Introduction
Postoperative infections associated with implants
are one of the most challenging complications after arthroplasty;
they may cause increased cost, hospitalization stays as well as
mortality and morbidity rates (1).
Any type of infection, including pneumonia, urinary tract
infections (UTI) and superficial surgical site infections (SSI)
should be cured during the perioperative period of arthroplasty and
may lead to periprosthetic joint infection. Thus, it is crucial to
detect any infections during the perioperative period of
arthroplasty in order to rapidly initiate adequate antimicrobial
therapy (2–4). In the diagnostic process for detecting
a perioperative infection, one of the most important steps is the
analysis of laboratory biomarkers of infection. Biomarkers, such as
white blood cell count (WBC), erythrocyte sedimentation rate (ESR)
and C-reactive protein (CRP) may be used to aid in the diagnosis,
therapeutic monitoring and risk stratification. However, these
blood parameters lack sensitivity and specificity in discriminating
inflammation due to a bacterial infection from that of a surgical
injury response (5–10). In addition, due to the low positivity
rate of pus culture, this gold standard has low sensitivity. Thus,
the search for realistic laboratory markers is essential. Previous
studies have reported that due to its high specificity,
procalcitonin (PCT) is comparatively more useful for diagnosing
bacterial infections, including sepsis, upper respiratory tract
infections, pneumonia, pancreatitis, pyelonephritis and burns
(11–13). PCT, the 116-amino acid prohormone of
calcitonin, is mainly produced as a precursor hormone of calcitonin
by the neuroendocrine cells of the thyroid and lung; alternative
pathological pathways in patients with inflammation and sepsis have
been described. Bacterial endotoxins have been reported to release
PCT directly into the circulation (14,15). PCT
typically increases 2 to 4 h following an appropriate exposure such
as sepsis (16–20); it reaches its peak after 6 h and has
a half-life of 25–30 h (21,22). A rapid decline occurs following
treatment or removal of the underlying trigger. Compared with CRP,
which only reaches a maximum after 36 h (23,24), PCT
may be detected sooner. The features of PCT suggests that detecting
PCT levels may be better than WBC and CRP for differentiating
between sepsis, aseptic inflammation and traumatic injury in
certain clinical settings, particularly following orthopaedic
surgery. However, only a small number of studies (25) have investigated the value of
diagnosing perioperative infection by detecting PCT levels during
the period of primary hip and knee arthroplasty and comparing them
with other biomarkers, such as WBC. Thus, it appeared worthwhile to
perform a study to determine the specificity and the sensitivity of
the characteristics of PCT for diagnosing infections during the
perioperative period of arthroplasty. As the serum levels of PCT
and WBC are measured in all patients undergoing arthroplasty at our
department, it was possible to perform a retrospective study. The
present study aimed to evaluate the serum PCT levels in patients
with perioperative infection following arthroplasty and to
determine the cut-off value that it may represent. The present
study hypothesized that the elevation of PCT levels in patients
undergoing primary arthroplasty may help to detect perioperative
infections.
Patients and methods
Study design and inclusion
criteria
The cohort analysis for this retrospective study was
approved by the local Ethics Committee of Huizhou Municipal Central
Hospital (Huizhou, China). Data were obtained from the hospital's
electronic medical record system. Two patient cohorts who were
treated between July 2014 and August 2015 at the Department of
Orthopaedic Surgery (Huizhou Municipal Central Hospital, Huizhou,
China) were enrolled. A consecutive series of patients undergoing
primary hip and knee arthroplasty was retrospectively reviewed and
all patients who developed postoperative infections, including
pneumonia and UTI occurring within two weeks and superficial SSI
occurring within 30 days (case group) were included. A tourniquet
was used during the knee surgery, and the duration was 60–90 min.
Antiseptic agent (iodine) was used on all patients and sterile
covering for preoperative skin preparation was performed;
furthermore, the skin incisions were mended using the same method
of suture. None of the patients of the present study received any
postoperative blood transfusion.
The inclusion criteria for the case group diagnosis
with pneumonia, UTI or superficial SSI. A diagnosis of pneumonia
required a new pulmonary infiltrate at the time of hospitalization,
and at least one of the following: New or increased cough,
leukocytosis, leukopenia or left shift pattern on WBC count, and a
body temperature of >37.8°C or <35.6°C. A diagnosis of UTI
required at least one of the following symptoms, such as fever
(>38°C), dysuria, pollakiuria, suprapubic tenderness and having
≥105 colony-forming units/ml of one or two types of
bacteria; for culture-negative patients, at least two of the
above-mentioned symptoms and one of the seven criteria defined by
the Centers for Disease Control and Prevention, such as nitrite
test positivity and pyuria, were required for inclusion in the
study (26). Asymptomatic patients
were excluded. The diagnosis of a superficial SSI included an
infection occurring within 30 days after the operation on a
surgical site, except for deep incisional SSI and periprosthetic
joint infections (PJI) (27,28). As presented in Table I, the classification of SSI
categorizes the infection into three groups, namely superficial
incisional, deep incisional and organ-space SSI (28).
 | Table I.Classification of SSI. |
Table I.
