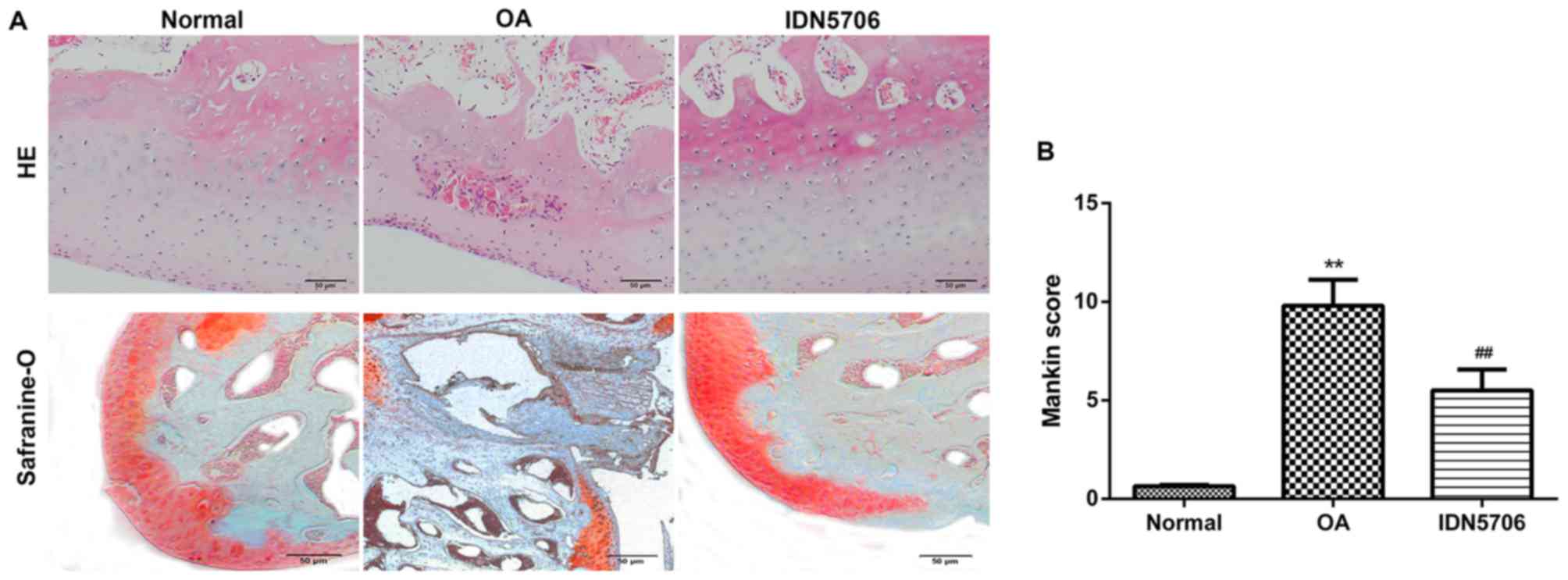
using H&E and safranin O staining, and Mankin scoring system.
H&E staining revealed normal morphology of joints in the normal
group, whereas the surface of the joint cartilage exhibited
defects, damage and structural breakage with a decreased number of
cartilage cells in the articular cartilage tissue of the OA group.
Treatment with IDN5706 alleviated the pathological changes in
edema, degeneration, clustering and necrosis in articular cartilage
of the knee joints, compared with the OA group (Fig. 1A). Safranin O staining demonstrated
that the nucleoli were stained brilliant red, and cytoplasm were
stained light red in the normal group. However, markedly reduced
staining was observed in articular cartilage of the knee joints
from OA rats, indicating that degeneration and denaturing of the
cartilage occurred. In addition, the nucleoli were stained
brilliant red in the IDN5706 group (Fig.
1A), suggesting a therapeutic effect of IDN5706 on OA.
Furthermore, Mankin scores were calculated in each group. As
presented in Fig. 1B, the score was
significantly elevated in the OA group compared with the normal
group, and IDN5706 treatment significantly decreased this score
compared with the OA group, indicating that IDN5706 may have a
therapeutic effect on OA (P<0.01).
