Introduction
Intervertebral disc degeneration is a major
pathological process that occurs in the lower back and is the main
cause of disc-associated diseases, including disc herniation and
spinal stenosis (1–4). Previous studies have indicated that the
underlying cause of disc degeneration is tissue weakening, which
occurs primarily due to genetic inheritance, aging, inadequate
nutritional status and loading history (5). Several other factors may also influence
the aging and degeneration of discs, including metabolite transport
impairment, cell senescence and death, genetic inheritance, changes
in matrix macromolecules and water content, alterations in enzyme
activity, structural failure and neurovascular ingrowth (5).
Several studies have reported the important role of
angiogenesis in degeneration of the intervertebral disc (6,7).
Degenerative intervertebral disc disorders are thought to be
characterized by angiogenesis and the increased expression of
vascular endothelial growth factor (VEGF), an angiogenic factor
(8). Wang et al (9) also concluded that degeneration of the
intervertebral disc was accompanied by angiogenesis. David et
al (10) demonstrated that
angiogenesis influences the pain intensity of intervertebral disc
hernias and negatively impacts postoperative pain improvement,
mobility and overall quality of life.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
composed of 20–22 nucleotides, which inhibit protein expression by
binding to the 3′-untranslated region of target mRNAs, leading to
transcriptional repression or degradation of the mRNA (11). MiR-21 is an oncogenic miRNA that is
overexpressed in several human tumors and has the ability to
modulate cancer-associated target gene expression (12). Notably, it has been reported that
miR-21 overexpression impairs angiogenesis in normal epithelial
cells (13). Zhao et al
(12) demonstrated that
arsenite-induced carcinogenesis involves angiogenesis mediated by
miR-21. Liu et al (14)
revealed that miR-21 overexpression induced tumor angiogenesis and
increased the expression of hypoxia inducible factor (HIF). HIF-1α
has an important role in angiogenesis and can stimulate the
expression of VEGF (15); HIF-1α has
also been reported to play an important role in the development of
degenerative processes in the intervertebral discs of mice
(16).
The aforementioned studies indicate the importance
of miR-21 in angiogenesis. The present study aimed to investigate
if miR-21 had a critical role in the progression of intervertebral
disc degeneration through HIF-1α and VEGF expression regulation. A
rat model of intervertebral disc degeneration was established and
the model rats were administered miR-21 inhibitor (antagomiR-21).
The vertebral pulp and annulus fibrosus were isolated for
immunohistochemical analysis to examine the effects of miR-21 on
HIF-1α and VEGF expression. The proteoglycan content in the lumbar
spines and disc cell apoptosis was also detected.
Materials and methods
Animals
A total of 60 1-year-old specific pathogen free
female Sprague-Dawley rats (150–200 g) were used in strict
accordance with the guidelines for the Care and Use of Laboratory
Animals (17). The present study was
approved by the Animal Ethics Association of The Affiliated Second
Hospital of Soochow University (Suzhou, China). The rats were
purchased from the Animal Laboratory of the Academy of Medical
Sciences (Beijing, China). They were kept in separate cages with
free access to food and water, and a 12/12 h light/dark cycle
(temperature, 25±1°C; humidity, 50%).
Experimental groups
Rats were randomly divided into four groups of 15.
The normal control group received a skin incision, which were
subsequently sutured. The rats in the three other groups underwent
a previously described surgical procedure to induce the development
of lumbar intervertebral disc degeneration (18). Briefly, the rats were anesthetized by
an intraperitoneal injection of 350 mg/kg 6.5% chloral hydrate. The
sacrospinal muscles, spinous processes, supraspinous ligaments,
interspinous ligaments and posterolateral halves of the bilateral
zygapophysial joints of the lumbar spine were removed.
Subsequently, rats in the model (control) group
received a tail vein injection of normal saline; rats in the
scramble group received a tail vein injection of 80 mg/kg/day
control oligonucleotides (Guangzhou RiboBio Co., Ltd., Guangzhou,
China); rats in the antagomiR-21 group received a tail vein
injection of 80 mg/kg/day of antagomiR-21 (Guangzhou RiboBio Co.,
Ltd.). Injections were administered for 8 weeks. Following this,
rats were euthanized by an intraperitoneal overdose of
pentobarbital sodium. Lumbar spines, including the L4 to L6 discs,
were removed en bloc; the paravertebral muscles and the posterior
columns were fully removed. The vertebral pulp and annulus fibrosus
were isolated for immunohistochemical analysis of HIF-1α and VEGF
expression.
