Introduction
The mortality rate of patients with colon cancer is
one of the highest of all types cancer, due to late tumor
presentation and rapid progression (1). Despite improvements in the diagnosis
and treatment of colon cancer, there are ~1,000,000 cases of colon
cancer reported annually, with >600,000 mortalities per year
(1). Patients are usually diagnosed
when they reach an advanced stage of colon cancer (2), when surgery is ineffective; however,
early stage surgery may be an effective method of attenuating its
progression (3). Therefore, novel
prognostic biomarkers and targeted therapeutics are required to
enable the early detection and treatment of colon cancer.
MicroRNAs (miRNAs) are a class of endogenous
noncoding RNAs 21–23 nucleotides long, which regulate the
expression of target genes in mammals either by degrading target
mRNA or inhibiting the translation of target genes (4). A total of 2,042 mature human miRNAs
have been identified, which may regulate >30% of target mRNAs
(5). The results of previous studies
have demonstrated that miRNAs serve important roles in
tumorigenesis by regulating the expression of various oncogenes and
tumor suppressor genes (6–8). miR-423-5p is an effective serum
biomarker for colon cancer. Fang et al (9) demonstrated that the concentration of
plasma miR-423-5p was decreased in patients with colon cancer and
benign lesions, including polyps and adenoma, compared with healthy
controls. Therefore, it has been suggested that plasma levels of
miR-423-5p may serve as a biomarker for colon cancer detection,
particularly for early stage colon cancer (9). Indeed, it was demonstrated that in
stage I–II colon cancer, serum miR-423-5p was significantly
elevated compared with controls, whereas in stage III–IV colon
cancer, the differences in miR-423-5p expression between patients
with colon cancer and healthy controls were not significant
(10).
However, the expression and function of miR-423-5p
in malignant colon tissues and colon cancer tumorigenesis remains
unclear. The aim of the present study was to evaluate the
expression of miR-423-5p in malignant colon tissues and colon
cancer cell lines. The potential regulatory role of miR-423-5p on
colon cancer cell proliferation and apoptosis was also determined.
These results may provide a novel target for the diagnosis and
treatment of colon cancer.
Materials and methods
Clinical samples
The present study was approved by the Ethics
Committee of Beijing Hospital (Beijing, China). A total of 25 pairs
of diagnostic primary malignant colon samples and adjacent normal
colon tissues (used as controls) were obtained from the Department
of General Surgery at the Beijing Hospital between May and October
2016. The 25 colon cancer patients, 11 male and 14 female, were
between 48 and 78 years (Table I).
Fasting peripheral blood (5 ml) was drawn from each patient and
placed in anticoagulative tubes at room temperature for 30 min,
followed by centrifugation at 1,500 × g for 5 min at 4°C. The
plasma supernatant was collected and stored at −80°C until use.
Written informed consent was obtained from the patients.
 | Table I.Clinicopathological characteristics of
patients with colon cancer. |
Table I.
Clinicopathological characteristics of
patients with colon cancer.
|
|
| miR-423-5P
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Number of
patients | Low | High | P-values |
|---|
| Sex (%) |
|
|
| >0.05 |
| Male | 11 (44) | 5 (45.5) | 6 (54.5) |
|
|
Female | 14 (56) | 6 (42.9) | 8 (57.1) |
|
| Age (%) |
|
|
| >0.05 |
| ≤60 | 6 (24) | 3 (50.0) | 3 (50.0) |
|
|
>60 | 19 (76) | 8 (42.1) | 11 (57.9) |
|
| TNM stage (%) |
|
|
| <0.05 |
| I | 6 (24) | 2 (33.3) | 4 (66.7) |
|
|
II–III | 11 (44) | 6 (54.5)a | 5 (45.5)a |
|
| IV | 8 (32) | 7 (87.5)b | 1 (12.5)b |
|
Cell culture
The human colon cancer cell lines HT29, SW480,
Caco-2, HCT116 and SW620 were obtained from the American Type
Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and
antibiotics (penicillin and streptomycin; Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a 100% humid incubator with 5%
CO2. Normal human colon epithelial cells (HCoEpiC) were
purchased from Shanghai Hongshun Biotechnology (Shanghai,
China).
