Introduction
According to GLOBOCAN statistics in 2012, lung
cancer is the most diagnosed cancer worldwide (1). As the leading cause of cancer death,
lung cancer accounted for 1.6 million deaths in 2012. Lung cancer
includes small cell lung cancer and non-small cell lung cancer
(NSCLC), and the latter accounts for 80–85% of total lung cancer
(2). Although improvements have made
in the diagnosis and treatment of NSCLC in previous decades, the
5-year survival rate of patients remains <15% (3). In addition, many current treatments of
NSCLC have various adverse effects including and easily induce
drug-resistance (4,5). For example, coexisting myasthenia
gravis, myositis, and polyneuropathy induced by ipilimumab and
nivolumab were exhibited in a patient with non-small-cell lung
cancer (6). Consequently, exploring
new therapeutic agents from Traditional Chinese Medicine has gained
increasing attention.
Dietary polyphenols constitute a large amount of
secondary metabolites and have been widely identified as
chemopreventive or anti-cancer agents (7). It has also been documented that dietary
polyphenols exert various biological activities, such as
anti-inflammatory, immunomodulatory and anti-tumor properties
(8–10). Ellagic acid
[2,3,7,8-tetrahydroxy-chromeno (5,4,3-cde) hromene-5,10-dione], a
natural polyphenolic compound, is widely found in grapes,
pomegranates, nuts, strawberries and green tea (11). Accumulating evidence has suggested an
anti-tumor activity of ellagic acid due to its ability to prevent
tumor growth (12,13), inhibit proliferation (14), arrest cell cycle (15), induce apoptosis (16) and suppress cell metastasis and
angiogenesis (13). Furthermore,
previous studies have elucidated that ellagic acid treatment
significantly ameliorated obstructive jaundice-induced lung damage
(17) and acute lung injury
(18), which suggests the pulmonary
protective effect of ellagic acid. Despite these biological
activities of ellagic acid, the effects of ellagic acid on human
NSCLC A549 cells remain unclear.
Therefore, the objective of the present study was to
investigate whether ellagic acid could inhibit human NSCLC A549
cells, and to reveal the potential underlying mechanism. It was
demonstrated that ellagic acid may suppress cell proliferation,
arrest cell cycle and induce apoptosis in human NSCLC A549 cells
via the suppression of the phosphoinositide 3-kinase (PI3K)/protein
kinase B (Akt) signaling pathway. The present findings provide
evidence that ellagic acid may be developed as a potential therapy
for the treatment of lung cancer.
Materials and methods
Chemicals and reagents
Ellagic acid, dimethylsulfoxide (DMSO), propidium
iodide (PI) and MTT were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). RPMI-1640 medium, fetal bovine serum (FBS),
penicillin and streptomycin were purchased from Invitrogen; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). The Annexin V-FITC
Apoptosis Detection kit and Cell cycle detection kit were obtained
from BD Biosciences (Franklin Lakes, NJ, USA). Antibodies against
phosphorylated (p)-PI3K, PI3K, p-Akt, Akt, cyclin D1, p21, B cell
lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax) and
cleaved-Caspase-3 were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Anti-GAPDH antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
The NSCLC line A549 was obtained from the Shanghai
Cell Bank of Chinese Academy of Sciences (Shanghai, China). A549
cells were maintained in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 10%
FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin in a
humidified incubator under 5% CO2 at 37°C.
MTT assay
The effect of ellagic acid on the viability of human
NSCLC A549 cells was measured by MTT assay. A total of
1×104 cells/well were cultured in 96-well plates and
stimulated with various concentrations of (5, 10 and 20 µM) of
ellagic acid for 48 h at 37°C. Then, the medium was removed, MTT
(100 µl) was added and incubated at 37°C for additional 4 h. Then
150 ml DMSO was added, and the absorbance was read at 570 nm with a
micro-plate reader (Thermo Fisher Scientific, Inc.).
Cell cycle assay
To detect the effect of ellagic acid in the cell
cycle, a PI cell cycle analysis kit was used. In brief, human NSCLC
A549 cells (5×105 cells per well) were cultured in
6-well plates and stimulated with various concentrations of (5, 10
and 20 µM) of ellagic acid for 48 h at 37°C. Cells were then fixed
in 70% ethanol at −20°C for 1 h, and 2 mg/ml RNaseA and 50 mg/ml PI
were added and incubated for 45 min at 37°C. Stained cells were
analyzed using the Guava easyCyte 5HT flow cytometry system (EMD
Millipore, Billerica, MA, USA).