Immunohistochemical analysis
The metaphysis of the vertebral pulp and annulus
fibrosus specimens were fixed in a 4% paraformaldehyde solution at
room temperature for 30 min following threes washes with PBS. Next,
tissues were dehydrated with a graded series of ethanol,
infiltrated with xylene, and then embedded in paraffin before being
cut into 6-µm-thick sections. The slides were then
deparaffinization and rehydration with a graded ethanol series.
Following this, the sections were depleted of endogenous peroxidase
activity through the addition of methanolic
H2O2 for 15 min and blocked with 10% normal
goat serum (Abcam, Cambridge, MA, USA) at 37°C for 30 min. The
samples were incubated overnight at 4°C with anti-HIF-1α (cat. no.
ab113642; 1:200; Abcam) and anti-VEGFA (cat. no. ab46154; 1:100;
Abcam). VEGFA is a common variant of VEGF, generally referred to as
VEGF. The samples were subsequently incubated with a biotinylated
rabbit secondary antibody (cat. no. BA1100; 1:375; Vector
Laboratories, Inc., Burlingame, CA, USA) at 37°C for 1 h. The bound
secondary antibody was amplified using the Elite ABC kit (Vector
Laboratories, Inc., Burlingame, CA, USA). The
antibody-biotin-avidin-peroxidase complex was visualized using
0.02% 3,3′-diaminobenzidine. The sections were mounted onto
gelatin-coated slides, air-dried overnight at room temperature and
the coverslips were mounted using Permount medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The slides were viewed using a
light microscope (Olympus BH-2; Olympus Corporation, Tokyo, Japan;
magnification, ×400) and the optical density (OD) was analyzed by
Image Pro Plus Version 6.0 image analyzing system (Media
Cybernetics, Inc., Rockville, MD, USA).
Detection of proteoglycan content
The proteoglycan content in the lumbar spines was
detected using the phloroglucinol method, as previously described
(19). Briefly, 0.6 g tissue was
ground, 5 ml 3% NaOH was added and the solution was placed in a
thermostatic oscillator at 40°C for 3 h. Trypsin (5 ml) was
subsequently added for 2 h at 37°C. The saccharide standard
concentration was formulated according to a Phloroglucinol solution
(Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) following
the manufacturer's protocol. Phloroglucinol solution (5 ml) was
added and the sample was placed in a water bath at 100°C for 8 min.
The absorbance of the solution was determined at 554 nm with a
UV-visible spectrophotometer (WFZ-UV 2800H; Unico, Shanghai,
China). Distilled water was used as a blank sample; the standard
tube A value was determined and a standard curve was drawn. The
proteoglycan content (mg/g) in the lumbar intervertebral disc
tissue was calculated relative to the value of A.
Apoptosis detection by terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
Lumbar spines were fixed in 4% paraformaldehyde at
4°C for 48 h, decalcified at 4°C in 20% EDTA for 5–7 weeks,
embedded in paraffin and cut into 4-µm thick sections along the
midsagittal plane. An in situ TUNEL reaction was performed
on two serial sections using the MK1020 apoptosis detection kit
(Wuhan Boster Biological Technology, Ltd., Wuhan, China), according
to the manufacturer's protocol. Apoptotic cells were imaged under a
light microscope (magnification, ×400). A total of 10 random fields
were selected and the number of TUNEL-positive disc cells were
compared with the total number of disc cells and expressed as a
percentage.
Statistical analysis
Statistical analysis was performed with one-way
analysis of variance followed by Tukey's test, using SPSS 11.5
software (SPSS, Inc., Chicago, IL, USA). The data are presented as
the mean ± standard deviation from three independent experiments.
Experiments were performed in triplicate. P<0.05 was considered
to indicate a statistically significant difference.