Classification of SSI.
| Type of SSI | Definition |
|---|
| Superficial
incisional SSI | Infection involves
only skin or subcutaneous tissue and at least one of the
following: |
|
| −Purulent drainage
from the superficial incision, with or without laboratory
result |
|
| −Isolated
microorganism from a culture of fluid or tissue |
|
| −Pain, swelling, heat
or redness at the surgical site |
|
| −Diagnosis of
superficial incisional SSI established by surgeon or attending
physician |
| Deep incisional
SSI | Infection involves
deep soft tissues such as fascia or muscle layer and at least one
of the following: |
|
| −Purulent drainage
from the deep incision but not from organ or organ space
component |
|
| −A deep incision
spontaneously dehisces or is deliberately opened by a surgeon when
the patient has at least one of the following signs: Fever
(>38°C), localized pain or tenderness |
|
| −An abscess or other
evidence of deep infection that is found on direct
histopathological or radiological examination |
|
| −Diagnosis of deep
incisional SSI made by a surgeon or attending physician |
| Organ or space
SSI | Infection involves
organs or spaces, other than the incision site and at least one of
the following: |
|
| −Purulent drainage
from the organ or space |
|
| −Isolated
microorganism from a culture of fluid or tissue |
|
| −An abscess or
other evidence of deep infection that is found on direct
examination or on histopathological or radiological
examination |
|
| −Diagnosis of
organ/space SSI by a surgeon or attending physician |
| SSI, surgical site
infection. |
|
A retrospective chart review was performed for each
surgical site to identify patients who met the inclusion criteria
for the case group. The control group was selected based on a
randomization table to gather a matched number of patients from
those treated during the same time span as the control group.
Patients in the control group underwent primary hip and knee
arthroplasty but did not develop any postoperative infection
complications during the inpatient stay, in the first month after
operation, and at the final 12–24-month follow-up exam. Patients of
the case and the control group were excluded from the study if they
met one of the following exclusion criteria: Possible precondition
for elevated inflammatory markers, including chronic inflammatory
diseases, obesity (body mass index, >30 kg/m2), viral
infections, malignancies, heavy smoking, inflammation other than an
orthopaedic infection (e.g., autoimmunity, intercurrent febrile
infection) for the purpose of avoiding interference with other
inflammatory processes, and patients with hepatic failure, or
deficiencies of the kidneys or the immune system.
Based on the inclusion criteria, a total of 500
patients were reviewed to obtain 25 patients with perioperative
infections in the case group, and 25 patients without any
associated complications were enrolled in the control group. The
mean levels of WBC and PCT were compared between these groups, and
the sensitivity, specificity and predictive values of WBC and PCT
were assessed.
Laboratory analyses
Blood samples for analysis had been collected at the
following time-points: Preoperatively (D0) and on day 4 (D4), D6
and D8 post operation. Blood was taken from the cubital vein. Serum
levels of PCT were measured using a KRYPTOR
electrochemiluminescence immunoassay and a Cobas 8000 modular
analyser (Roche Diagnostics GmbH, Mannheim, Germany) and WBC were
measured using an XE-5000 automated hematology system (Sysmex
Corp., Kobe, Japan) at the Institute of Clinical Chemistry of
Huizhou Municipal Central Hospital (Huizhou, China).
Statistical analysis
Descriptive data analyses were performed using SPSS
version 19.0 for Windows (IBM Corp., Armonk, NY, USA). Prior to
assessing the association or difference, a one-Sample
Kolmogorov-Smirnov test was used to assess descriptive values for
normality. For comparing the difference between two independent
samples, the Mann-Whitney U-test was used if the descriptive
statistics had an abnormal distribution; otherwise, the Student's
t-test was used. For comparing the constituent ratio difference
between two independent samples, Pearson's Chi-square test was
used. A box plot was used to display the distribution range of PCT
levels. Receiver operator characteristic (ROC) curves were
generated to determine the best cut-off values and to calculate
individual specificity and sensitivity for PCT and WBC. P<0.01
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 50 patients were included in the present
retrospective cohort study. In the case group (n=25), 4 patients
had pneumonia, 7 had UTI and 14 had superficial SSI. Indwelling
catheters occurred in 6 patients of the case group and 2 patients
of the control group. Among the 7 patients with UTI, 4 had
indwelling catheters. Of the 25 patients in the case group, 20
underwent total hip arthroplasty (THA) and the other 5 underwent
total knee arthroplasty (TKA). Of the 25 patients in the control
group, 14 underwent THA and the other 11 underwent TKA. Except at
D8 in the control group (P<0.01), no significant difference was
identified in the PCT levels between patients who underwent THA and
those who underwent TKA, including D0, D4, D6 and D8 (P>0.01).