Cell apoptosis assay
Human NSCLC A549 cells were treated with various
concentrations of ellagic acid (5, 10 and 20 µM) for 48 h at 37°C.
Cell apoptosis was assessed using flow cytometry with staining of
the cells using an Annexin V/propidium iodide (PI) kit (cat. no.
556547; BD Biosciences, Franklin Lakes, NJ, USA). Cells were
incubated with Annexin V-FITC (5 µl) and PI (5 µl) at room
temperature for 30 min in the dark. Then a flow cytometer (Cytomics
FC 500 MPL; Beckman Coulter, Inc., Brea, CA, USA) was used to
measure the apoptosis rates by detecting the relative amount of
Annexin V-FITC positive and PI negative cells.
Western blotting
After A549 cells were treated with 5, 10, or 20 µM
ellagic acid for 48 h at 37°C, total proteins were extracted from
the A549 cells with radio immunoprecipitation assay lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.). Protein samples were then
quantified with a BCA Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein (25 µg/pore) were separated by 10%
SDS-PAGE, transferred to polyvinylidene difluoride membranes. The
membrane was then blocked overnight with skimmed milk at 4°C. and
incubated with antibodies against p-PI3K (1:1,000; cat. no. 4228),
PI3K (1:1,000; cat. no. 4257), p-Akt (1:1,000; 4060), Akt (1:1,000;
cat. no. 4685), cyclin D1 (1:1,000; cat. no. 2978), p21 (1:1,000;
cat. no. 2947), Bax (1:1,000; cat. no. 5023), Bcl-2 (1:1,000; cat.
no. 15071), cleaved-caspase-3 (1:1,000; cat. no. 9661) and GAPDH
(1:2,000, cat. no. 5174). After washing with PBST, the membrane was
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000; cat. no. ab191866 and ab218695; Abcam,
Cambridge, UK) and visualized with an enhanced chemiluminescence
kit (Thermo). GAPDH acted as an internal control, and quantified
using the Bio-Rad GS-700 imaging densitometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the means + standard
deviation. The differences between control and treatment groups
were analyzed using one-way analysis of variance followed by
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Treatment of ellagic acid inhibits
cell viability in A549 cells
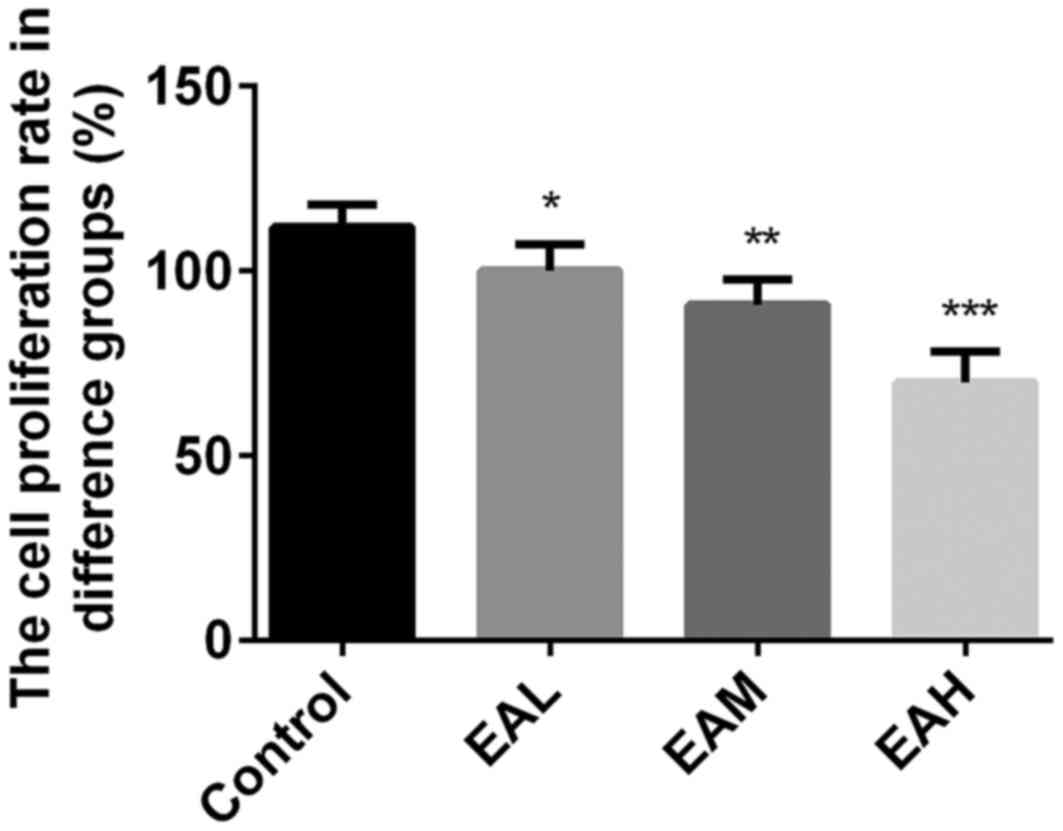
Initially, the effects of ellagic acid on viability
of A549 cells were measured by MTT assay. As presented in Fig. 1, the viability rates were
significantly reduced by ellagic acid treatment in a dose-dependent
manner, compared with the control group (5 µM, P<0.05; 10 µM,
P<0.01, 20 µM; P<0.001). These results indicate that ellagic
acid significantly inhibited the viability of human NSCLC A549
cells.
