Introduction
The implantation site of the fertilized egg in the
fallopian tube, is known as tubal pregnancy and is the most common
ectopic pregnancy (1). The early
diagnostic methods of tubal pregnancy include transvaginal
ultrasonography, laparoscopy, detection of progesterone, serum
β-human chorionic gonadotropin (β-HCG) and other biochemical
indexes. The study of the correlation between Chlamydia
trachomatis immunoglobulin G (CT-IgG) antibody and salpingitis,
tubal pregnancy and tubal infertility has been reported (2,3). The
clinical manifestations of early tubal pregnancy are not specific
and similar to the symptoms of early intrauterine pregnancy and
abortion, and in addition to the individual differences of pregnant
women, sometimes the single detection method is not enough to
diagnose tubal pregnancy. Life will be threatened once the rupture
or abortion occurs (4,5).
In this study, tubal pregnancy was diagnosed via the
detection of serum β-HCG and CT-IgG combined with transvaginal
ultrasonography, to explore a safe, effective and highly accurate
diagnostic scheme to guide the clinical practice.
Patients and methods
Patients
A total of 55 patients with early tubal pregnancy
treated in Linyi People's Hospital (Linyi, China) from September
2015 to September 2016 were collected as the tubal pregnancy group,
while 55 subjects of early intrauterine pregnancy were collected as
the intrauterine pregnancy group. Before the study, patients signed
the informed consent. The general data, such as age, pregnancy
times and past medical history, had no statistically significant
differences between the two groups and were comparable. The Ethics
Committee of Linyi People's Hospital approved this study
[201502-007]. Signed informed consents were obtained from all the
patients or guardians.
Inclusion criteria and exclusion
criteria
Inclusion criteria were: i) A slightly larger uterus
and an appendage or thickening of the uterus with obvious pain in
gynecologic examination; ⅱ) a history of menopause; ⅲ) β-HCG test
results were positive; and ⅳ) there was no obvious echo in the womb
of the patient's uterus, and the echo of abnormal mass was found in
the accessory area of the para uteri.
Exclusion criteria were: Ultrasound examination
shows the echo of the intrauterine gestation sac, which shows the
yolk sac, the germ, the intrauterine pregnancy of the primitive
heart tube pulsation.
Detection protocols
Transvaginal ultrasonography was performed using the
LOGIQ-3 digital color Doppler ultrasound diagnostic apparatus
(General Electric, Schenectady, NY, USA). Fasting blood (2 ml) was
drawn from the two groups of subjects in the morning for the
detection of IgG. Serum CT-IgG was detected using the enzyme-linked
immunosorbent assay (ELISA) kit (Biovision, Beijing, China). Serum
β-HCG was detected using the ELECSYS 2010 electrochemiluminescence
full automated immunoassay analyzer (Roche Diagnostics, Basel,
Switzerland).
Observation indexes
The detection results of serum β-HCG and CT-IgG in
subjects were observed, and the endometrial thickness was detected
via ultrasound. Levels of serum β-HCG in subjects were detected
again after 48 h, and whether the chronic pelvic adhesion existed
and its degree in patients with tubal pregnancy were detected
during operation. Degree I: No adhesion or mild membranous
adhesion; degree II: Adhesion easy to be separated; degree III:
Severe adhesion that cannot be separated. The diagnostic
coincidence rate was based on the mean values of the three kinds of
detection methods of normal pregnancy. Endometrial thickness
<11.2 mm and β-HCG level <1,500 U/l may indicate early tubal
pregnancy.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (IBM Corp., Armonk, NY, USA) was used for the
statistical treatment and analysis of research data. Measurement
data are presented as mean ± standard deviation. Comparison between
groups was done using one-way ANOVA test followed by post hoc test
(Least Significant Difference). Chi-square test was used for the
comparison of enumeration data. P<0.05 indicates that the
difference is statistically significant.
Results
Comparison of clinical data
Basic data for the patients in tubal pregnancy and
intrauterine pregnancy groups are shown in Table I. There was no significant difference
in age, pregnancy times, history of pelvic inflammation, history of
spontaneous abortion and history of implanting intrauterine device
(IUD) between the two groups (p>0.05).
 | Table I.Comparisons of general clinical data
between tubal and intrauterine pregnancy group. |
Table I.