However, 5 patients required additional surgical debridement, and
all of the 14 patients with SSI recovered following treatments
including prolonged hospital stay and antibiotics.
The patient demographics are provided in Table II. There were no significant
differences in age (P=0.056), patient gender (P=0.508) or joint
distribution (P=0.069) between the groups.
 | Table II.Patient demographics. |
Table II.
Patient demographics.
| Group | Patients (n) | Mean age
(years) | Sex
(female/male) | Hip/knee
arthroplasty |
|---|
| Case | 25 | 69.12±9.94 | 18/7 | 20/5 |
| Control | 25 | 63.28±11.13 | 20/5 | 14/11 |
| t-test |
| 1.956 | 0.439 | 3.309 |
| P-value |
| 0.056 | 0.508 | 0.069 |
All data of the present study followed a normal
distribution. Comparison of the means of the PCT levels between the
case and the control group indicated a significant difference at D8
(P=0.007), while no significant difference was observed at D0
(P=0.01), D4 (P=0.069) and D6 (P=0.093). In addition, no
statistical significance was observed regarding the differences in
WBC levels between the two groups (P>0.01; Table III).
 | Table III.Descriptive statistics of parameters
at various perioperative time-points. |
Table III.
Descriptive statistics of parameters
at various perioperative time-points.
|
| PCT (ng/ml) | WBC
(×109/l) |
|---|
|
|
|
|
|---|
| Group | D0 | D4 | D6 | D8 | D0 | D4 | D6 | D8 |
|---|
| Case | 0.121±0.166 | 0.510±1.208 | 0.527±1.360 | 0.686±1.117 | 7.556±3.037 | 7.932±2.266 | 8.336±2.777 | 8.148±2.013 |
| Control | 0.031±0.011 | 0.062±0.020 | 0.051±0.019 | 0.032±0.015 | 7.036±2.537 | 7.636±1.681 | 8.892±2.013 | 8.548±2.252 |
| t-test | 2.697 | 1.859 | 1.750 | 2.925 | 0.657 | 0.525 | −0.811 | −0.662 |
| P-value | 0.01 | 0.069 | 0.093 | 0.007 | 0.514 | 0.602 | 0.422 | 0.511 |
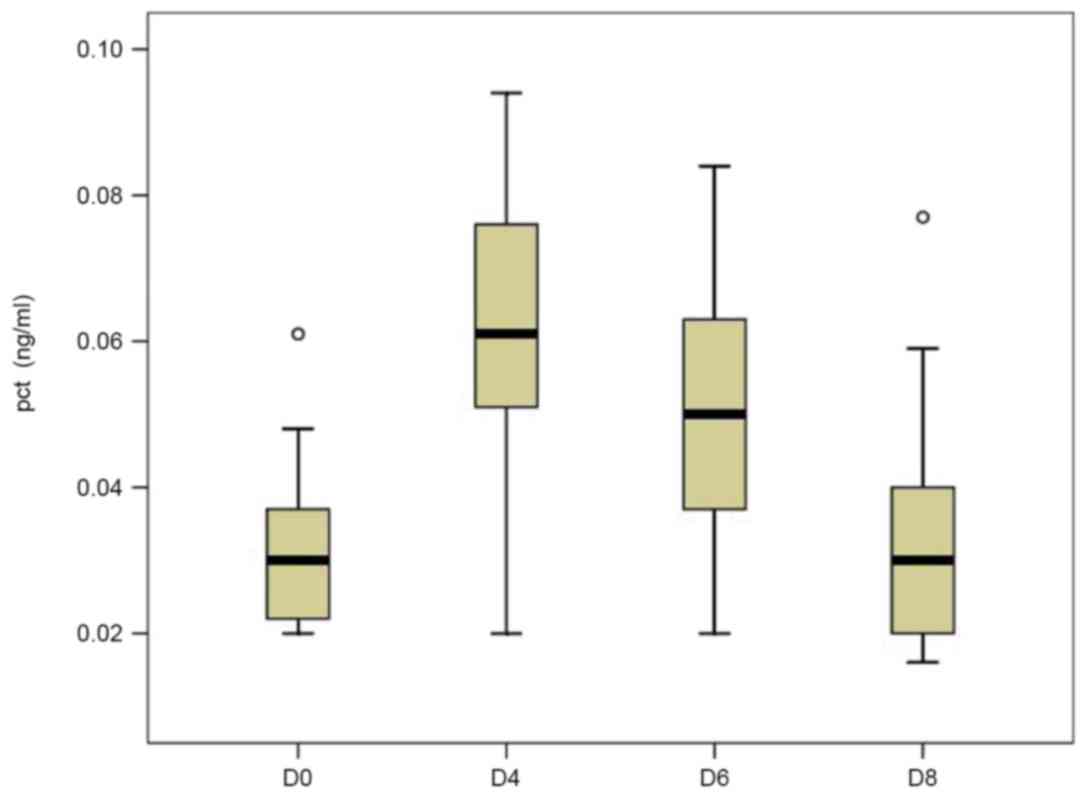
In the control group, the mean serum levels of PCT
at D0 (0.031±0.011 ng/ml) were in the normal range (0–0.05 ng/ml)
and had increased by two-fold by D4 (0.062±0.020 ng/ml). However,