IDN5706 may protect articular
cartilage and affect autophagy
To elucidate the influence of IDN5706 in OA, the
degeneration of chondrocytes and autophagosome were observed using
electron microscopy. In the normal group, a intact cell structure
was observed, the rough endoplasmic reticulum was abundant and
concentrated in the perinuclear regions, and surrounded various
types of organelles and autophagosomes (Fig. 2A). Decreased numbers of organelles,
swelling mitochondria and vacuolar degeneration were observed in
the cytoplasm of the OA group, and autophagosomes with double
membranes were also decreased (Fig.
2A). Following treated with IDN5706, autophagosomes with double
membranes were increased compared with the OA group (Fig. 2A). These results indicated that
IDN5706 alleviated the degeneration of chondrocytes caused by
OA.
 | Figure 2.Detection of autophagy and
degeneration of chondrocytes in the three groups. Normal, normal
cartilage tissue of the right knee joint; OA, received
intra-articular injection of collagenase solution in the right knee
joint; IDN5706, following treatment with collagenase solution, the
rats were intragastrically administered with IDN5706. (A) Autophagy
and degeneration of chondrocytes were examined using transmission
electron microscopy of cartilage tissues from rats in each group.
The yellow arrows indicate autolysosmes and the red arrows indicate
autophagosomes. Scale bar, 0.5 µm. N=5 in each group. (B)
Expression of MMP-13 and VEGF in cartilage tissue was analyzed by
reverse transcription-quantitative polymerase chain reaction. N=10
in each group. (C) Expression of mTOR, Atg5, Beclin-1, LC3-I,
LC3-II and p-mTOR, measured by western blotting. Data are presented
as the mean + standard deviation. N=10 in each group. **P<0.01
vs. normal group; ##P<0.01 vs. OA group. OA,
osteoarthritis; IDN5706, tetrahydrohyperforin; MMP-13, matrix
metalloproteinase-13; VEGF, vascular endothelial growth factor;
mTOR, mammalian target of rapamycin; Atg5, autophagy-related
protein 5; LC3, microtubule-associated protein 1 light chain 3; p,
phosphorylated. |
To further investigate the detailed role of IDN5706
in autophagy and OA, OA- and autophagy-related genes and proteins
were detected by RT-qPCR and western blotting. The results
demonstrated that the expression of MMP-13 and VEGF in the OA group
was significantly increased compared with the normal group
(P<0.01), and the relative levels of MMP-13 and VEGF were
significantly reduced in the IDN5706 group compared with the OA
group (P<0.01) (Fig. 2B). The
results mentioned above indicated that IDN5706 may have a
protective role in articular cartilage. As presented in Fig. 2C, the phosphorylation of mTOR was
markedly increased in the OA group compared with the normal group;
however, IDN5706 treatment resulted in a marked amelioration in the
levels of p-mTOR. Furthermore, the protein levels of
autophagy-related proteins, including LC3, Beclin-1 and Atg5, were
reduced in the OA group compared with the normal group, and
displayed a marked increase in the IDN5706 group compared with the
OA group. These findings suggest that IDN5706 had a protective
effect on articular cartilage and affected the level of autophagy
in articular cartilage from OA model rats.
IDN5706 reduces the injury of
chondrocyte
To further confirm the therapeutic effect of IDN5706
in OA, chondrocytes were isolated from the knee joints of OA rats
and cultured with IDN5706 (50, 100, 150 and 200 µmol/l). As
presented in Fig. 3A, cell viability
in the five groups of chondrocytes exhibited no difference compared
with the chondrocytes without treatment of IDN5706. The finding
indicated that there was no injury of IDN5706 for the chondrocytes.
Thus, 50 µmol/l IDN5706 was chosen for the subsequent assays. The
results from ELISA indicated that the levels of MMP-13 and IL-6
were significantly decreased in the IDN5706 group compared with the
control group (P<0.01; Fig. 3B).
