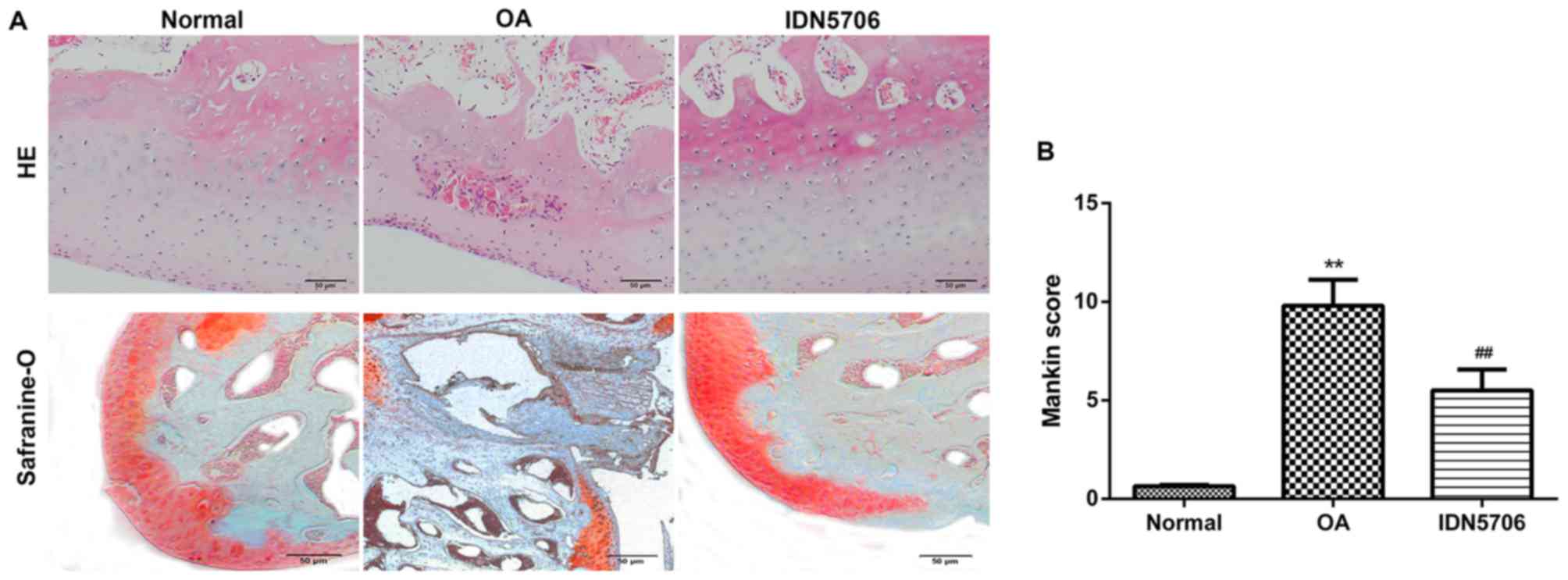
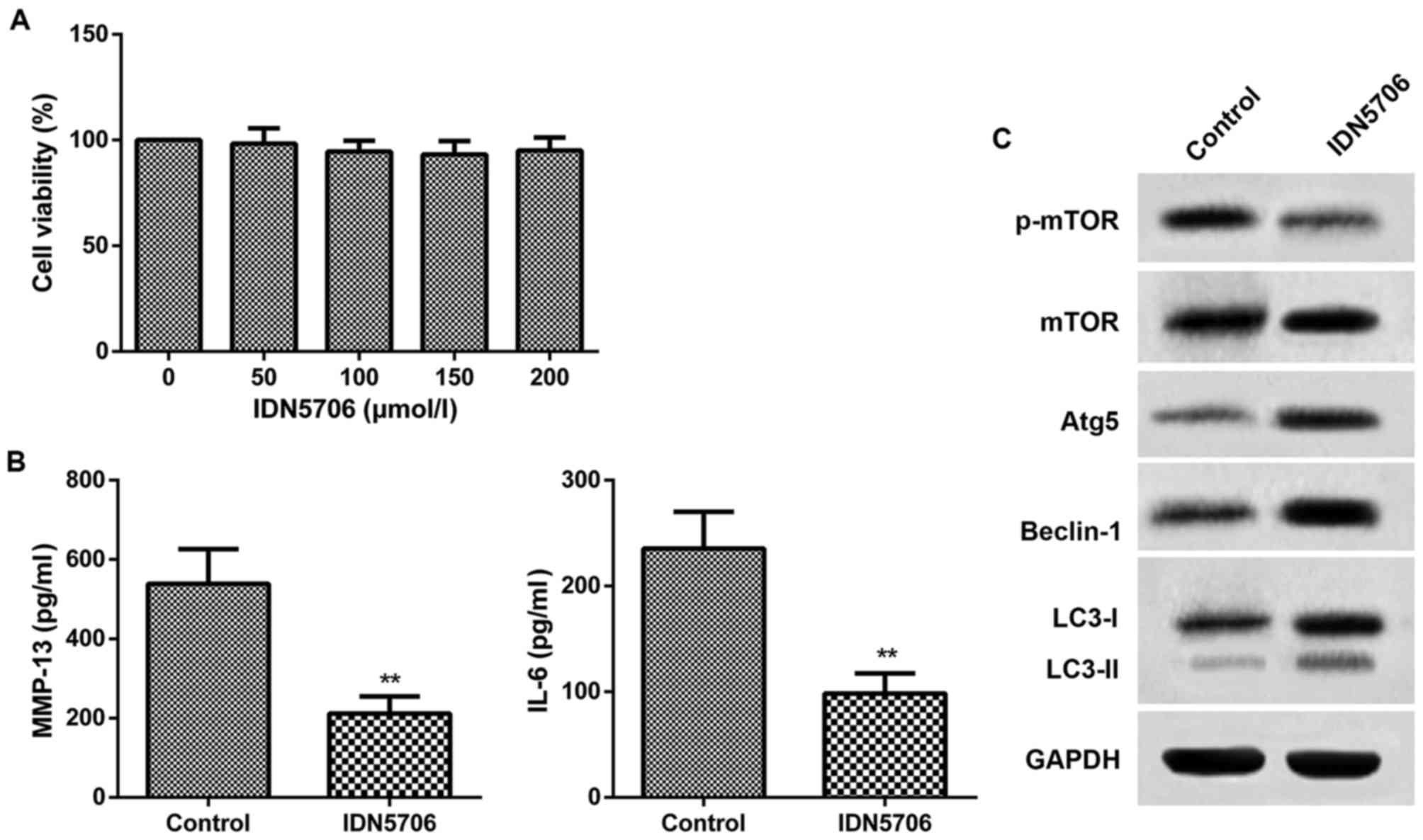
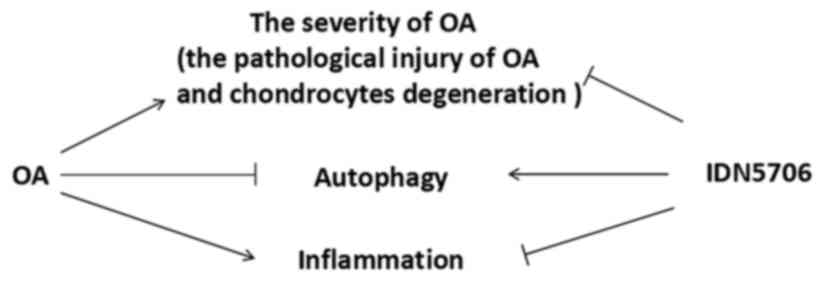
|
1
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blazek K, Favre J, Asay J, Erhart-Hledik J
and Andriacchi T: Age and obesity alter the relationship between
femoral articular cartilage thickness and ambulatory loads in
individuals without osteoarthritis. J Orthop Res. 32:394–402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barenius B, Ponzer S, Shalabi A, Bujak R,
Norlén L and Eriksson K: Increased risk of osteoarthritis after
anterior cruciate ligament reconstruction: A 14-year follow-up
study of a randomized controlled trial. Am J Sports Med.