it had rapidly decreased by D6 (0.051±0.019 ng/ml) and returned to
the normal range on D8 (0.032±0.015 ng/ml; Fig. 1). Although the preoperative mean
serum levels of PCT in the case group (0.121±0.166 ng/ml) were
higher than those in the control group (0.031±0.011 ng/ml), no
statistical significance was observed. Similarly to those in the
control group, the PCT levels in the case group had rapidly
increased on D4 (0.510±1.208 ng/ml); however, they continuously
increased on D6 (0.527±1.360 ng/ml) and D8 (0.686±1.117 ng/ml;
Fig. 2). From a clinical point of
view, infection events were indicated in these patients during the
postoperative period.
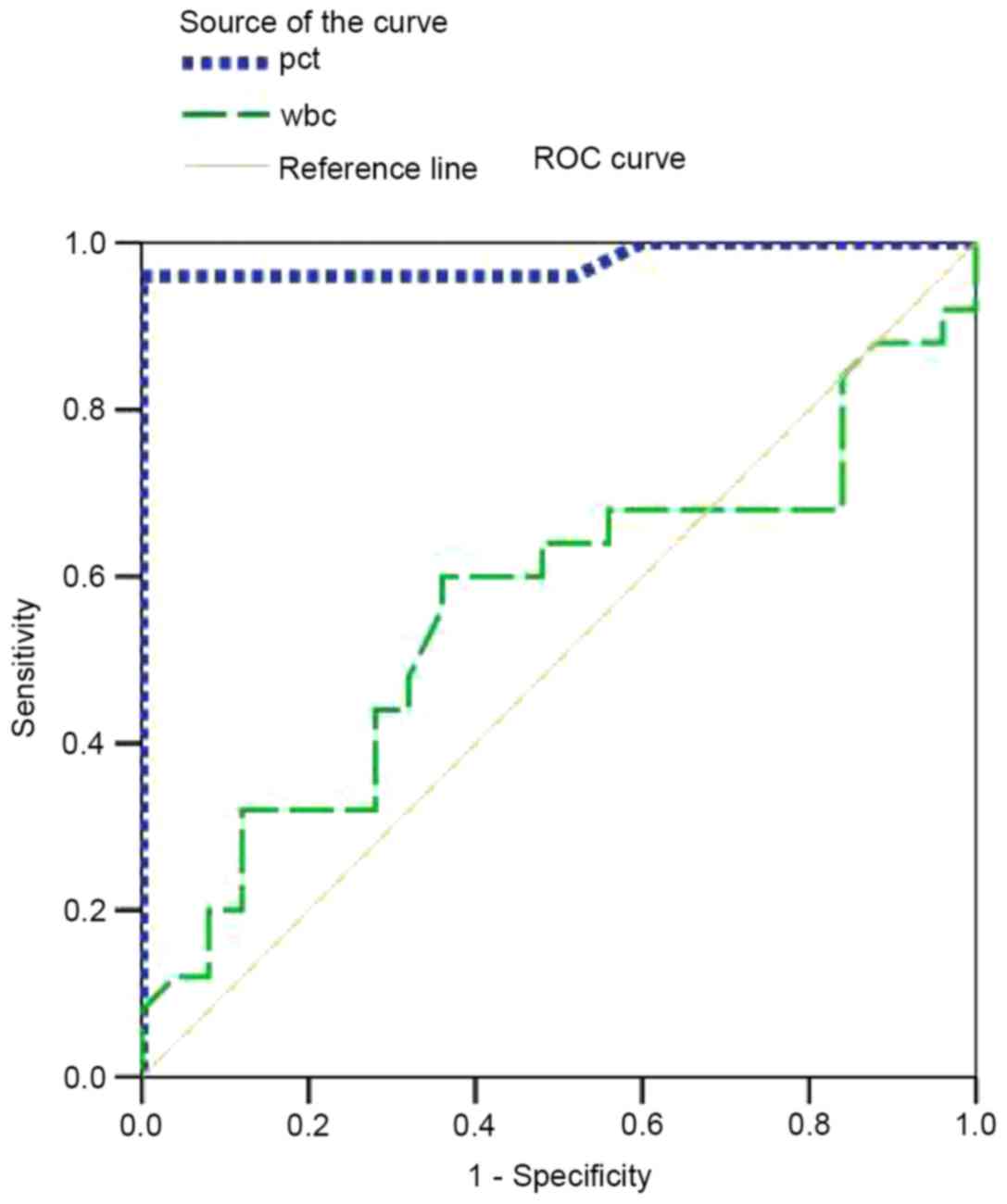
To measure the best cut-off values and calculate the
individual specificity and sensitivity of PCT and WBC, ROC curves
were generated (Fig. 3). A summary
of the results is provided in Table
IV. To compare the capability of the two biomarkers to
distinguish between patients without infection from those with
infection, the area under the curve (AUC) was calculated. For PCT,
the AUC was 0.978 [95% confidence interval (CI), 0.933–1.022]; for
WBC, the AUC was 0.562 (95% CI, 0.398–0.0.726). Based on the above
data, the PCT value was a significant predictor of infection
(AUC>0.9). For PCT, the cut-off point of 0.0995 ng/ml was
associated with a sensitivity of 96% and a specificity of 100%
(Fig. 3). At a cut-off of 0.526
ng/ml, PCT was found to be 36% sensitive and 100% specific in
diagnosing infections. However, WBC was not a significant predictor
of infection (0.5<AUC<0.7). A WBC value of
7.05×109/l was associated with a sensitivity of 64% and
a specificity of 44%. For combining different parameters, the point
with the minimal distance on the ROC curve was considered to be the
optimal threshold. Obviously, PCT had a higher sensitivity and
specificity compared with WBC.
 | Table IV.Results of the receiver operating
characteristic curve analysis. |
Table IV.
Results of the receiver operating
characteristic curve analysis.