As presented in Fig. 3C, IDN5706
treatment resulted in a marked decrease in the protein levels of
p-mTOR, and a significant increase in the protein levels of LC3,
Beclin-1 and Atg5, in the IDN5706 group compared with the control
group. These findings suggest that IDN5706 had a protective effect
on chondrocytes and affected the level of autophagy in
vitro.
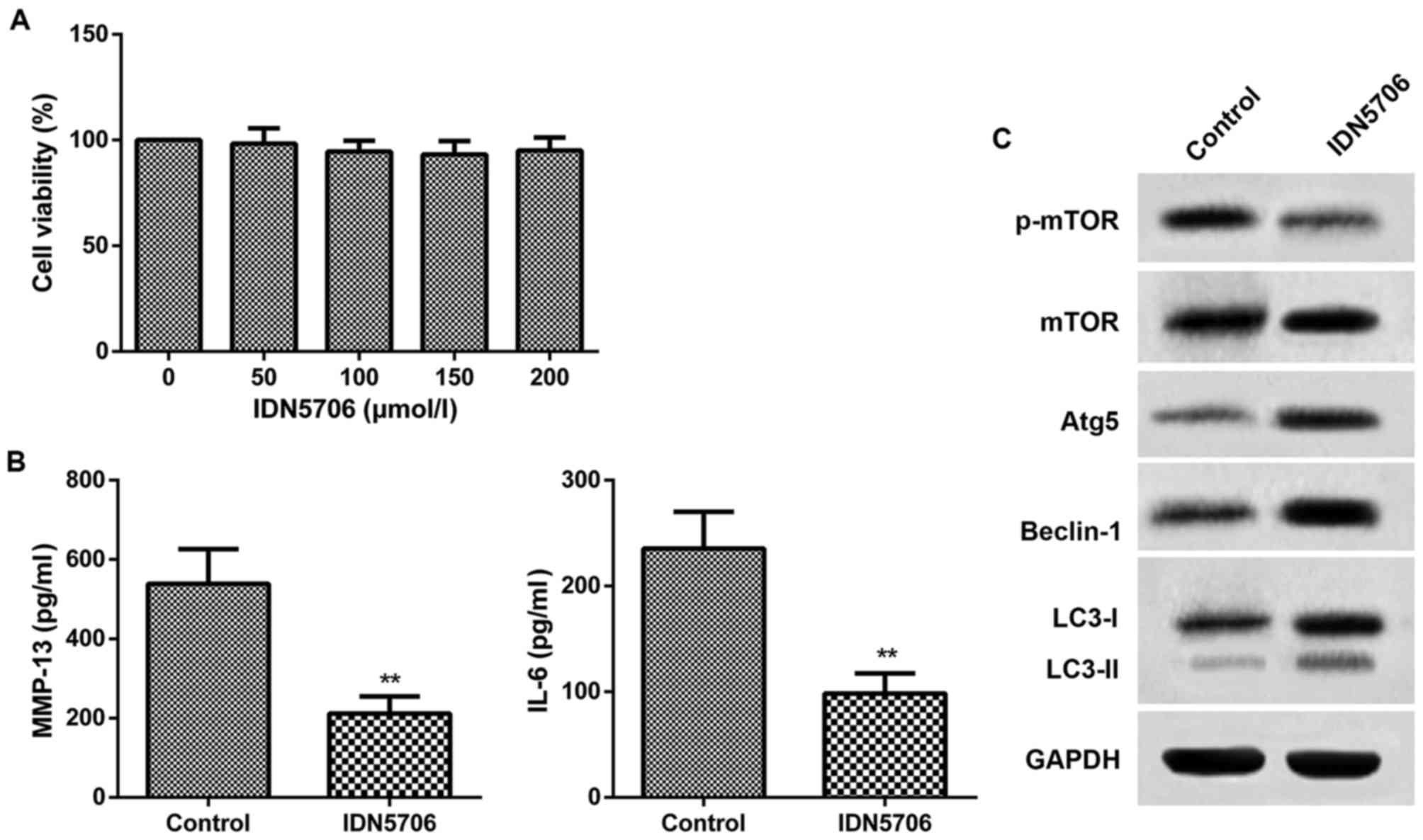 | Figure 3.Chondrocytes were cultured with
IDN5706 (0, 50, 100, 150 and 200 µmol/l). Control, normal
chondrocytes without IDN5706 treatment; IDN5706, chondrocytes
cultured with IDN5706 (50 µmol/l). (A) Cell viability was assessed
using a cell counting kit-8 assay. (B) Chondrocytes were cultured
with IDN5706 (50 µmol/l) and the levels of MMP-13 and IL-6 were
detected with ELISA. (C) Expression of mTOR, Atg5, Beclin-1, LC3-I,
LC3-II and p-mTOR was measured by western blotting. Data are
presented as the mean + standard deviation (n=5). **P<0.01 vs.