Comparisons of general clinical data
between tubal and intrauterine pregnancy group.
| Pregnancy Group | No. | Age | Pregnant times | History of pelvic
inflammation | History of
spontaneous abortion | History of implanting
IUD |
|---|
| Tubal | 55 | 25.2±3.6 | 1.4±0.1 | 14 (25.5%) | 4 (7.2%) | 8 (14.5%) |
| Intrauterine | 55 | 25.9±4.1 | 1.5±0.5 | 11 (20.0%) | 3 (5.5%) | 7 (12.7%) |
| P-value |
| 0.563 | 0.472 | 0.338 | 0.415 | 0.511 |
Comparison of serum β-HCG and CT-IgG
between tubal and intrauterine pregnancy group
The levels of serum β-HCG and CT-IgG of patients
with early tubal pregnancy on the admission day and after 48 h were
significantly lower than those in women with intrauterine pregnancy
(p<0.01) (Table II). The level
of serum β-HCG in women with intrauterine pregnancy after 48 h was
significantly higher than that on the admission day (p<0.01).
There was no statistically significant difference in the comparison
of serum β-HCG level in patients with early tubal pregnancy on the
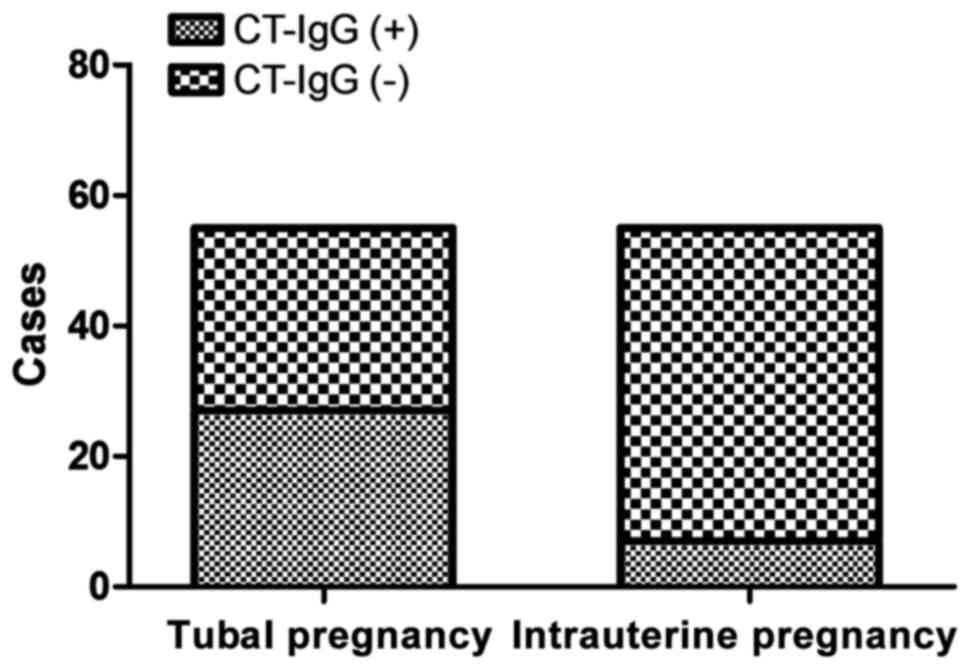
admission day and after 48 h (p>0.05). The serum CT-IgG
antibody-positive rate of patients in tubal pregnancy group (49.1%)
was significantly higher than that in intrauterine pregnancy group
(12.7%) (p<0.01) (Fig. 1). that
in intrauterine pregnancy group (12.7%) (p<0.01) (Fig. 1). Human monoclonal CT-IgG antibody
(dilution: 1:2,000; cat. no. ab108720) was purchased from Abcam
(Cambridge, MA, USA).
 | Table II.Comparison of serum β-HCG level
between tubal and intrauterine pregnancy group. |
Table II.
Comparison of serum β-HCG level
between tubal and intrauterine pregnancy group.
|
|
| β-HCG (U/l) |
|---|
|
|
|
|
|---|
| Group | No. | On admission | 48 h | t | P-value |
|---|
| Tubal pregnancy
group | 55 | 776±109 | 758±111 | 0.707 | 0.415 |
| Intrauterine
pregnancy group | 55 | 5,598±187 | 10,997±1,798 | 19.898 | 0.008 |
| t |
| 150.166 | 45.733 |
|
|
| P-value |
| <0.001 | <0.001 |
|
|
Comparison of endometrial thickness
and mass size in adnexa area between tubal and intrauterine
pregnancy groups
Endometrial thickness in patients with early tubal
pregnancy on the admission day and after 48 h was significantly
lower than those in women with intrauterine pregnancy (p<0.01).
Mass size in adnexa area of patients with early tubal pregnancy on
the admission day and after 48 h was significantly bigger than
those in women with intrauterine pregnancy (p<0.01). There was
no statistically significant difference in the comparison of pelvic
effusion between the two groups on the admission day or after 48 h
(p>0.05) (Table III).
 | Table III.Comparison of transvaginal ultrasound
examination results between tubal and intrauterine pregnancy
group. |
Table III.