Results
AntagomiR-21 treatment decreased the
expression of HIF-1α in the vertebral pulp and annulus
fibrosus
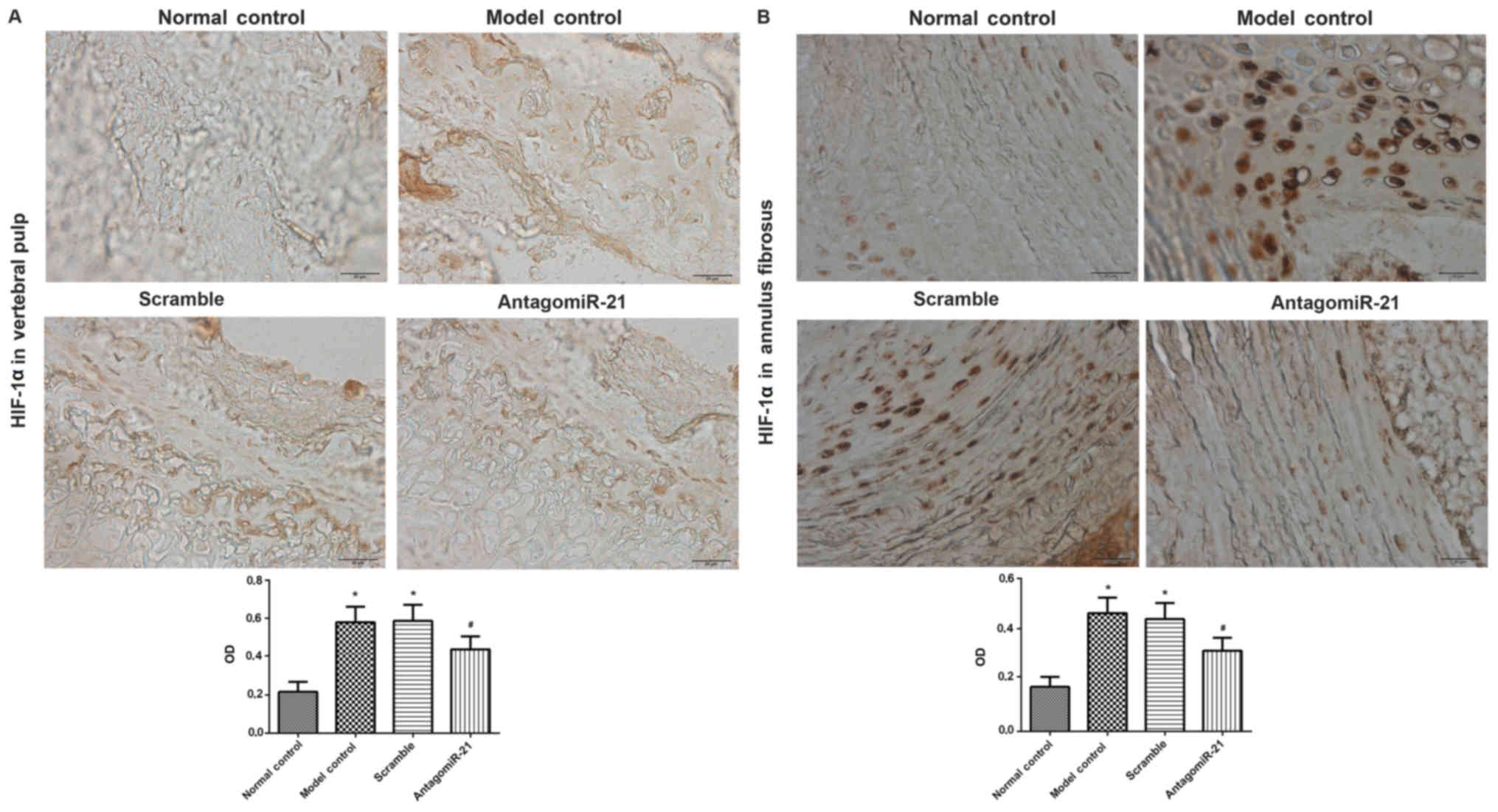
The vertebral pulp and annulus fibrosus from rats in
each group were isolated for immunohistochemical analysis of HIF-1α
expression. In the model and scramble groups, positive staining for
HIF-1α (brown and yellow) was observed in the nucleus and cytoplasm
(Fig. 1). The model and scramble
groups exhibited significantly increased expression of HIF-1α in
the vertebral pulp and annulus fibrosus compared with the control
group. Compared with the scramble group, antagomiR-21 treatment
significantly decreased HIF-1α expression in the vertebral pulp and
annulus fibrosus.