control group. IDN5706, tetrahydrohyperforin; MMP-13, matrix
metalloproteinase-13; IL, interleukin; mTOR, mammalian target of
rapamycin; Atg5, autophagy-related protein 5; LC3,
microtubule-associated protein 1 light chain 3; p,
phosphorylated. |
Discussion
OA is a highly prevalent disease and principally
affects the biomechanical and structural properties of the focal
regions of articular cartilage tissue (39). It is characterized by limited
intra-articular inflammation with synovitis, degeneration of
articular cartilage, and changes in subchondral bone and
periarticular bone (5). Substantial
progress has been achieved in understanding pathogenesis pathways
in established OA, and a large number of drug candidates have been
effective in OA animal models (40–43). In
the present study, justification was provided for the therapeutic
use of IDN5706, a synthetic derivative of the chemical compound
hyperforin of the St John's Wort plant, which may lessen symptoms
of OA and alleviate the degeneration of articular cartilage.
Intra-articular injection of collagenase has been
widely used to induce the murine model of OA (18,44–47).
Intra-articular injection with collagenase induces damage to
collagen type I-containing joint structures, such as tendons,
ligaments and menisci, leading to the osteoarthritic joint lesions
observed in this model (48). It is
a rapid and economic method to induce OA-like lesions (47,48). In
addition, collagenase-induced OA was characterized by severe
degenerative cartilage lesions on the medial side of the
femorotibial joint associated with patellar dislocation to the
medial side of the joint, sclerosis of subchondral bone below the
cartilage erosions, osteophyte formation and consequent deformity
of the knee joints. The collagenase-induced OA model offers the
possibility of studying experimental OA in large animal groups
(47). In the present study, OA was
induced in rats with intra-articular injection with collagenase.
Largely consistent with previous studies that successfully
established OA rat models, the collagenase-induced OA rats
displayed degeneration and denature of the articular cartilage.
In the experimental OA model, it was observed that
treatment with IDN5706 caused a marked reduction in OA severity.
The Mankin scoring system of OA indicated the degree of the
cartilage lesions, which reflect extracellular matrix degradation
and cell loss. It was observed that the Mankin score was
significantly elevated in the OA group compared with the normal
group, whereas IDN5706 treatment significantly decreased the score,
indicating that IDN5706 exerted a definite effect on OA.
Furthermore, transmission electron microscopy revealed that IDN5706
alleviated the degeneration of chondrocytes caused by OA. The
expression of VEGF and MMP-13 was significantly decreased in
cartilage from IDN5706-treated OA rats, compared with the OA group.
The association of increased production of proteinases, such as
MMP-13, with cartilage damage has been previously established
(49,50). Furthermore, expression of VEGF and
MMP-13 may disrupt normal homeostasis, resulting in abnormal
cartilage and bone metabolism (51).
As autophagy level was closely associated with OA, autophagosomes
were examined using transmission electron microscopy and protein
levels of autophagy-related proteins Beclin-1 and LC3 were detected
using western blotting. Results from electron microscopy
demonstrated decreased autophagosomes in the OA rats. Furthermore,
autophagosomes with double membranes were increased in
IDN5706-treated rats compared with the experimental OA rats.