42:1049–1057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flores R and Hochberg M: Definition and
classification of osteoarthritis. Osteoarthritis. 2:1–8. 1998.
|
|
7
|
Moskowitz RW, Kelly MA and Lewallen DG:
Understanding osteoarthritis of the knee-causes and effects. Am J
Orthop (Belle Mead NJ). 33 2 Suppl:S5–S9. 2004.
|
|
8
|
Spaans AJ, van Minnen LP, Kon M, Schuurman
AH, Schreuders AR and Vermeulen GM: Conservative treatment of thumb
base osteoarthritis: A systematic review. J Hand Surg Am.
40:16–21.e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermeulen GM, Slijper H, Feitz R, Hovius
SE, Moojen TM and Selles RW: Surgical management of primary thumb
carpometacarpal osteoarthritis: A systematic review. J Hand Surg
Am. 36:157–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hara T, Nakamura K, Matsui M, Yamamoto A,
Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I,
Okano H and Mizushima N: Suppression of basal autophagy in neural
cells causes neurodegenerative disease in mice. Nature.
441:885–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komatsu M, Waguri S, Ueno T, Iwata J,
Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et
al: Impairment of starvation-induced and constitutive autophagy in
Atg7-deficient mice. J Cell Biol. 169:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibata M, Lu T, Furuya T, Degterev A,
Mizushima N, Yoshimori T, MacDonald M, Yankner B and Yuan J:
Regulation of intracellular accumulation of mutant huntingtin by
beclin 1. J Biol Chem. 281:14474–14485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF,
Gao SG and Lei GH: Autophagy in osteoarthritis. Joint Bone Spine.
83:143–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Hu J, Pan Y, Shan Y, Jiang L, Qi X
and Jia L: miR-140-5p/miR-149 affects chondrocyte proliferation,
apoptosis, and autophagy by targeting FUT1 in osteoarthritis.
Inflammation. Feb 27–2018.(Epub ahead of print). View Article : Google Scholar
|
|
18
|
Cheng N, Meng H, Ma L, Zhang L, Yu HM,
Wang ZZ and Guo A: Role of autophagy in the progression of
osteoarthritis: The autophagy inhibitor, 3-methyladenine,
aggravates the severity of experimental osteoarthritis. Int J Mol
Med. 39:1224–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wangyang Y, Zheng X, Liu GW, Li DY, Feng
YB, Guo TY, Ma C and Wang T: Upregulation of P63 inhibits
chondrocyte autophagy thereby enhancing the malignant progression
of osteoarthritis. Pharmazie. 72:361–364. 2017.PubMed/NCBI
|
|
20
|
Settembre C, Arteaga-Solis E, Mckee MD, de
Pablo R, Al Awqati Q, Ballabio A and Karsenty G: Proteoglycan
desulfation determines the efficiency of chondrocyte autophagy and
the extent of FGF signaling during endochondral ossification. Genes
Dev. 22:2645–2650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Research. 17:666–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabers CJ, Martin MM, Brunn GJ, Williams
JM, Dumont FJ, Wiederrecht G and Abraham RT: Isolation of a protein
target of the FKBP12-rapamycin complex in mammalian cells. J Biol
Chem. 270:815–822. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shigemitsu K, Tsujishita Y, Hara K,
Nanahoshi M, Avruch J and Yonezawa K: Regulation of translational
effectors by amino acid and mammalian target of rapamycin signaling
pathways. Possible involvement of autophagy in cultured hepatoma
cells. J Biol Chem. 274:1058–1065. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdulrahman BA, Khweek AA, Akhter A,
Caution K, Kotrange S, Abdelaziz DH, Newland C, Rosales-Reyes R,
Kopp B, McCoy K, et al: Autophagy stimulation by rapamycin
suppresses lung inflammation and infection by Burkholderia
cenocepacia in a model of cystic fibrosis. Autophagy. 7:1359–1370.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aso E and Ferrer I: It may be possible to
delay the onset of neurodegenerative diseases with an
immunosuppressive drug (rapamycin). Expert Opin Biol Ther.