Treatment of ellagic acid induces
apoptosis in A549 cells
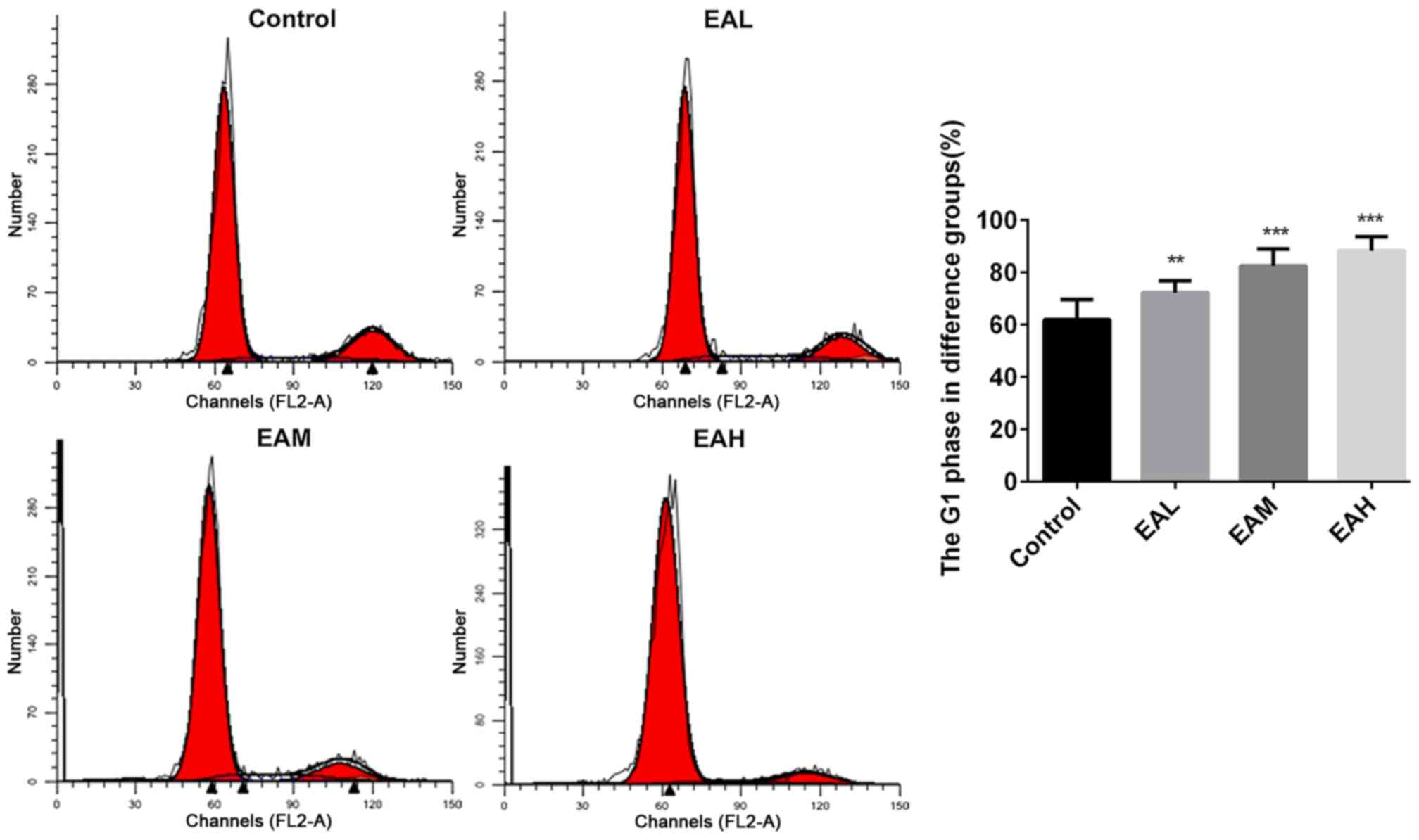
The cell cycle between different groups was measured
with PI cell cycle analysis kit, and the relative proportion of
A549 cells in the G1 phase was calculated. Fig. 2 demonstrated that in the control
group, the number of cells in G1 phase were low, whereas, after
treatment with ellagic acid for 48 h, the rates of cells in G1