AntagomiR-21 treatment decreased VEGF
expression in the vertebral pulp and annulus fibrosus
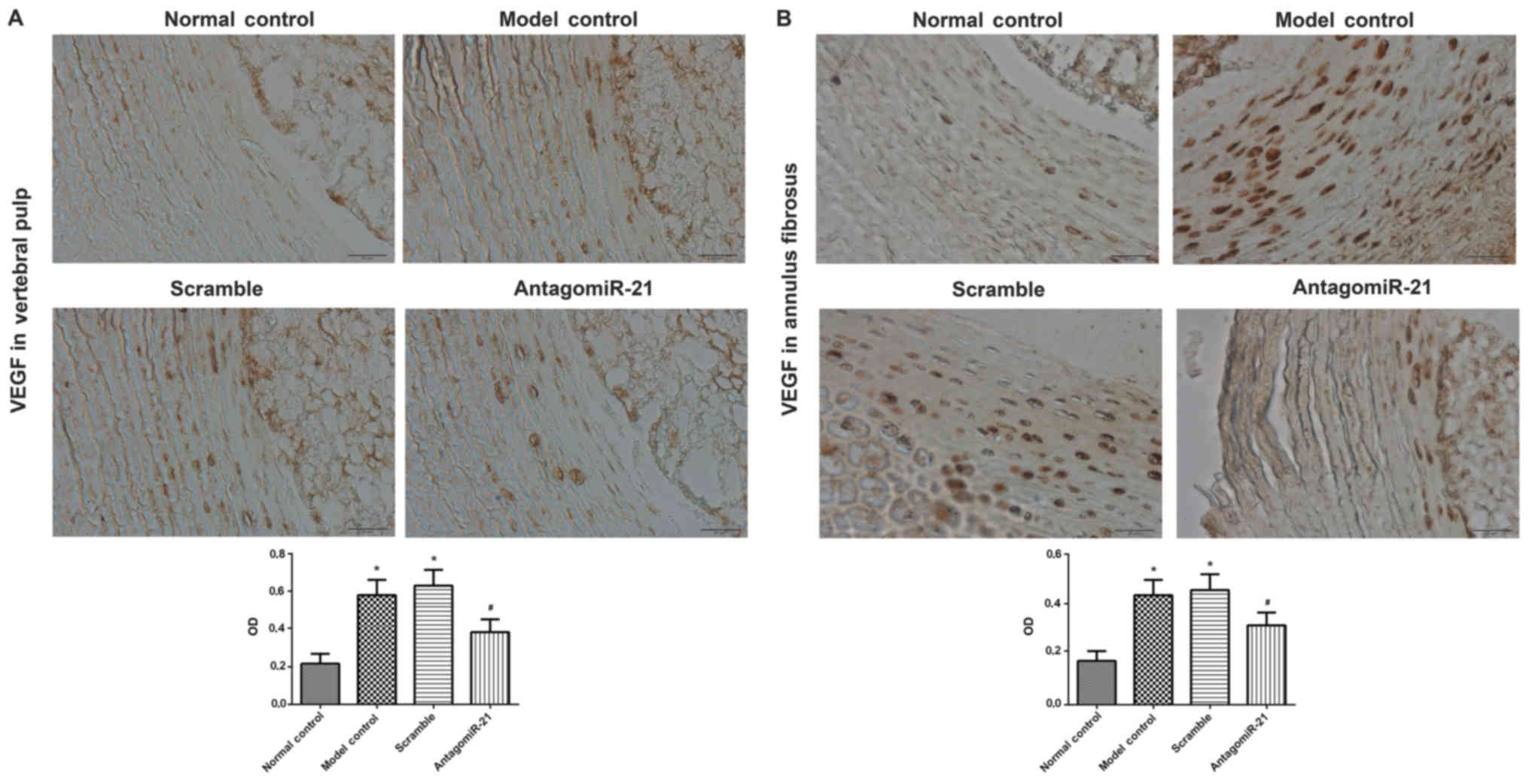
The vertebral pulp and annulus fibrosus were also
isolated for the immunohistochemical analysis of VEGF expression.
Positive staining for the expression of VEGF (brown and yellow) was
predominantly observed in the cytoplasm. Similar to the expression
pattern of HIF-1α, VEGF expression in the model and scramble groups
was significantly increased compared with the control group and
VEGF expression was significantly decreased in the antagomiR-21
treatment group compared with the scramble group (Fig. 2).