Furthermore, it has been reported recently that autophagy
activation by IDN5706 is dependent on mTOR inactivation and Atg5
levels (30). Consistent with this,
the present results demonstrated that IDN5706 treatment increased
the number of autophagosomal structures, and levels of LC3-II,
Beclin-1 and Atg5, and decreased the levels of p-mTOR, which
further indicated that autophagy loss partially resulted in the
degeneration of articular cartilage following induction of OA with
collagenase injection.
To confirm this hypothesis, the impact of IDN5706 on
chondrocytes was detected in vitro. At the onset of OA,
articular cartilage differentiates into hypertrophic cartilage, and
the hypertrophic chondrocytes express MMP-13 and further degrade
the cartilage matrix (52–54). MMP-13 was previously confirmed to be
associated with the regulation of chondrocyte proliferation and
chondrocyte hypertrophy (55,56). In
addition, OA in experimental models and humans is associated with
inflammatory changes (5). Previous
research has demonstrated that IL-6 can increase inflammatory cells
and stimulate the proliferation of chondrocytes (57). In the present study, it was
demonstrated that IDN5706 also reduced the levels of MMP-13 and
IL-6 in chondrocytes. Meanwhile, the levels of LC3-II, Beclin-1 and
Atg5 were increased and the level of p-mTOR was decreased. The main
findings of the present study are presented in Fig. 4. These findings proved once again
that IDN5706 prevented articular cartilage degeneration in rats
with OA and affected autophagy.
However, an increasing number of studies have
demonstrated that, monitoring autophagic flux in vivo or in
organs is limited at present, and ideal methods associated with
cell culture techniques may not exist (58,59). One
potential method for in vivo studies is the analysis of
green fluorescent protein-light chain 3/Atg8 using fluorescence
microscopy, and another is immunohistochemical staining (59). Therefore, the use of transmission
electron microscopy in the present study is not sufficient to
monitor autophagy in vivo. Furthermore, other
autophagy-related proteins, such as Atg8/LC3, which is an excellent
marker for autophagic structures, are recommended to be measured.
In addition, another factor that should be considered is that the
effect of intra-articular injection of collagenase solution itself
on the change of autophagy level. All of these factors affect the
interpretation of the present results. The detected level of
autophagy may be the result of comprehensive effects mediated by
both known and unknown factors. Collectively, these are limitations
of the present study. Detection of in vivo autophagy using
other methods should to be performed in future studies.
In conclusion, the present study is, to our
knowledge, the first to establish the efficacy of IDN5706 in an
animal model of OA. The present data demonstrated that IDN5706
reduced the severity of experimental OA, alleviated the
degeneration of articular cartilage, and affects autophagy. These
results suggest that IDN5706 is a potentially effective therapeutic
approach for OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS and JZ conceived and designed the study. JZ, SW,
GR, BG and FC performed the experiments. JZ and SW wrote the paper.
CS reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
All experiments related to the use of animals were
approved by the Institutional Animal Care and Use Committee of The
First Affiliated Hospital of Anhui Medical University (Hefei,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blazek K, Favre J, Asay J, Erhart-Hledik J
and Andriacchi T: Age and obesity alter the relationship between
femoral articular cartilage thickness and ambulatory loads in
individuals without osteoarthritis. J Orthop Res. 32:394–402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barenius B, Ponzer S, Shalabi A, Bujak R,
Norlén L and Eriksson K: Increased risk of osteoarthritis after
anterior cruciate ligament reconstruction: A 14-year follow-up
study of a randomized controlled trial. Am J Sports Med.
42:1049–1057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flores R and Hochberg M: Definition and
classification of osteoarthritis. Osteoarthritis. 2:1–8. 1998.
|
|
7
|
Moskowitz RW, Kelly MA and Lewallen DG:
Understanding osteoarthritis of the knee-causes and effects. Am J
Orthop (Belle Mead NJ). 33 2 Suppl:S5–S9. 2004.
|
|
8
|
Spaans AJ, van Minnen LP, Kon M, Schuurman
AH, Schreuders AR and Vermeulen GM: Conservative treatment of thumb
base osteoarthritis: A systematic review. J Hand Surg Am.