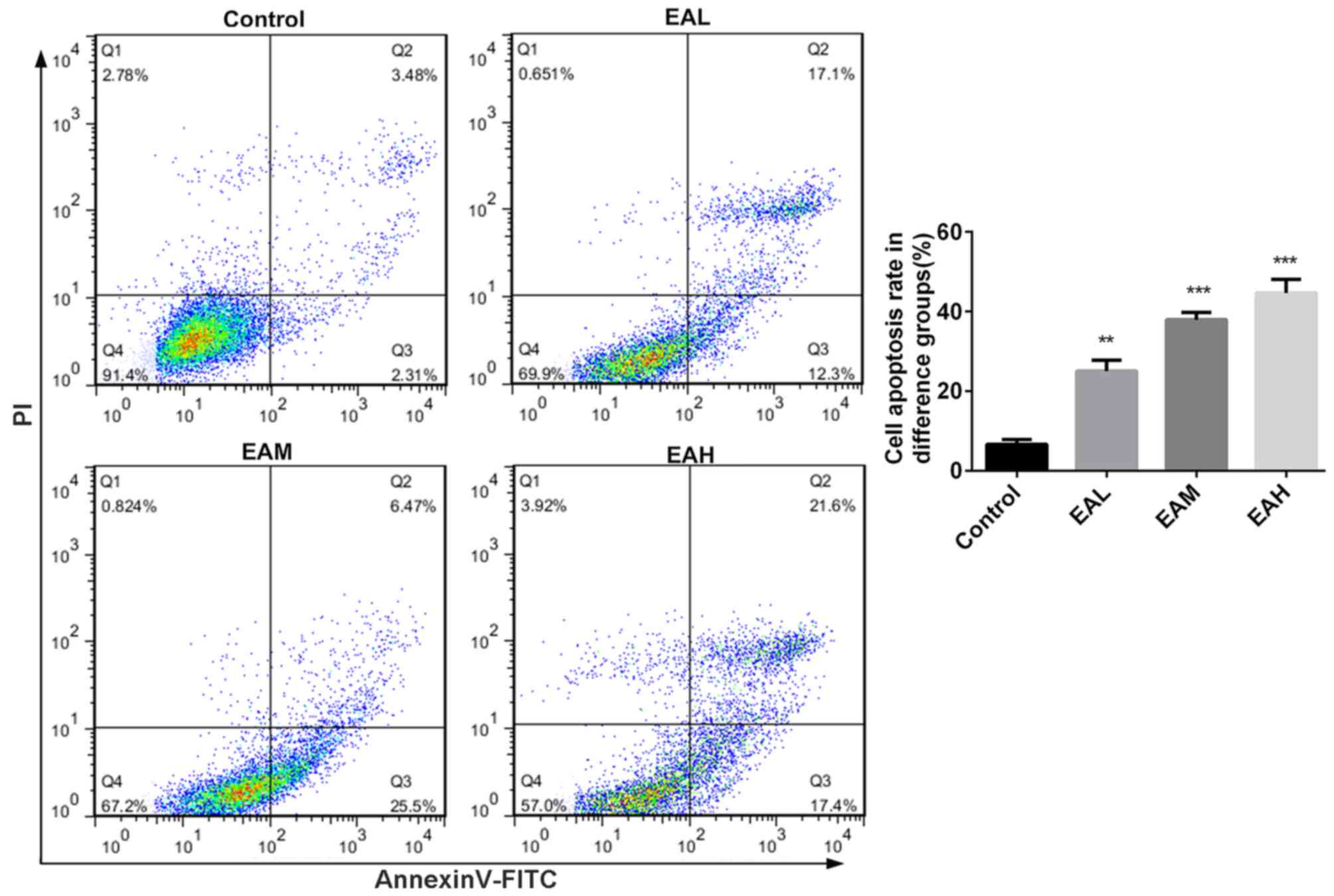
phase were significantly increased (P<0.05). Annexin V and PI
double staining results in Fig. 3
demonstrated that the ellagic acid-treated groups exhibited a
significantly increased apoptosis rate in comparison with controls
(P<0.01). These observations indicate that the inhibitory effect
of ellagic acid on human NSCLC A549 cells may be due to increased
apoptosis.