AntagomiR-21 treatment increased the
lumbar spine proteoglycan content
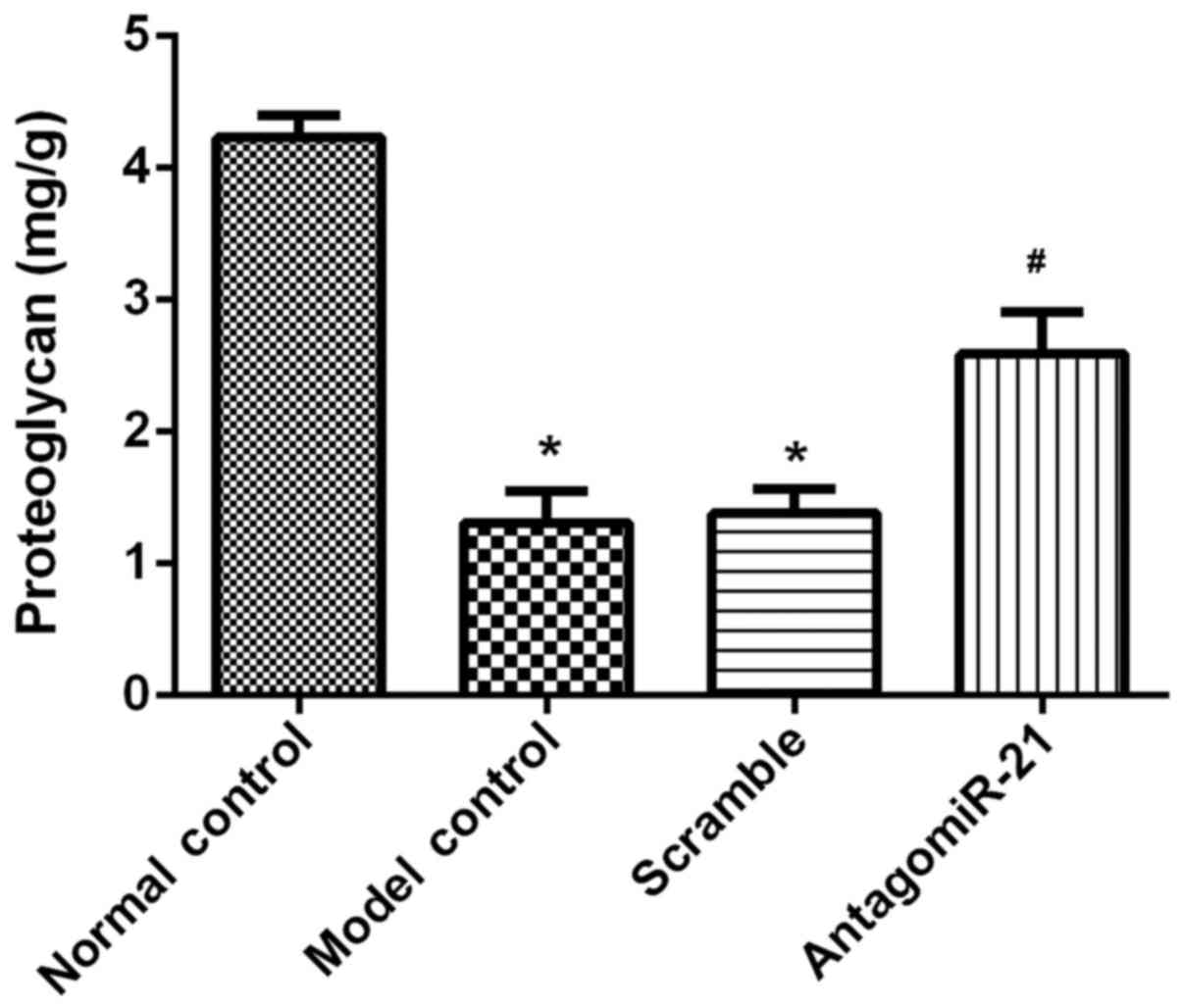
The phloroglucinol method was used to evaluate the
effect of antagomiR-21 on the lumbar spine proteoglycan content.
The results revealed that the proteoglycan content of the lumbar
spine was significantly decreased in the model group compared with
the control group. AntagomiR-21 treatment significantly increased
the proteoglycan content in lumbar spines compared with the
scramble group (Fig. 3).