Comparison of transvaginal ultrasound
examination results between tubal and intrauterine pregnancy
group.
|
|
| Transvaginal
ultrasonography |
|---|
|
|
|
|
|---|
| Group | No. | Endometrial thickness
(mm) | Mass size in adnexa
area (cm2) | Pelvic effusion
(cm) |
|---|
| Tubal pregnancy
group | 55 |
6.9±1.7 | 60.5±46.2 | 2.4±1.2 |
| Intrauterine
pregnancy group | 55 | 11.6±1.2 | 34.2±25.7 | 2.0±1.4 |
| P-value |
| 0.003 | 0.002 | 0.368 |
Pelvic adhesion grading of tubal and
intrauterine pregnancy groups
The serum CT-IgG antibody-positive rates of patients
with grade I, II and III of pelvic adhesion in tubal pregnancy
group were 28.6, 75.0 and 81.8%, respectively. These results
suggested that the more severe the pelvic adhesion was, the higher
the CT-IgG positive rate would be (Fig.
2).
Comparison of diagnostic results of
serum β-HCG, CT-IgG and endometrial thickness
The diagnostic coincidence rate of endometrial
thickness was higher than that of the detection of serum β-HCG and
CT-IgG, and the difference was statistically significant
(p<0.05). The diagnostic coincidence rates of single detection
of serum β-HCG, CT-IgG and transvaginal ultrasonography in the
tubal pregnancy and intrauterine pregnancy groups were
significantly lower than those of the combined detection
(p<0.05) (Table IV).
 | Table IV.Comparison of diagnostic coincidence
rates of different detection methods between tubal and intrauterine
pregnancy group. |
Table IV.
Comparison of diagnostic coincidence
rates of different detection methods between tubal and intrauterine
pregnancy group.
| Group | No. | β-HCG | CT-IgG | Endometrial
thickness | Combined
detection |
|---|
| Tubal pregnancy
group | 55 | 28 (50.9%) | 41 (74.5%) | 45 (81.8%) | 53 (96.4%) |
| P-valuea |
| 0.0152 | 0.0454 |
|
|
| P-valueb |
| 0.0113 | 0.0325 | 0.0374 |
|
| Intrauterine
pregnancy group | 55 | 34 (61.8%) | 43 (78.2%) | 49 (89.1%) | 54 (98.2%) |
| P-valuea |
| 0.0207 | 0.0416 |
|
|
| P-valueb |
| 0.0127 | 0.0289 | 0.0473 |
|
Discussion
Tubal pregnancy is a common acute abdominal disease
in gynecology and obstetrics, but its pathogenesis is not clear.
The salpingitis, fallopian tube abnormalities, contraception,
fertilized egg migration, and endocrine abnormalities may lead to
the occurrence of tubal pregnancy (1). The incidence of tubal pregnancy is
currently on the increase. However, the number of deaths due to
rupture has been reduced rather than increased, and the subsequent
fertility function can also be retained as much as possible
(1). The main reason is due to the
rapid and effective development of early diagnosis and treatment
technology.
The determination of HCG is the most commonly used
diagnostic method of tubal pregnancy. β-HCG is produced by
syncytiotrophoblast cells and reflects the activity of villus.
Continuous detection of blood β-HCG can identify intrauterine
pregnancy and ectopic pregnancy. It is important for early
diagnosis and is also an important monitoring method for
conservative treatment of tubal pregnancy (6–8). As the
variation range of absolute value of serum β-HCG is large, it is
used to diagnose ectopic pregnancy clinically via monitoring its
doubling time. It is reported that the doubling time of serum β-HCG
in patients with ectopic pregnancy is later than that in women with
normal intrauterine pregnancy (1.4–2.2 vs. 3–8 days) (9). The serum β-HCG level can directly
reflect the viability of trophoblasts, and the high β-HCG level
indicates the high proliferation activity of trophoblasts and high
invasion into fallopian tube. Dynamic monitoring of serum β-HCG
changes can predict the prognosis of tubal pregnancy. If the serum
β-HCG level is <2,000 IU/l, the drug therapy and non-surgical
conservative treatment can be chosen. If the serum β-HCG level
continues to rise >8,000 IU/l, the rupture of tubal pregnancy
should be identified and treated by surgery as early as possible
(10–12). The results of this study showed that
the serum β-HCG level in women with early tubal pregnancy was
significantly lower than that in women with normal intrauterine
pregnancy. Moreover, the serum β-HCG level in women with normal
intrauterine pregnancy was obviously increased on the visiting
date, suggesting that the serum β-HCG level in women with normal
intrauterine pregnancy can be doubled after 48 h. However, there
was no significant change in serum β-HCG in the early stage of
tubal pregnancy, which suggested that the early pregnancy of
oviduct is not able to implant in the endometrium, and the decidua
reaction cannot be completed normally. Insufficient blood supply
affects the development of trophoblastic cells, so the synthesis of
β-HCG is reduced.