| Parameter | AUC (95% CI) | Cut-off (ng/ml or
1/1) | Sensitivity
(%) | Specificity
(%) |
|---|
| PCT | 0.978
(0.933–1.022) | 0.0565 | 100 | 40 |
|
|
| 0.059 | 96 | 48 |
|
|
| 0.0995 | 96 | 100 |
|
|
| 0.526 | 36 | 100 |
| WBC | 0.562
(0.398–0.726) |
3.2×109 | 100 | 0 |
|
|
|
7.05×109 | 64 | 44 |
|
|
|
9.2×109 | 36 | 72 |
|
|
|
10.9×109 | 8 | 100 |
Discussion
Any infections, including pneumonia, UTI and
superficial SSI, are dangerous during the perioperative period of
arthroplasty, as they may lead to the occurrence of PJI, a serious
postoperative complication following arthroplasty (29–31). To
prevent unnecessary PJI following perioperative infections, an
early and correct diagnosis is important. The present study
retrospectively analysed the plasma concentration of PCT and the
WBC with regard to their sensitivity and specificity for detecting
perioperative infections.
PCT, the 116-amino-acid prohormone of calcitonin, is
mainly produced by the parafollicular cells of the thyroid in
healthy individuals, while alternative pathological pathways have
been described in patients with inflammation and sepsis. PCT is
widely used as a diagnostic marker of sepsis and systemic
inflammatory response syndrome (32), and has been demonstrated to be a more
accurate marker for the detection of early postoperative infection
after cardiac, intestinal and major neural surgeries compared with
standard laboratory parameters, such as CRP and WBC (33–36). In
these cases, inflammatory cytokines, such as tumor necrosis
factor-α, interleukin-1β and fragments of cell walls or membranes
of microbes, such as lipopolysaccharides or peptidoglycans, may
induce PCT production. In the control group, the mean serum
concentrations of PCT peaked at D4 and had decreased on D6 post
surgery. Surgical trauma may have been the cause of the transient
elevation of PCT. Due to the consistent and rapid increase in PCT
levels post surgery in the case group, the PCT trend may be used as
a marker for possible infection in the early postoperative period.