AntagomiR-21 treatment inhibited cell
apoptosis in lumbar spines
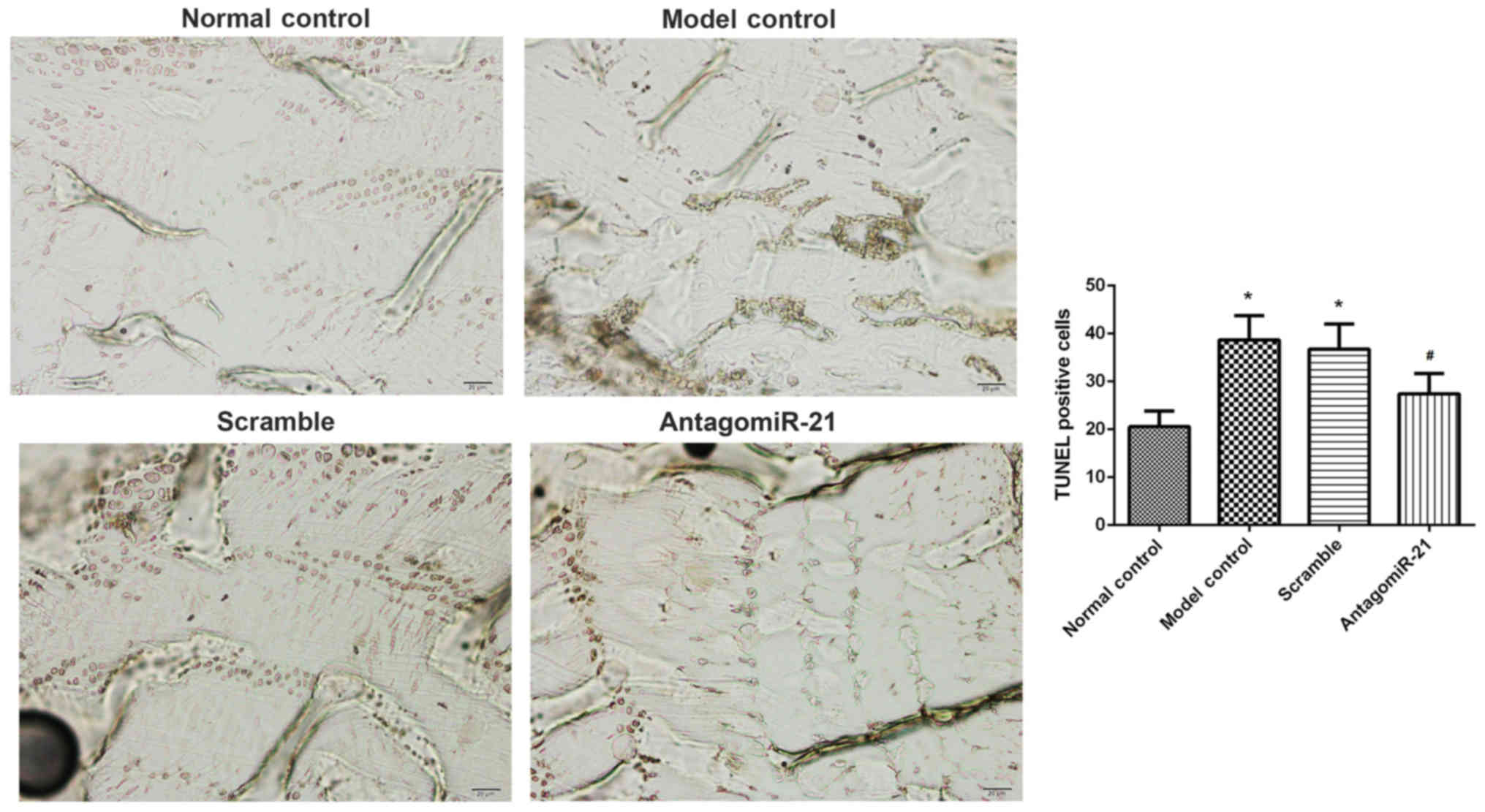
The effects of antagomiR-21 treatment on cell
apoptosis in lumbar spines were investigated. The number of
TUNEL-positive cells (brown) in the model group was significantly
increased compared with the control group (Fig. 4). As expected, antagomiR-21 treatment
significantly decreased the number of TUNEL-positive cells compared
with the scramble group.
Discussion
In the present study, antagomiR-21 treatment was
demonstrated to decrease the expression of HIF-1α and VEGF in the
vertebral pulp and annulus fibrosus of a rat model of
intervertebral disc degeneration. Nucleus pulposus cell death
mediated through apoptosis is involved in extracellular matrix
degradation, which is a deleterious consequence of intervertebral
disc degeneration (20). Liu et
al (21) reported that miR-21 is
upregulated in degenerative human nucleus pulposus tissues compared
with normal tissues. Furthermore, Liu et al (21) demonstrated that miR-21 administration
promotes nucleus pulposus cell proliferation.
To the best of our knowledge, the present study
provided novel evidence to demonstrate the role of miR-21 in the
regulation of angiogenesis, as evidenced by the decreased
expression of HIF-1α and VEGF following antagomiR-21 treatment in a
rat model of intervertebral disc degeneration. Consistent with
these findings, Liu et al (14) reported that miR-21 overexpression
increases HIF-1α and VEGF expression and induces tumor
angiogenesis. Zhao et al (22) suggested that inhibition of
angiogenesis with antagomiR-21 occurs through the HIF-1α/VEGF/VEGF
receptor 2 signaling pathway.