40:16–21.e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermeulen GM, Slijper H, Feitz R, Hovius
SE, Moojen TM and Selles RW: Surgical management of primary thumb
carpometacarpal osteoarthritis: A systematic review. J Hand Surg
Am. 36:157–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hara T, Nakamura K, Matsui M, Yamamoto A,
Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I,
Okano H and Mizushima N: Suppression of basal autophagy in neural
cells causes neurodegenerative disease in mice. Nature.
441:885–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komatsu M, Waguri S, Ueno T, Iwata J,
Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et
al: Impairment of starvation-induced and constitutive autophagy in
Atg7-deficient mice. J Cell Biol. 169:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibata M, Lu T, Furuya T, Degterev A,
Mizushima N, Yoshimori T, MacDonald M, Yankner B and Yuan J:
Regulation of intracellular accumulation of mutant huntingtin by
beclin 1. J Biol Chem. 281:14474–14485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF,
Gao SG and Lei GH: Autophagy in osteoarthritis. Joint Bone Spine.
83:143–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Hu J, Pan Y, Shan Y, Jiang L, Qi X
and Jia L: miR-140-5p/miR-149 affects chondrocyte proliferation,
apoptosis, and autophagy by targeting FUT1 in osteoarthritis.
Inflammation. Feb 27–2018.(Epub ahead of print). View Article : Google Scholar
|
|
18
|
Cheng N, Meng H, Ma L, Zhang L, Yu HM,
Wang ZZ and Guo A: Role of autophagy in the progression of
osteoarthritis: The autophagy inhibitor, 3-methyladenine,
aggravates the severity of experimental osteoarthritis. Int J Mol
Med. 39:1224–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wangyang Y, Zheng X, Liu GW, Li DY, Feng
YB, Guo TY, Ma C and Wang T: Upregulation of P63 inhibits
chondrocyte autophagy thereby enhancing the malignant progression
of osteoarthritis. Pharmazie. 72:361–364. 2017.PubMed/NCBI
|
|
20
|
Settembre C, Arteaga-Solis E, Mckee MD, de
Pablo R, Al Awqati Q, Ballabio A and Karsenty G: Proteoglycan
desulfation determines the efficiency of chondrocyte autophagy and
the extent of FGF signaling during endochondral ossification. Genes
Dev. 22:2645–2650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Research. 17:666–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabers CJ, Martin MM, Brunn GJ, Williams
JM, Dumont FJ, Wiederrecht G and Abraham RT: Isolation of a protein
target of the FKBP12-rapamycin complex in mammalian cells. J Biol
Chem. 270:815–822. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shigemitsu K, Tsujishita Y, Hara K,
Nanahoshi M, Avruch J and Yonezawa K: Regulation of translational
effectors by amino acid and mammalian target of rapamycin signaling
pathways. Possible involvement of autophagy in cultured hepatoma
cells. J Biol Chem. 274:1058–1065. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdulrahman BA, Khweek AA, Akhter A,
Caution K, Kotrange S, Abdelaziz DH, Newland C, Rosales-Reyes R,
Kopp B, McCoy K, et al: Autophagy stimulation by rapamycin
suppresses lung inflammation and infection by Burkholderia
cenocepacia in a model of cystic fibrosis. Autophagy. 7:1359–1370.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aso E and Ferrer I: It may be possible to
delay the onset of neurodegenerative diseases with an
immunosuppressive drug (rapamycin). Expert Opin Biol Ther.
13:1215–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esposito C, Villa L, Grosjean F, Mangione
F, Esposito V, Castoldi F, Serpieri N, Arra M, Pertile E, Maggi N,
et al: Rapamycin reduces proteinuria and renal damage in the rat
remnant kidney model. Transplant Proc. 41:1370–1371. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldshmit Y, Kanner S, Zacs M, Frisca F,
Pinto AR, Currie PD and Pinkas-Kramarski R: Rapamycin increases
neuronal survival, and reduces inflammation and astrocyte
proliferation after spinal cord injury. Mol Cell Neurosci.
68:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caramés B, Hasegawa A, Taniguchi N, Miyaki
S, Blanco FJ and Lotz M: Autophagy activation by rapamycin reduces
severity of experimental osteoarthritis. Ann Rheum Dis. 71:575–581.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavieres VA, González A, Muñoz VC, Yefi
CP, Bustamante HA, Barraza RR, Tapia-Rojas C, Otth C, Barrera MJ,
González C, et al: Tetrahydrohyperforin Inhibits the proteolytic
processing of amyloid precursor protein and enhances its
degradation by Atg5-dependent autophagy. PLoS One. 10:e01363132015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koeberle A, Rossi A, Bauer J, Dehm F,
Verotta L, Northoff H, Sautebin L and Werz O: Hyperforin, an
anti-inflammatory constituent from St. John's Wort, inhibits
microsomal prostaglandin E(2) synthase-1 and suppresses
prostaglandin E(2) formation in vivo. Front Pharmacol. 2:72011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bombardelli E, Morazzoni P and Riva A: IDN
5491 (Hyperforin trimethoxybenzoate): A new antidepressive drug.
Eur Neuropsychopharm. 12:2402002. View Article : Google Scholar
|
|
33
|
Chiang IT, Chen WT, Tseng CW, Chen YC, Kuo
YC, Chen BJ, Weng MC, Lin HJ and Wang WS: Hyperforin inhibits cell
growth by inducing intrinsic and extrinsic apoptotic pathways in
hepatocellular carcinoma cells. Anticancer Res. 37:161–167. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39(199): 208–111. 1996.
|
|
35
|
Miyauchi S, Machida A, Onaya J, Sakamoto
T, Tokuyasu K and Iwata H: Alterations of proteoglycan synthesis in
rabbit articular cartilage induced by intra-articular injection of
papain. Osteoarthritis Cartilage. 1:253–262. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mankin HJ, Johnson ME and Lippiello L:
Biochemical and metabolic abnormalities in articular cartilage from
osteoarthritic human hips. III. Distribution and metabolism of
amino sugar-containing macromolecules. J Bone Joint Surg Am.
63:131–139. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang J, Cai H, Lin L, Xie P, Zhong W and
Tang M: Increased expression of CD24 is associated with tumor
progression and prognosis in patients suffering osteosarcoma. Clin
Transl Oncol. 15:541–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marks R: Osteoarthritis and articular
cartilage: Biomechanics and novel treatment paradigms. Adv Aging
Res. 03:297–309. 2014. View Article : Google Scholar
|
|
40
|
Lotz MK and Kraus VB: New developments in
osteoarthritis. Posttraumatic osteoarthritis: Pathogenesis and
pharmacological treatment options. Arthritis Res Ther. 12:2112010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cornelis S, Kersse K, Festjens N, Lamkanfi
M and Vandenabeele P: Inflammatory caspases: Targets for novel
therapies. Curr Pharm Des. 13:367–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
DiMicco MA, Patwari P, Siparsky PN, Kumar
S, Pratta MA, Lark MW, Kim YJ and Grodzinsky AJ: Mechanisms and
kinetics of glycosaminoglycan release following in vitro cartilage
injury. Arthritis Rheum. 50:840–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inoue A, Takahashi KA, Arai Y, Tonomura H,
Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y and Kubo T: The
therapeutic effects of basic fibroblast growth factor contained in
gelatin hydrogel microspheres on experimental osteoarthritis in the
rabbit knee. Arthritis Rheum. 54:264–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khatab S, van Buul G, Kops N,
Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA and van Osch GJ:
Intra-articular injections of platelet-rich plasma releasate reduce
pain and synovial inflammation in a mouse model of osteoarthritis.