Within 1–2 weeks after the fertilized eggs are
implanted outside the uterine cavity, the serum β-HCG levels in
women with early tubal pregnancy and normal intrauterine pregnancy
are similar, so other clinical detection means are often needed to
make up for this shortcoming. Due to the high resolution,
transvaginal ultrasonography can clearly identify the location of
tubal pregnancy and show the small lesions in organs and tissues in
pelvic cavity without being affected by the bladder filling and
abdominal wall thickness (13). Kirk
et al (14) conducted
statistical research on transvaginal ultrasonography results of
5,240 menopausal women. The results showed that the positive
predictive value and negative predictive value of diagnosis of
tubal pregnancy are 96.7 and 99.4%. Ectopic pregnancy is divided
into different types according to the symptoms and outcome. No
gestational sac but tubal ring sign with specific diagnostic value
found in uterine cavity is the important sign of tubal pregnancy,
and trophoblastic blood flow can be seen around the tubal ring. In
addition, fake gestational sac can occur in ectopic pregnancy due
to the hematocele in uterine cavity, and the blood flow resistance
is higher because of the blood flow from the endometrial spiral
artery, while the trophoblastic blood flow around the true
gestational sac is from the original placenta with low resistance
(15,16). The results of this study showed that
the endometrial thickness in patients with early tubal pregnancy
was smaller than that in women with normal intrauterine pregnancy
(p<0.05); the diagnostic coincidence rate of endometrial
thickness was higher than that of serum β-HCG and CT-IgG detection,
and the difference was statistically significant (p<0.05).
One of the main pathogenesis of female urethritis
and salpingitis is CT infection. There are often no obvious
subjective symptoms in the early stage. CT damages the uterus and
adnexa upwards from the infected cervix, leading to tubal
pregnancy, tubal infertility and other sequelae (17,18). It
is reported that the serum CT-IgG positive antibody can be detected
in women with ectopic pregnancy and normal pregnancy, and the rates
are 32–71 and 4–39%, respectively. The relative risk of tubal
pregnancy in women with serum CT-IgG positive is 2.4–7.9 (19). Dunne et al (20) studied and suggested that with the
decline in CT infection positive rate, the incidence rate of
ectopic pregnancy is also rapidly reduced. The results of this
study showed that the serum CT-IgG antibody positive rate of
patients in tubal pregnancy group was significantly higher than
that in intrauterine pregnancy group (p<0.01). At the same time,
it was found that the serum CT-IgG antibody-positive rates of
patients with degree I, II and III of pelvic adhesion were 28.6,
75.0 and 81.8%, respectively. The more severe the pelvic adhesion
was, the higher the CT-IgG positive rate would be. CT infection
often shows the subclinical state with no obvious subjective
symptoms. If it is not treated and controlled in time, endometrial
and endosalpinx injury is caused, further leading to pelvic
adhesion, tubal obstruction and other permanent injuries, which is
a basic cause of tubal pregnancy.
In conclusion, the results of the present study have
shown that the diagnostic coincidence rates of single detection of
serum β-HCG, CT-IgG and transvaginal ultrasonography in tubal and
intrauterine pregnancy group were significantly lower than those of
combined detection. Therefore, the detection of serum β-HCG and
CT-IgG combined with transvaginal ultrasonography can improve the
diagnostic accuracy of suspected early tubal pregnancy, and provide
a reliable basis for clinical treatment and prognosis, which has
important application values. However, the sample size of this
study is insufficient, and the result is limited. A larger number
of samples is needed to confirm the conclusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HX and PL designed the study, WL performed the
ultrasonography, WL and HX collected the data, and WL and PL
analyzed the data. HX prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Linyi People's Hospital (Linyi, China). Signed written informed
consents were obtained from the patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stulberg DB, Cain LR, Dahlquist I and
Lauderdale DS: Ectopic pregnancy rates in the Medicaid population.
Am J Obstet Gynecol. 208:274.e1–274.e7. 2013. View Article : Google Scholar
|
|
2
|
Ma LK, Cao DY, Yang JX, Liu JT, Shen K and
Lang JH: Pregnancy outcome and obstetric management after vaginal
radical trachelectomy. Eur Rev Med Pharmacol Sci. 18:3019–3024.