Treatment of ellagic acid suppresses
the PI3K/Akt signaling pathway in A549 cells
The effects of ellagic acid treatment on the
PI3K/Akt signaling pathway in A549 cells were detected by western
blotting. Fig. 4 indicates that,
compared with the control group, the phosphorylation of PI3K and
Akt were significantly downregulated by the treatment of ellagic
acid in a dose-dependent manner (5 µM, P<0.05; 10 µM, P<0.01,
20 µM; P<0.001).
Treatment of ellagic acid regulates
apoptosis regulator protein expression in A549 cells
Finally, the effect of ellagic acid on expression of
apoptosis-related protein in A549 cells was detected. As presented
in Fig. 5, compared with the control
group, ellagic acid stimulation (5, 10 and 20 µM) for 48 h
significantly increased p21, Bax and cleaved caspase-3 protein
expression in a dose-dependent manner in A549 cells (5 µM,
P<0.05; 10 µM, P<0.01, 20 µM; P<0.001). Conversely, cyclin
D1 and Bcl-2 protein levels were significantly downregulated after
the treatment of ellagic acid (5 µM, P<0.05; 10 µM, P<0.01;
20 µM, P<0.001).
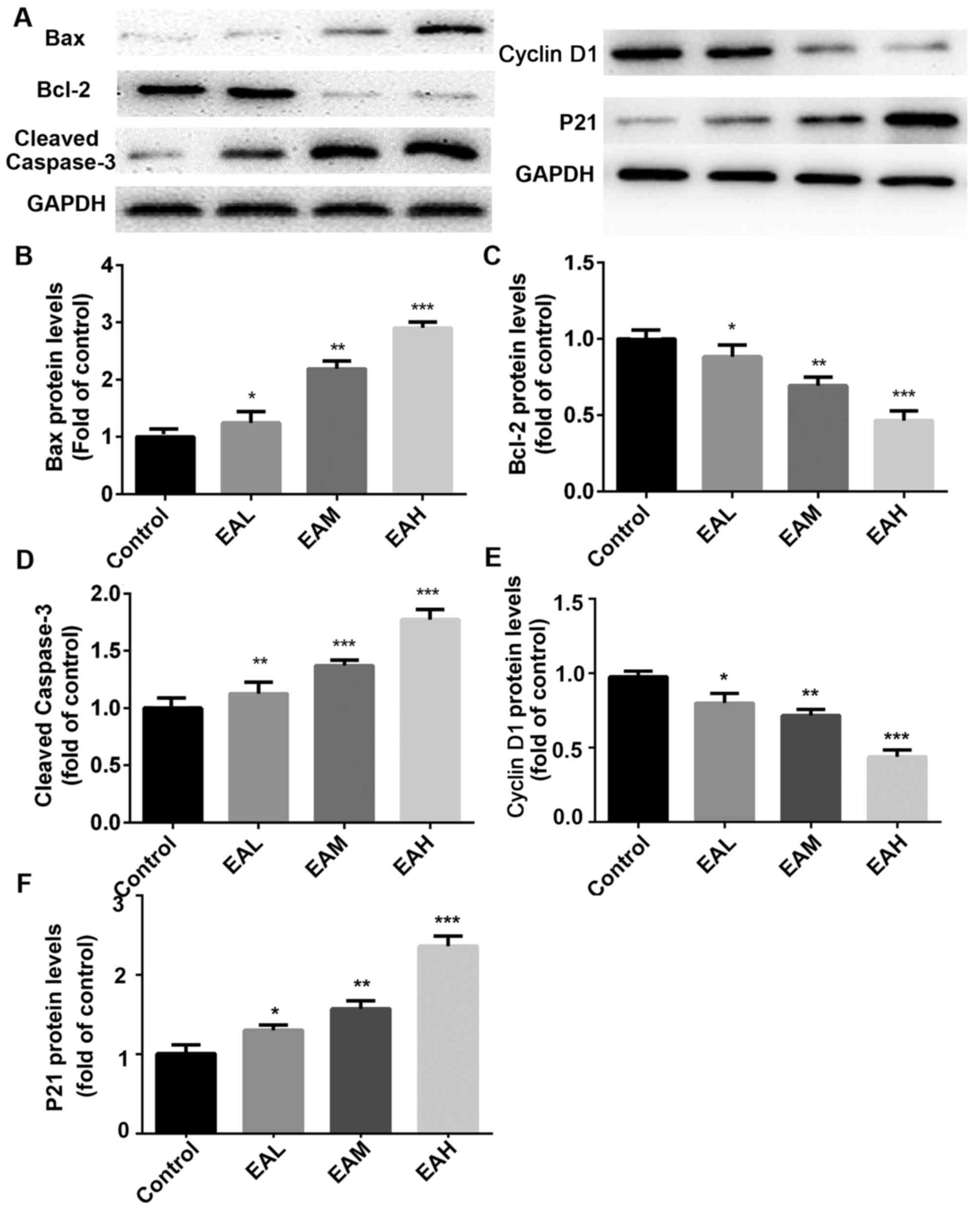 | Figure 5.(A) Apoptosis-associated protein
expression. Compared with the control group, p21, Bax and cleaved
caspase-3 protein expression was increased in a dose-dependent
manner following treatment. Conversely, cyclin D1 and Bcl-2 protein
levels were downregulated after the treatment with ellagic acid.