In the control group, none of the patients had any perioperative
infection. The preoperative results in the control group
convincingly demonstrated that aseptic pathological changes in hip
and knee did not cause any significant increases in PCT. These
findings indicated that a sudden elevation at D6 is suggestive of a
bacterial infection; this was indicated in the case group, in which
a sudden and continuous elevation of PCT levels was identified. In
addition, the post-operative levels of PCT in the case group were
much higher than those commonly seen in the control group. In the
case group, the most common type of infection was superficial SSI,
which was seen in 14 patients (56%), while 7 patients (28%) had a
UTI and 4 patients (16%) had pneumonia. Among the 7 patients with a
UTI, 4 patients had indwelling catheters; therefore, an indwelling
catheter was likely to be one of the factors causing UTI. Previous
studies reported that 70–80% of complicated UTIs are attributable
to indwelling catheters in the US (37), accounting for ~1 million cases per
year (38). All of the infections
were caused by various bacteria. Bacterial endotoxins have been
reported to release PCT directly into the circulation (14,15). PCT
increases at 2–4 h following an insult such as sepsis (16–20),
reaches its peak after 6 h and has a half-life of 25–30 h (21,22). A
rapid decline occurs following treatment or removal of the
underlying trigger. Based on the results of the present study, the
serum PCT levels continue to increase from D6 to D8 post surgery if
an infection is present; therefore, any significant elevations of
PCT at D6 may suggest the occurrence of infection or a different
type of inflammation. Based on these results, attention should be
paid on the SSI, the most common type of perioperative infection,
and approaches to reduce the risk of SSI, including antimicrobial
prophylaxis, preoperative optimization of anaemia and diabetes,
preoperative chlorhexidine washes and adjustment of operation
duration should be pursued. In addition, routine use of an
indwelling catheter is not recommended due to it being one of the
factors causing UTI.
Although numerous studies have reported that the
highest serum PCT values occur in patients with sepsis (32), they are also increased in
inflammatory conditions, such as trauma following extensive surgery
(25,39,40). In
addition, unspecific or trauma-associated induction of PCT has been
reported during the perioperative period. Thus, it is important to
determine cut-off values suitable for different types of surgery
and local bacterial infections. In the present study, the kinetics
of PCT were analysed in patients during the perioperative period of
arthroplasty, including infection and aseptic cases. Previous
studies suggested that PCT values of >2 ng/ml are strongly
indicative of sepsis or severe bacterial infection, while these
condition are unlikely if PCT levels are <0.5 ng/ml (41,42).
Hügle et al (43)
demonstrated that PCT has high sensitivity but low specificity at a
cut-off value of 0.25 ng/ml. However, the present study identified
that the cut-off point of 0.0995 ng/ml was associated with the
highest sensitivity (96%) and specificity (100%). In addition,
other studies reported that PCT is a marker with poor sensitivity
but high specificity at a cut-off of 0.5 ng/ml (22,44,45).
Bottner et al (46) reported
a high specificity (98%) but low sensitivity (33%) in the detection
of deep chronic periprosthetic infection for PCT levels with a
cut-off at 0.3 ng/ml. Similarly, in the present study, at a cut-off
of 0.526 ng/ml, PCT had 36% sensitivity and 100% specificity for
diagnosing infections. For any novel diagnostic marker, balancing
sensitivity and specificity is essential. In the present study,
0.0995 ng/ml was taken as the cut-off for diagnosing perioperative
infection.
Of note, the present study had several limitations.
Data were collected retrospectively from a single centre, and the
sample size was low for a study investigating perioperative
infections in patients undergoing primary hip and knee
arthroplasty.
In summary, the present study suggested that PCT is
a promising marker for diagnosing bacterial infections due to its
high specificity. Based on the sensitivity and the specificity of
PCT, detecting PCT may be more valuable than using WBC in the
diagnosis of septic pathological changes in the perioperative
period. Large retrospective studies analysing the serum PCT levels
in patients with infections following primary hip and knee
arthroplasty may provide further insight into the diagnostic value
of PCT in the future.
References
|
1
|
Dale H, Hallan G, Hallan G, Espehaug B,
Havelin LI and Engesaeter LB: Increasing risk of revision due to
deep infection after hip arthroplasty. Acta Orthop. 80:639–645.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costerton JW, Post JC, Ehrlich GD, Hu FZ,
Kreft R, Nistico L, Kathju S, Stoodley P, Hall-Stoodley L, Maale G,
et al: New methods for the detection of orthopedic and other
biofilm infection. FEMS Immunol Med Microbiol. 61:133–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savarino L, Baldini N, Tarabusi C,
Pellacani A and Giunti A: Diagnosis of infection after total hip
replacement. J Biomed Mater Res B Appl Biomater. 70:139–145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Kleunen JP, Knox D, Garino JP and Lee
GC: Irrigation and dèbridement and prosthesis retention for
treating acute periprosthetic infections. Clin Orthop Relat Res.
468:2024–2028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Leeuwen MA and van Rijswijk MH: Acute
phase proteins in the monitoring of inflammatory disorders.