13:1215–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esposito C, Villa L, Grosjean F, Mangione
F, Esposito V, Castoldi F, Serpieri N, Arra M, Pertile E, Maggi N,
et al: Rapamycin reduces proteinuria and renal damage in the rat
remnant kidney model. Transplant Proc. 41:1370–1371. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldshmit Y, Kanner S, Zacs M, Frisca F,
Pinto AR, Currie PD and Pinkas-Kramarski R: Rapamycin increases
neuronal survival, and reduces inflammation and astrocyte
proliferation after spinal cord injury. Mol Cell Neurosci.
68:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caramés B, Hasegawa A, Taniguchi N, Miyaki
S, Blanco FJ and Lotz M: Autophagy activation by rapamycin reduces
severity of experimental osteoarthritis. Ann Rheum Dis. 71:575–581.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavieres VA, González A, Muñoz VC, Yefi
CP, Bustamante HA, Barraza RR, Tapia-Rojas C, Otth C, Barrera MJ,
González C, et al: Tetrahydrohyperforin Inhibits the proteolytic
processing of amyloid precursor protein and enhances its
degradation by Atg5-dependent autophagy. PLoS One. 10:e01363132015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koeberle A, Rossi A, Bauer J, Dehm F,
Verotta L, Northoff H, Sautebin L and Werz O: Hyperforin, an
anti-inflammatory constituent from St. John's Wort, inhibits
microsomal prostaglandin E(2) synthase-1 and suppresses
prostaglandin E(2) formation in vivo. Front Pharmacol. 2:72011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bombardelli E, Morazzoni P and Riva A: IDN
5491 (Hyperforin trimethoxybenzoate): A new antidepressive drug.
Eur Neuropsychopharm. 12:2402002. View Article : Google Scholar
|
|
33
|
Chiang IT, Chen WT, Tseng CW, Chen YC, Kuo
YC, Chen BJ, Weng MC, Lin HJ and Wang WS: Hyperforin inhibits cell
growth by inducing intrinsic and extrinsic apoptotic pathways in
hepatocellular carcinoma cells. Anticancer Res. 37:161–167. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39(199): 208–111. 1996.
|
|
35
|
Miyauchi S, Machida A, Onaya J, Sakamoto
T, Tokuyasu K and Iwata H: Alterations of proteoglycan synthesis in
rabbit articular cartilage induced by intra-articular injection of
papain. Osteoarthritis Cartilage. 1:253–262. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mankin HJ, Johnson ME and Lippiello L:
Biochemical and metabolic abnormalities in articular cartilage from
osteoarthritic human hips. III. Distribution and metabolism of
amino sugar-containing macromolecules. J Bone Joint Surg Am.
63:131–139. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang J, Cai H, Lin L, Xie P, Zhong W and
Tang M: Increased expression of CD24 is associated with tumor
progression and prognosis in patients suffering osteosarcoma. Clin
Transl Oncol. 15:541–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marks R: Osteoarthritis and articular
cartilage: Biomechanics and novel treatment paradigms. Adv Aging
Res. 03:297–309. 2014. View Article : Google Scholar
|
|
40
|
Lotz MK and Kraus VB: New developments in
osteoarthritis. Posttraumatic osteoarthritis: Pathogenesis and
pharmacological treatment options. Arthritis Res Ther. 12:2112010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cornelis S, Kersse K, Festjens N, Lamkanfi
M and Vandenabeele P: Inflammatory caspases: Targets for novel
therapies. Curr Pharm Des. 13:367–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
DiMicco MA, Patwari P, Siparsky PN, Kumar
S, Pratta MA, Lark MW, Kim YJ and Grodzinsky AJ: Mechanisms and
kinetics of glycosaminoglycan release following in vitro cartilage
injury. Arthritis Rheum. 50:840–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inoue A, Takahashi KA, Arai Y, Tonomura H,
Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y and Kubo T: The
therapeutic effects of basic fibroblast growth factor contained in
gelatin hydrogel microspheres on experimental osteoarthritis in the
rabbit knee. Arthritis Rheum. 54:264–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khatab S, van Buul G, Kops N,
Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA and van Osch GJ:
Intra-articular injections of platelet-rich plasma releasate reduce
pain and synovial inflammation in a mouse model of osteoarthritis.