(B-F) Quantitation of Western blot signal intensities. Data are
presented as the mean ± standard deviation (n=3). *P<0.05,
**P<0.01, ***P<0.001 vs. control. Bcl-2, B cell lymphoma 2;
Bax, Bcl-2 associated X protein; EAL, low concentration (5 µM)
ellagic acid; EAM, middle concentration (10µM) ellagic acid; EAH,
high concentration (20 µM) ellagic acid. |
Discussion
In the present study, the inhibitory influence of
ellagic acid on the viability of NSCLC A549 cells was investigated.
The data demonstrated that stimulation with ellagic acid for 48 h
significantly inhibited cell viability in a dose-dependent manner.
Additionally, ellagic acid significantly induced cell apoptosis in
human NSCLC A549 cells in a dose-dependent manner. Ho et al
(19) have previously reported that
ellagic acid may inhibit viability and promote apoptosis in human
bladder cancer cells.
Increasing evidence has demonstrated that the
PI3K/Akt signaling pathway is associated with a large number of
pathophysiological processes including cell viability, cell cycle
progression, survival, apoptosis and metastasis (20,21). The
aberrant activation of the PI3K/Akt signaling pathway has also been
observed in NSCLC cell lines and NSCLCs, and has been demonstrated
to serve a key role in the initiation and development of this
cancer (22,23). In addition, it has previously been
reported that ellagic acid may inhibit viability and induce
apoptosis via the Akt signaling pathway in HCT-15 colon
adenocarcinoma cells (24). These
findings suggest that the PI3K/Akt signaling pathway is a novel
potential target of NSCLC therapy. In accordance with these
studies, the present study demonstrated that the phosphorylation of
PI3K and Akt were significant lower in ellagic acid-treated groups
than the control group, identifying the inhibitory effect of
ellagic acid on the PI3K/Akt signaling pathway in A549 cells. These
findings suggest that ellagic acid inhibits the cell viability and
promotes cell apoptosis in A549 cells via the suppression of
PI3K/Akt signaling pathway. As the present findings indicated,
after the treatment of ellagic acid for 48 h, the proportion of
cells in G1 phase increased significantly in ellagic acid-treated
groups, and ellagic acid-treated groups exhibited a significant
increase in apoptosis rate in comparison with controls, suggesting
that ellagic acid may accelerate cell apoptosis in a dose-dependent
manner. Western blot analysis revealed that the phosphorylation of
PI3K and Akt were downregulated by the treatment of ellagic acid,
indicating that PI3K and Akt signaling pathway was inhibited. p21,
Bax and cleaved Caspase-3 protein expression were increased with
ellagic acid treatment in a dose-dependent manner, consistent with
the results of apoptosis rate increase. Conversely, cyclin D1 and
Bcl-2 protein levels were downregulated after treatment with
ellagic acid, further indicating that ellagic acid promotes cell
apoptosis at the protein level.
Ellagic acid antitumor activity was initially
suggested after the observation that aromatase, a key enzyme in
breast cancer development which converts androgens to estrogens, is
inhibited by polyphenols derived from fresh pomegranate juice
(25). Akt, the serine/threonine
kinase downstream effector of PI3K, causes tumor cell survival and
inhibition of apoptosis, induces viability and cell growth, and
stimulates angiogenesis by phosphorylating numerous downstream
targets in the presence of different apoptotic stimuli (26). These previous findings suggest that
increased constitutive phosphorylation of Akt is associated with
decreased apoptosis, whereas Akt inhibition increased apoptosis.
Therefore, the Akt signaling pathway is becoming a promising target
for cancer chemoprevention and therapy (27). The aim of the present study was to
address the cytotoxic effects of ellagic acid on A549 cells. PI3K
activates the downstream target Akt to mediate several biological
effects. Upon activation, Akt inactivates several downstream
targets including Bcl-2 family members and caspase-3, thereby
blocking apoptosis (28).