2014.PubMed/NCBI
|
|
3
|
Hoenderboom BM, van Oeffelen AA, van
Benthem BH, van Bergen JE, Dukers-Muijrers NH, Götz HM, Hoebe CJ,
Hogewoning AA, van der Klis FR, van Baarle D, et al: The
Netherlands Chlamydia cohort study (NECCST) protocol to assess the
risk of late complications following Chlamydia trachomatis
infection in women. BMC Infect Dis. 17:2642017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cain LR and Stulberg D: Ectopic pregnancy
rates in a non-Medicaid population are lower than previously
reported. Am J Obstet Gynecol. 209:5922013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akaba GO, Agida TE and Onafowokan O:
Ectopic pregnancy in Nigeria's federal capital territory: A six
year review. Niger J Med. 21:241–245. 2012.PubMed/NCBI
|
|
6
|
Senapati S and Barnhart KT: Biomarkers for
ectopic pregnancy and pregnancy of unknown location. Fertil Steril.
99:1107–1116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mäkinen J: Current treatment of ectopic
pregnancy. Ann Med. 31:197–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor AH, Finney M, Lam PM and Konje JC:
Modulation of the endocannabinoid system in viable and non-viable
first trimester pregnancies by pregnancy-related hormones. Reprod
Biol Endocrinol. 9:1522011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gevaert O, De Smet F, Kirk E, Van Calster
B, Bourne T, Van Huffel S, Moreau Y, Timmerman D, De Moor B and
Condous G: Predicting the outcome of pregnancies of unknown
location: Bayesian networks with expert prior information compared
to logistic regression. Hum Reprod. 21:1824–1831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goksedef BP, Kef S, Akca A, Bayik RN and
Cetin A: Risk factors for rupture in tubal ectopic pregnancy:
Definition of the clinical findings. Eur J Obstet Gynecol Reprod
Biol. 154:96–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Mello NM, Mol F, Ankum WM, Mol BW, van
der Veen F and Hajenius PJ: Ectopic pregnancy: How the diagnostic
and therapeutic management has changed. Fertil Steril.
98:1066–1073. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Mello NM, Mol F, Verhoeve HR, van Wely
M, Adriaanse AH, Boss EA, Dijkman AB, Bayram N, Emanuel MH,
Friederich J, et al: Methotrexate or expectant management in women
with an ectopic pregnancy or pregnancy of unknown location and low
serum hCG concentrations? A randomized comparison. Hum Reprod.
28:60–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reid S, Casikar I, Barnhart K and Condous
G: Serum biomarkers for ectopic pregnancy diagnosis. Expert Opin
Med Diagn. 6:153–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kirk E, Papageorghiou AT, Condous G, Tan
L, Bora S and Bourne T: The diagnostic effectiveness of an initial
transvaginal scan in detecting ectopic pregnancy. Hum Reprod.
22:2824–2828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szabó I, Csabay L, Belics Z, Fekete T and
Papp Z: Assessment of uterine circulation in ectopic pregnancy by
transvaginal color Doppler. Eur J Obstet Gynecol Reprod Biol.
106:203–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thoma ME: Early detection of ectopic
pregnancy visualizing the presence of a tubal ring with
ultrasonography performed by emergency physicians. Am J Emerg Med.
18:444–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zele-Starcević L, Plecko V, Budimir A and
Kalenić S: Choice of antimicrobial drug for infections caused by
Chlamydia trachomatis and Chlamydophila pneumoniae.
Acta Med Croatica. 58:329–333. 2004.(In Croatian). PubMed/NCBI
|
|
18
|
Stamatopoulos N, Casikar I, Reid S, Roy B,
Branley J, Mongelli M and Condous G: Chlamydia trachomatis
in fallopian tubes of women undergoing laparoscopy for ectopic
pregnancy. Aust N Z J Obstet Gynaecol. 52:377–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Meng CX, Sun LL, Zhao WH, Zhang M,
Zhang J and Cheng L: Reduced prevalence of chronic tubal
inflammation in tubal pregnancies after levonorgestrel emergency
contraception failure. Pharmacoepidemiol Drug Saf. 24:548–554.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dunne EF, Chapin JB, Rietmeijer CA, Kent
CK, Ellen JM, Gaydos CA, Willard NJ, Kohn R, Lloyd L, Thomas S, et
al: Rate and predictors of repeat Chlamydia trachomatis
infection among men. Sex Transm Dis. 35 Suppl:S40–S44. 2008.
View Article : Google Scholar : PubMed/NCBI
|