Am J Sports Med. 46:977–986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cremers NAJ, van den Bosch MHJ, van Dalen
S, Di Ceglie I, Ascone G, van de Loo F, Koenders M, van der Kraan
P, Sloetjes A, Vogl T, et al: S100A8/A9 increases the mobilization
of pro-inflammatory Ly6Chigh monocytes to the synovium during
experimental osteoarthritis. Arthritis Res Ther. 19:2172017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Adães S, Almeida L, Potes C, Ferreira AR,
Castro-Lopes JM, Ferreira-Gomes J and Neto FL: Glial activation in
the collagenase model of nociception associated with
osteoarthritis. Mol Pain. 13:17448069166882192017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeh TT, Wen ZH, Lee HS, Lee CH, Yang Z,
Jean YH, Wu SS, Nimni ME and Han B: Intra-articular injection of
collagenase induced experimental osteoarthritis of the lumbar facet
joint in rats. Eur Spine J. 17:734–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
van der Kraan PM, Vitters EL, van
Beuningen HM, van de Putte LB and van den Berg WB: Degenerative
knee joint lesions in mice after a single intra-articular
collagenase injection. A new model of osteoarthritis. J Exp Pathol
(Oxford). 71:19–31. 1990.PubMed/NCBI
|
|
49
|
Cawston TE and Wilson AJ: Understanding
the role of tissue degrading enzymes and their inhibitors in
development and disease. Best Pract Res Clin Rheumatol.
20:983–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plaas A, Osborn B, Yoshihara Y, Bai Y,
Bloom T, Nelson F, Mikecz K and Sandy JD: Aggrecanolysis in human
osteoarthritis: Confocal localization and biochemical
characterization of ADAMTS5-hyaluronan complexes in articular
cartilages. Osteoarthritis Cartilage. 15:719–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tamamura Y, Otani T, Kanatani N, Koyama E,
Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici
M, et al: Developmental regulation of Wnt/beta-catenin signals is
required for growth plate assembly, cartilage integrity, and
endochondral ossification. J Biol Chem. 280:19185–19195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kamekura S, Hoshi K, Shimoaka T, Chung U,
Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K and
Kawaguchi H: Osteoarthritis development in novel experimental mouse
models induced by knee joint instability. Osteoarthritis Cartilage.
13:632–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kamekura S, Kawasaki Y, Hoshi K, Shimoaka
T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G,
et al: Contribution of runt-related transcription factor 2 to the
pathogenesis of osteoarthritis in mice after induction of knee
joint instability. Arthritis Rheum. 54:2462–2470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
von der Mark K, Kirsch T, Nerlich A, Kuss
A, Weseloh G, Glückert K and Stöss H: Type X collagen synthesis in
human osteoarthritic cartilage. Indication of chondrocyte
hypertrophy. Arthritis Rheum. 35:806–811. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takeda S, Bonnamy JP, Owen MJ, Ducy P and
Karsenty G: Continuous expression of Cbfa1 in nonhypertrophic
chondrocytes uncovers its ability to induce hypertrophic
chondrocyte differentiation and partially rescues Cbfa1-deficient
mice. Genes Dev. 15:467–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ueta C, Iwamoto M, Kanatani N, Yoshida C,
Liu Y, Enomoto-Iwamoto M, Ohmori T, Enomoto H, Nakata K, Takada K,
et al: Skeletal malformations caused by overexpression of Cbfa1 or
its dominant negative form in chondrocytes. J Cell Biol.
153:87–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kinoshita H, Minamitani K, Komiya S,
Higuchi F, Yamanaka K, Inoue A, Matsuo K, Yoshimoto K and Yokoyama
M: Immunohistochemical studies on the distribution of IL-1 and IL-6
producing cells in the synovial membranes of patients with joint
disorders. Orth Traumatol. 39:622–625. 1990–1991.
|
|
58
|
Seiliez I, Belghit I, Gao Y, Skiba-Cassy
S, Dias K, Cluzeaud M, Rémond D, Hafnaoui N, Salin B, Camougrand N
and Panserat S: Looking at the metabolic consequences of the
colchicine-based in vivo autophagic flux assay. Autophagy.
12:343–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena Acevedo A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|