HIF-1 is a key transcription factor expressed in
response to hypoxic stress and is closely associated with
angiogenesis. HIF-1 is a heterodimeric transcription factor
containing HIF-1α and HIF-1β subunits (23). Under hypoxic conditions, HIF-1α
accumulates in the cytoplasm and subsequently translocates into the
nucleus. HIF-1α and HIF-1β can then dimerize and bind to hypoxia
response elements to stimulate the transcription of a large number
of genes, including prostaglandin synthase, angiopoietin, protein
tyrosine phosphatase, erythropoietin and VEGF (24,25). Zhu
et al (26) suggested that
mutual promotion of HIF-1α expression occurs during the process of
lumbar intervertebral disc degeneration and that the expression of
HIF-1α is significantly associated with microvessel density, which
provides evidence for the association between HIF-1α expression and
angiogenesis in lumbar intervertebral disc degeneration of
rats.
The present study also revealed that antagomiR-21
treatment increased proteoglycan content and inhibited cell
apoptosis in lumbar spines. A decrease in proteoglycan content is
consistently detected with degeneration, particularly in the center
of the disc (27). The induction of
disc cell apoptosis is closely associated with intervertebral disc
degeneration (28). Thus, it was
concluded that antagomiR-21 treatment exerted a protective role in
the rat model of intervertebral disc degeneration. However, the
present study only discussed the effect of antagomiR-21 treatment
on expression of HIF-1α and VEGF in the vertebral pulp, annulus
fibrosus, lumbar spine proteoglycan content, and lumbar spine cell
apoptosis of a rat model of intervertebral disc degeneration. The
association of an antagomiR-21-mediated decrease in the expression
of HIF-1α and VEGF with proteoglycan content and/or cell apoptosis
in lumbar spines was not fully elucidated, which was the limitation
of the present study. In addition, the underlying mechanism by
which antagomiR-21 treatment decreased expression of HIF-1α and
VEGF also requires further investigation.
In conclusion, the present study demonstrated that
antagomiR-21 treatment exerted a protective role in the rat model
of intervertebral disc degeneration by increasing the proteoglycan
content and inhibiting cell apoptosis, at least in part through
HIF-1α and VEGF expression regulation. The findings of the current
study demonstrate that antagomiR-21 may be a novel approach for the
treatment of intervertebral disc degeneration.
Acknowledgements
Not applicable.
Funding
The present research was supported by the National
Natural Science Foundation of China (grant no. 81572179), the
Jiangsu Provincial Grant (grant no. BL 2014044), the pre-research
project of the Affiliated Second Hospital (grant no. SDFEYQN1608)
and the Jiangsu Province's Young Medical Talents Program (grant no.
QNRC2016880).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and YX conceived and designed the study. XS, QG,
and JY performed the experiments. XS and QG wrote the paper. YX
reviewed and edited the manuscript. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Association of The Affiliated Second Hospital of Soochow University
(Suzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kang JD, Stefanovic-Racic M, Mcintyre LA,
Georgescu HI and Evans CH: Toward a biochemical understanding of
human intervertebral disc degeneration and herniation.
Contributions of nitric oxide, interleukins, prostaglandin E2, and
matrix metalloproteinases. Spine (Phila Pa 1976). 22:1065–1073.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freemont AJ, Watkins A, Le Maitre C,
Jeziorska M and Hoyland JA: Current understanding of cellular and
molecular events in intervertebral disc degeneration: Implications
for therapy. J Pathol. 196:374–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fairbank J: Clinical importance of the
intervertebral disc, or back pain for biochemists. Biochem Soc
Trans. 30:829–831. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Binch ALA, Phillips KL, Chiverton N, Cole
A, Michael AR, Breakwell L, Cross AK and Le Maitre CL: Role of
semaphorins in angiogenesis and innervation in human intervertebral
disc degeneration. Global Spine Journal. 04:1834–1841. 2014.