Alternatively, the inhibition of phosphorylated Akt increases the
expression of proapoptotic Bax, a fact that favors progress of the
apoptotic process. Bcl-2 and its helpers compete with Bax and other
proapoptotic proteins to regulate the release of cytochrome c from
mitochondria, which in turn activates initiator caspases including
caspase-3 (29). The present
findings suggest that in A549 cells, ellagic acid blocks PI3K
phosphorylation, thus decreasing Akt phosphorylation, therefore the
PI3K/Akt signaling pathway was blocked. This trigged cell apoptosis
as evidenced by increased p21, Bax and cleaved caspase-3 protein
levels, and decreased Bcl-2 and cyclin D1 levels.
In conclusion, the results of the present study
demonstrated the inhibition of human NSCLC cell viability and
induction of apoptosis by ellagic acid treatment. The findings
demonstrated that ellagic acid decreased PI3K and AKT
phosphorylation, and promoted A549 cell apoptosis. The apoptotic
induction capacity of ellagic acid may be attributed to its effect
to regulate the apoptosis-related proteins Bax, Bcl-2, and
caspase-3 through downregulating the PI3K/Akt pathway. The present
study revealed that the mechanism by which ellagic acid exhibits
its growth inhibitory effect on human NSCLC in vitro is by
inhibiting cell viability and inducing apoptosis. Further studies
are required to understand the different molecular mechanisms of
action of ellagic acid, which will help provide useful information
for its possible application in cancer prevention, and perhaps
novel therapies for cancer and other diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangdong
Provincial Key Platform and Major Scientific Research Projects
(grant no. 2016GXJK213) and Xinhua Institute Teachers Research Fund
Project of Sun Yat-sen University (grant no. 2016YB002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL wrote the manuscript and interpreted the data. XL
analyzed the data and revised the manuscript, CN searched the
literature and collected the data. XW designed the study.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Tieulent Lortet J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Subramaniam S, Thakur RK, Yadav VK, Nanda
R, Chowdhury S and Agrawal A: Lung cancer biomarkers: State of the
art. J Carcinog. 12:32013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Huang Y, Zhuo W, Zhu Y, Zhu B and
Chen Z: Fisetin, a dietary phytochemical, overcomes
Erlotinib-resistance of lung adenocarcinoma cells through
inhibition of MAPK and AKT pathways. Am J Transl Res. 8:4857–4868.
2016.PubMed/NCBI
|
|
4
|
Lim SM, Syn NL, Cho BC and Soo RA:
Acquired resistance to EGFR targeted therapy in non-small cell lung
cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev.
65:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalvi MP, Wang L, Zhong R, Kollipara RK,
Park H, Bayo J, Yenerall P, Zhou Y, Timmons BC, Rodriguez-Canales
J, et al: Taxane-platin-resistant lung cancers Co-develop
hypersensitivity to jumonjic demethylase inhibitors. Cell Rep.
19:1669–1684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen JH, Lee KY, Hu CJ and Chung CC:
Coexisting myasthenia gravis, myositis, and polyneuropathy induced
by ipilimumab and nivolumab in a patient with non-small-cell lung
cancer: A case report and literature review. Medicine (Baltimore).
96:e92622017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mocanu MM, Nagy P and Szöllősi J:
Chemoprevention of breast cancer by dietary polyphenols. Molecules.
20:22578–22620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkar S, Siddiqui AA, Mazumder S, De R,
Saha SJ, Banerjee C, Iqbal MS, Adhikari S, Alam A, Roy S and
Bandyopadhyay U: Ellagic acid, a dietary polyphenol inhibits
tautomerase activity of human macrophage migration inhibitory
factor and its pro-inflammatory responses in human peripheral blood
mononuclear cells. J Agr Food Chem. 63:4988–4998. 2015. View Article : Google Scholar
|
|
9
|
Benvenuto M, Fantini M, Masuelli L, De
Smaele E, Zazzeroni F, Tresoldi I, Calabrese G, Galvano F, Modesti
A and Bei R: Inhibition of ErbB receptors, Hedgehog and NF-kappaB
signaling by polyphenols in cancer. Front Biosci (Landmark Ed).