Baillieres Clin Rheumato. 8:531–552. 1994. View Article : Google Scholar
|
|
6
|
Rafiq M, Worthington T, Tebbs SE, Treacy
RB, Dias R, Lambert PA and Elliott TS: Serological detection of
Gram-positive bacterial infection around prostheses. J Bone Joint
Surg Br. 82:1156–1161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itasaka T, Kawai A, Sato T, Mitani S and
Inoue H: Diagnosis of infection after total hip arthroplasty. J
Orthop Sci. 6:320–326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Cesare PE, Chang E, Preston CF and Liu
CJ: Serum interleukin-6 as a marker of periprosthetic infection
following total hip and knee arthroplasty. J Bone Joint Surg Am.
87:1921–1927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitaka C: Clinical laboratory
differentiation of infectious versus non-infectious systemic
inflammatory response syndrome. Clin Chim Acta. 351:17–29. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Falcoz PE, Laluc F, Toubin MM, Puyraveau
M, Clement F, Mercier M, Chocron S and Etievent JP: Usefulness of
procalcitonin in the early detection of infection after thoracic
surgery. Eur J Cardiothorac Surg. 27:1074–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delèvaux I, André M, Colombier M,
Albuisson E, Meylheuc F, Bègue RJ, Piette JC and Aumaître O: Can
procalcitonin measurement help in differentiating between bacterial
infection and other kinds of inflammatory processes? Ann Rheum Dis.
62:337–340. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eberhard K, Haubitz M, Brunkhorst M, Kliem
V and Koch M: Usefulness of procalcitonin for differentiation
between activity of systemic autoimmune disease and invasive
bacterial infection. Arthritis Rheum. 40:1250–1256. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Becker KL, Snider R and Nylen ES:
Procalcitonin assay in systemic inflammation, infection and sepsis:
Clinical utility and limitations. Crit Care Med. 36:941–952. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dandona P, Nix D, Wilson MF, Aljada A,
Love J, Assicot M and Bohuon C: Procalcitonin increase after
endotoxin injection in normal subjects. J Clin Endocrinol Metab.
79:1605–1608. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reinhart K, Karzai W and Meisner M:
Procalcitonin as a marker of the systemic inflammatory response to
infection. Intensive Care Med. 26:1193–1200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carrol ED, Thomson AP and Hart CA:
Procalcitonin as a marker of sepsis. Int J Antimicrob Agents.
20:1–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunziker S, Hügle T, Schuchardt K,
Groeschl I, Schuetz P, Mueller B, Dick W, Eriksson U and Trampuz A:
The value of serum procalcitonin level for differentiation of
infectious from noninfectious causes of fever after orthopaedic
surgery. J Bone Joint Surg Am. 92:138–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nascimento-Carvalho CM, Cardoso MR, Barral
A, Araujo-Neto CA, Guerin S, Saukkoriipi A, Paldanius M, Vainionpaa
R, Lebon P, Leinonen M, et al: Procalcitonin is useful in
identifying bacteraemia among children with pneumonia. Scand J
Infect Dis. 42:644–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pramod J and Singh A: Sepsis biomarkers.
Am J Med. 121:e112008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruiz-Alvarez MJ, García-Valdecasas S, De
Pablo R, Sanchez García M, Coca C, Groeneveld TW, Roos A, Daha MR
and Arribas I: Diagnostic efficacy and prognostic value of serum
procalcitonin concentration in patients with suspected sepsis. J
Intensive Care Med. 24:63–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gendrel D and Bohuon C: Procalcitonin, a
marker of bacterial infection. Infection. 25:133–134. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinot M, Sordet C, Soubrier M, Puéchal
X, Saraux A, Lioté F, Guggenbuhl P, Legre V, Jaulhac B, Maillefert
JF, et al: Diagnostic value of serum and synovial procalcitonin in
acute arthritis: A prospective study of 42 patients. Clin Exp
Rheumato. 23:303–310. 2005.
|
|
23
|
Simon L, Gauvin F, Amre DK, Saint-Louis P
and Lacroix J: Serum procalcitonin and C-reactive protein levels as
markers of bacterial infection: A systematic review and
meta-analysis. Clin Infect Dis. 39:206–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foglar C and Lindsey RW: C-reactive
protein in orthopedics. Orthopedics. 21:687–691. 1998.PubMed/NCBI
|
|
25
|
Meisner M, Tschaikowsky K, Hutzler A,
Schick C and Schüttler J: Postoperative plasma concentrations of
procalcitonin after different types of surgery. Intensive Care
Medicine. 24:680–684. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garner JS, Jarvis WR, Emori TG, Horan TC
and Hughes JM: CDC definitions for nosocomial infections, 1988. Am
J Infect Control. 16:128–140. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
CDC: Surgical site infections: Resources
for patients and healthcare providers. http://www.cdc.gov/ncidod/dhqp/dpac_ssiMarch
10–2010
|
|
28
|
Mangram AJ, Horan TC, Pearson ML, Silver
LC and Jarvis WR: Guideline for prevention of surgical site
infection. Infect Control Hosp Epidemiol. 20:247–264. 1999.