View Article : Google Scholar
|
|
7
|
Ali R, Le-Maitre CL, Richardson SM,
Hoyland JA and Freemont AJ: Connective tissue growth factor
expression in human intervertebral disc: Implications for
angiogenesis in intervertebral disc degeneration. Biotech
Histochem. 83:239–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JM, Song JY, Baek M, Jung HY, Kang H,
Han IB, Kwon YD and Shin DE: Interleukin-1β induces angiogenesis
and innervation in human intervertebral disc degeneration. J Orthop
Res. 29:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Chen H, Yuan W, Cao P, Shi L, Li R
and Zang F: Analysis on angiogenesis in degenerative intervertebral
disc and relevant factors. Zhonghua Guke Zazhi. 35:1200–1205.
2015.(In Chinese).
|
|
10
|
David G, Ciurea AV, Iencean SM and Mohan
A: Angiogenesis in the degeneration of the lumbar intervertebral
disc. J Med Life. 3:154–161. 2010.PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Xu Y, Luo F, Xu W, Wang B, Pang Y,
Zhou J, Wang X and Liu Q: Angiogenesis, mediated by miR-21, is
involved arsenite-induced carcinogenesis. Toxicol Lett. 223:35–41.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabatel C, Malvaux L, Bovy N, Deroanne C,
Lambert V, Gonzalez ML, Colige A, Rakic JM, Noël A, Martial JA and
Struman I: MicroRNA-21 exhibits antiangiogenic function by
targeting RhoB expression in endothelial cells. PLoS One.
6:e169792011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R,
Jiang Y, Kung HF, Lai L and Jiang BH: MiR-21 induced angiogenesis
through AKT and ERK activation and HIF-1α expression. PLoS One.
6:e191392011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Liu J, He B, Li Y, Liu S, Wu B,
Wang S, Zhang S, Xu X and Wang J: Vascular endothelial growth
factor (VEGF) regulation by hypoxia inducible factor-1 alpha
(HIF1A) starts and peaks during endometrial breakdown, not repair,
in a mouse menstrual-like model. Hum Reprod. 30:2160–2170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu WJ, Zhang XK, Zheng XF, Yang YH, Jiang
SD and Jiang LS: SHH-dependent knockout of HIF-1 alpha accelerates
the degenerative process in mouse intervertebral disc. Int J
Immunopathol Pharmacol. 26:601–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bayne K: Revised guide for the care and
use of laboratory animals available. american physiological
society. Physiologist. 39(199): 208–111. 1996.
|
|
18
|
Zhao CQ, Zhang YH, Jiang SD, Jiang LS and
Dai LY: Both endoplasmic reticulum and mitochondria are involved in
disc cell apoptosis and intervertebral disc degeneration in rats.
Age (Dordr). 32:161–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu B, Meng C, Wang H, Jia C and Zhao Y:
Proteoglycan and collagen type II in the adjacent intervertebral
disc of the cervical instability models. Zhongguo Zuzhi Gongcheng
Yanjiu. 17:5421–5426. 2013.(In Chinese).
|
|
20
|
Wei A, Brisby H, Chung SA and Diwan AD:
Bone morphogenetic protein-7 protects human intervertebral disc
cells in vitro from apoptosis. Spine J. 8:466–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Huang X, Liu X, Xiao S, Zhang Y,
Xiang T, Shen X, Wang G and Sheng B: miR-21 promotes human nucleus
pulposus cell proliferation through PTEN/AKT signaling. Int J Mol
Sci. 15:4007–4018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao D, Tu Y, Wan L, Bu L, Huang T, Sun X,
Wang K and Shen B: In vivo monitoring of angiogenesis inhibition
via down-regulation of mir-21 in a VEGFR2-luc murine breast cancer
model using bioluminescent imaging. PLoS One. 8:e714722013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Semenza GL: Vascular responses to hypoxia
and ischemia. Arterioscler Thromb Vasc Biol. 30:648–652. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rey S and Semenza GL: Hypoxia-inducible
factor-1-dependent mechanisms of vascularization and vascular
remodelling. Cardiovasc Res. 86:236–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu L, Ye W and Cao Y: The relationship
between the expression of cyclooxygenase-2, hypoxia-inducible
factor-1α and angiogenesis in lumbar intervertebral disc
degeneration of rats. Jiang Yaotong Zazhi. 33:174–178. 2012.(In
Chinese).
|
|
27
|
Lyons G, Eisenstein SM and Sweet MB:
Biochemical changes in intervertebral disc degeneration. Biochim
Biophys Acta. 673:443–453. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kohyama K, Saura R, Doita M and Mizuno K:
Intervertebral disc cell apoptosis by nitric oxide: Biological
understanding of intervertebral disc degeneration. Kobe J Med Sci.
46:283–295. 2000.PubMed/NCBI
|