18:1290–1310. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marzocchella L, Fantini M, Benvenuto M,
Masuelli L, Tresoldi I, Modesti A and Bei R: Dietary flavonoids:
Molecular mechanisms of action as anti-inflammatory agents. Recent
Pat Inflamm Allergy Drug Discov. 5:200–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talcott ST and Lee JH: Ellagic acid and
flavonoid antioxidant content of muscadine wine and juice. J Agr
Food Chem. 50:3186–3192. 2002. View Article : Google Scholar
|
|
12
|
Zhao M, Tang SN, Marsh JL, Shankar S and
Srivastava RK: Ellagic acid inhibits human pancreatic cancer growth
in Balb c nude mice. Cancer Lett. 337:210–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ceci C, Tentori L, Atzori MG, Lacal PM,
Bonanno E, Scimeca M, Cicconi R, Mattei M, de Martino MG,
Vespasiani G, et al: Ellagic acid inhibits bladder cancer
invasiveness and in vivo tumor growth. Nutrients. 8:pii: E744.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malik A, Afaq S, Shahid M, Akhtar K and
Assiri A: Influence of ellagic acid on prostate cancer cell
proliferation: A caspase-dependent pathway. Asian Pac J Trop Med.
4:550–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narayanan BA, Geoffroy O, Willingham MC,
Re GG and Nixon DW: p53/p21 (WAF1/CIP1) expression and its possible
role in G1 arrest and apoptosis in ellagic acid treated cancer
cells. Cancer Lett. 136:215–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ho CC, Huang AC, Yu CS, Lien JC, Wu SH,
Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, et al: Ellagic acid
induces apoptosis in TSGH8301 human bladder cancer cells through
the endoplasmic reticulum stress-and mitochondria-dependent
signaling pathways. Environ Toxicol. 29:1262–1274. 2014.PubMed/NCBI
|
|
17
|
Gul M, Aliosmanoglu I, Uslukaya O, Firat
U, Yüksel H, Gümüs M and Ulger BV: The protective effect of ellagic
acid on lung damage caused by experimental obstructive jaundice
model. Acta Chir Belg. 113:285–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Favarin Cornelio D, Teixeira Martins M, de
Andrade Lemos E, de Freitas Alves C, Chica Lazo JE, Sorgi Artério
C, Faccioli LH and Rogerio Paula A: Anti-inflammatory effects of
ellagic acid on acute lung injury induced by acid in mice.
Mediators Inflamm. 2013:1642022013.PubMed/NCBI
|
|
19
|
Ho CC, Huang AC, Yu CS, Lien JC, Wu SH,
Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, et al: Ellagic acid
induces apoptosis in TSGH8301 human bladder cancer cells through
the endoplasmic reticulum stress- and mitochondria-dependent
signaling pathways. Environ Toxicol. 29:1262–1274. 2014.PubMed/NCBI
|
|
20
|
Morgan TM, Koreckij TD and Corey E:
Targeted therapy for advanced prostate cancer: Inhibition of the
PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 9:237–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye G, Lu Q, Zhao W, Du D, Jin L and Liu Y:
Fucoxanthin induces apoptosis in human cervical cancer cell line
HeLa via PI3K/Akt pathway. Tumor Biol. 35:11261–11267. 2014.
View Article : Google Scholar
|
|
22
|
Scrima M, De Marco C, Fabiani F, Franco R,
Pirozzi G, Rocco G, Ravo M, Weisz A, Zoppoli P, Ceccarelli M, et
al: Signaling networks associated with AKT activation in non-small
cell lung cancer (NSCLC): New insights on the role of
phosphatydil-inositol-3 kinase. PLoS One. 7:e304272012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu CX, Jin H, Shin JY, Kim JE and Cho MH:
Roles of protein kinase B/Akt in lung cancer. Front Biosci (Elite
Ed). 2:1472–1484. 2010.PubMed/NCBI
|
|
24
|
Umesalma S, Nagendraprabhu P and
Sudhandiran G: Ellagic acid inhibits proliferation and induced
apoptosis via the Akt signaling pathway in HCT-15 colon
adenocarcinoma cells. Mol Cell Biochem. 399:303–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim ND, Mehta R, Yu W, Neeman I, Livney T,
Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, et al:
Chemopreventive and adjuvant therapeutic potential of pomegranate
(Punica granatum) for human breast cancer. Breast Cancer Res Treat.
71:203–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mitsiades CS, Mitsiades N and Koutsilieris
M: The Akt pathway: Molecular targets for anti-cancer drug
development. Current Cancer Drug Targets. 4:235–256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saglam O, Garrett CR, Boulware D, Sayegh
Z, Shibata D, Malafa M, Yeatman T, Cheng JQ, Sebti S and Coppola D:
Activation of the serine/threonine protein kinase AKT during the
progression of colorectal neoplasia. Clinical Colorectal Cancer.
6:652–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D WR: The hallmarks of cancer.
Cell. 2000. View Article : Google Scholar
|