Am J Sports Med. 46:977–986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cremers NAJ, van den Bosch MHJ, van Dalen
S, Di Ceglie I, Ascone G, van de Loo F, Koenders M, van der Kraan
P, Sloetjes A, Vogl T, et al: S100A8/A9 increases the mobilization
of pro-inflammatory Ly6Chigh monocytes to the synovium during
experimental osteoarthritis. Arthritis Res Ther. 19:2172017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Adães S, Almeida L, Potes C, Ferreira AR,
Castro-Lopes JM, Ferreira-Gomes J and Neto FL: Glial activation in
the collagenase model of nociception associated with
osteoarthritis. Mol Pain. 13:17448069166882192017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeh TT, Wen ZH, Lee HS, Lee CH, Yang Z,
Jean YH, Wu SS, Nimni ME and Han B: Intra-articular injection of
collagenase induced experimental osteoarthritis of the lumbar facet
joint in rats. Eur Spine J. 17:734–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
van der Kraan PM, Vitters EL, van
Beuningen HM, van de Putte LB and van den Berg WB: Degenerative
knee joint lesions in mice after a single intra-articular
collagenase injection. A new model of osteoarthritis. J Exp Pathol
(Oxford). 71:19–31. 1990.PubMed/NCBI
|
|
49
|
Cawston TE and Wilson AJ: Understanding
the role of tissue degrading enzymes and their inhibitors in
development and disease. Best Pract Res Clin Rheumatol.
20:983–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plaas A, Osborn B, Yoshihara Y, Bai Y,
Bloom T, Nelson F, Mikecz K and Sandy JD: Aggrecanolysis in human
osteoarthritis: Confocal localization and biochemical
characterization of ADAMTS5-hyaluronan complexes in articular
cartilages. Osteoarthritis Cartilage. 15:719–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tamamura Y, Otani T, Kanatani N, Koyama E,
Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici
M, et al: Developmental regulation of Wnt/beta-catenin signals is
required for growth plate assembly, cartilage integrity, and
endochondral ossification. J Biol Chem. 280:19185–19195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kamekura S, Hoshi K, Shimoaka T, Chung U,
Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K and
Kawaguchi H: Osteoarthritis development in novel experimental mouse
models induced by knee joint instability. Osteoarthritis Cartilage.
13:632–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kamekura S, Kawasaki Y, Hoshi K, Shimoaka
T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G,
et al: Contribution of runt-related transcription factor 2 to the
pathogenesis of osteoarthritis in mice after induction of knee
joint instability. Arthritis Rheum. 54:2462–2470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
von der Mark K, Kirsch T, Nerlich A, Kuss
A, Weseloh G, Glückert K and Stöss H: Type X collagen synthesis in
human osteoarthritic cartilage. Indication of chondrocyte
hypertrophy. Arthritis Rheum. 35:806–811. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takeda S, Bonnamy JP, Owen MJ, Ducy P and
Karsenty G: Continuous expression of Cbfa1 in nonhypertrophic
chondrocytes uncovers its ability to induce hypertrophic
chondrocyte differentiation and partially rescues Cbfa1-deficient
mice. Genes Dev. 15:467–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ueta C, Iwamoto M, Kanatani N, Yoshida C,
Liu Y, Enomoto-Iwamoto M, Ohmori T, Enomoto H, Nakata K, Takada K,
et al: Skeletal malformations caused by overexpression of Cbfa1 or
its dominant negative form in chondrocytes. J Cell Biol.
153:87–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kinoshita H, Minamitani K, Komiya S,
Higuchi F, Yamanaka K, Inoue A, Matsuo K, Yoshimoto K and Yokoyama
M: Immunohistochemical studies on the distribution of IL-1 and IL-6
producing cells in the synovial membranes of patients with joint
disorders. Orth Traumatol. 39:622–625. 1990–1991.
|
|
58
|
Seiliez I, Belghit I, Gao Y, Skiba-Cassy
S, Dias K, Cluzeaud M, Rémond D, Hafnaoui N, Salin B, Camougrand N
and Panserat S: Looking at the metabolic consequences of the
colchicine-based in vivo autophagic flux assay. Autophagy.
12:343–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena Acevedo A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|