View Article : Google Scholar
|
|
29
|
Pulido L, Ghanem E, Joshi A, Purtill JJ
and Parvizi J: Periprosthetic joint infection: The incidence,
timing, and predisposing factors. Clin Orthop Relat Res.
466:1710–1715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choong PF, Dowsey MM, Carr D, Daffy J and
Stanley P: Risk factors associated with acute hip prosthetic joint
infections and outcome of treatment with a rifampinbased regimen.
Acta Orthop. 78:755–765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Phillips JE, Crane TP, Noy M, Elliott TS
and Grimer RJ: The incidence of deep prosthetic infections in a
specialist orthopaedic hospital: A 15-year prospective survey. J
Bone Joint Surg Br. 88:943–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Assicot M, Gendrel D, Carsin H, Raymond J,
Guilbaud J and Bohuon C: High serum procalcitonin concentrations in
patients with sepsis and infection. Lancet. 341:515–518. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laffey JG, Boylan JF and Cheng DC: The
systemic inflammatory response to cardiac surgery. Anesthesiology.
97:215–252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jebali MA, Hausfater P, Abbes Z, Aouni Z,
Riou B and Ferjani M: Assessment of the accuracy of procalcitonin
to diagnose postoperative infection after cardiac surgery.
Anesthesiology. 107:232–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laifer G, Wasner M, Sendi P, Graber P,
Gratzl O, Huber P, Fluckiger U and Zimmerli W: Dynamics of serum
procalcitonin in patients after major neurosurgery. Clin Microbiol
Infect. 11:679–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oberhofer D, Rumenjak V, Lazić J and Vucić
N: Inflammatory indicators in patients after surgery of the large
intestine. Acta Medica Croatica. 60:429–433. 2006.(In Croatian).
PubMed/NCBI
|
|
37
|
Lo E, Nicolle LE, Coffin SE, Gould C,
Maragakis LL, Meddings J, Pegues DA, Pettis AM, Saint S and Yokoe
DS: Strategies to prevent catheter-associated urinary tract
infections in acute care hospitals: 2014 update. Infect Control
Hosp Epidemiol. 35:464–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Foxman B: The epidemiology of urinary
tract infection. Nature Rev Urol. 7:653–660. 2010. View Article : Google Scholar
|
|
39
|
Carsin H, Assicot M, Feger F, Roy O,
Pennacino I, Le Bever H, Ainaud P and Bohuon C: Evolution and
significance of circulating procalcitonin levels compared with
IL-6, TNF alpha and endotoxin levels early after thermal injury.
Burns. 23:218–224. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wanner GA, Keel M, Steckholzer U, Beier W,
Stocker R and Ertel W: Relationship between procalcitonin plasma
levels and severity of injury, sepsis, organ failure and mortality
in injured patients. Crit Care Med. 28:950–957. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Uçkay I, Garzoni C, Ferry T, Harbarth S,
Stern R, Assal M, Hoffmeyer P, Lew D and Bernard L: Postoperative
serum pro-calcitonin and C-reactive protein levels in patients with
orthopedic infections. Swiss Med Wkly. 140:w131242010.PubMed/NCBI
|
|
42
|
Boussekey N, Leroy O, Georges H, Devos P,
D'Escrivan T and Guery B: Diagnostic and prognostic values of
admission procalcitonin levels in community-acquired pneumonia in
an intensive care unit. Infection. 33:257–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hügle T, Schuetz P, Mueller B, Laifer G,
Tyndall A, Regenass S and Daikeler T: Serum procalcitonin for
discrimination between septic and non-septic arthritis. Clin Exp
Rheumato. 26:453–456. 2008.
|
|
44
|
Fottner A, Birkenmaier C, von Schulze PC,
Wegener B and Jansson V: Can serum procalcitonin help to
differentiate between septic and nonseptic arthritis? Arthroscopy.
24:229–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Faesch S, Cojocaru B, Hennequin C, Pannier
S, Glorion C, Lacour B and Chéron G: Can procalcitonin measurement
help the diagnosis of osteomyelitis and septic arthritis? A
prospective trial. Ital J Pediatr. 35:332009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bottner F, Wegner A, Winkelmann W, Becker
K, Erren M and Götze C: Interleukin-6, procalcitonin and TNF-alpha:
Markers of peri-prosthetic infection following total joint
replacement. J Bone Joint Surg Br. 89:94–99. 2007. View Article : Google Scholar : PubMed/NCBI
|