Cell transfection
The pU6 vector-based miR-423-5p overexpression
plasmid and miR-negative control (NC) expression plasmid were
customized and purchased from GenePharma (Shanghai, China). A total
of 2 µg miR-423-5p overexpression plasmid or miR-NC were
transfected into SW620 and HCT116 cells using 5 µl FuGENE
HP® (Promega Corporation, Madison, WI, USA), following
the manufacturer's protocol. A total of 48 h after cell
transfection, the cells were collected for further analysis.
z-VAD-FMK was purchased from Selleck Chemicals (Shanghai, China)
and used to treat cells at a concentration of 50 µM for 24 h
following transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Human colon samples and transfected cells were lysed
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
and total RNA was extracted following the manufacturer's protocol.
Reverse transcription was performed on the isolated total RNA using
a PrimeScript® RT Master Mix kit (Perfect Real Time;
cat. no. RR036A; Takara Bio, Inc., Kusatsu, Japan) and qPCR was
performed using a SYBR Green I Real Time PCR kit (cat. no. RR420A;
Takara Bio, Inc.), according to the manufacturer's protocol.
Reverse transcription was performed at 65°C for 5 min, 30°C for 10
min, 42°C for 10 min and 2°C for 3 min. qPCR conditions were as
follows: Denaturation at 94°C for 2 min, amplification for 30
cycles at 94°C for 30 sec, annealing at 59°C for 30 sec and
extension at 72°C for 1 min, followed by a terminal elongation step
at 72°C for 10 min. The primers for miR-423-5p were purchased from
Guangzhou RiboBio Co., Ltd. (cat no. S170721170309; Guangzhou,
China). Sequences were not supplied due to the rules of the
company. U6 was used as an internal control. qPCR analysis was
performed using a Bio-Rad CFX96 thermal cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Data were quantified using
the 2−ΔΔCq method (11,12).
Cell proliferation assay
Following transfection, cells were cultured in a
96-well plate at a density of 800 cells/well. A Cell Counting Kit-8
(CCK-8) assay was performed to measure cell proliferation. At 0,
24, 48 and 72 h post transfection, CCK-8 (Beyotime Institute of
Biotechnology, Haimen, China) was added to the wells and cells were
incubated at 37°C for 4 h. Optical density was measured at 450 nm
using a microplate reader. Experiments were performed four
times.
Colony formation assay
SW620 and HCT116 cells (1,000 cells/well) were
seeded in a 6-well plate following transfection. Cells were
cultured with DMEM containing 10% FBS. Following 12 days, cells
were fixed with 4% paraformaldehyde at room temperature for 15 min
and stained with crystal violet (Beyotime Institute of
Biotechnology) at room temperature for 10 min. The number of
colonies in 5 random fields were counted and analyzed with inverted
microscope (BX51; Olympus, Tokyo, Japan) at a magnification of ×10.
Experiments were performed three times.
Apoptosis analysis
Following transfection for 48 h, cells were
harvested and stained using an Annexin V-fluorescein
isothiocyanate/Propidium iodide Apoptosis Detection kit (Nanjing
Keygentec Biotech, Nanjing, China) following the manufacturer's
protocol. Apoptotic cells were assessed using a flow cytometer (BD
FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA) and analyzed
by FlowJo (version 10.4.1; FlowJo LLC, Ashland, OR, USA).
Experiments were performed three times.
Western blot analysis
Following transfection for 48 h, cells were
harvested and lysed in ice-cold radioimmunopreciptation assay lysis
buffer (Beyotime Institute of Biotechnology) containing 1% protease
inhibitor cocktail (Pierce; Thermo Fisher Scientific, Inc.) for 30
min and were then centrifuged at 13,000 × g for 15 min at 4°C. A
BCA kit (Beyotime Institute of Biotechnology) was used to determine
protein concentration and SDS-PAGE loading buffer (Beyotime
Institute of Biotechnology) was added to prepare the loading
sample. A total of 15 µg/lane protein was subjected- to
electrophoresis on 10–12% SDS-PAGE gels and transferred to PVDF
membranes (Merck KGaA, Darmstadt, Germany). Membranes were blocked
with PBS containing 5% non-fat milk for 1 h at room temperature and
then incubated with primary antibodies against caspase 3 (cat no.
9662; 1:1,000), caspase 8 (cat. no. 4790; 1:800), caspase 9 (cat.
no. 9508; 1:800), p53 (cat. no. 2524; 1:1,000) and GAPDH (cat no.
5174; 1:5,000; all Cell Signaling Technology, Inc., Danvers, MA,
USA) at 4°C overnight. Following washing, membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit (cat. no.
TA140003) and mouse secondary antibodies (cat. no. TA140002; both
1:10,000, OriGene Technologies, Inc., Rockville, MD, USA) for 60
min at room temperature. The bands were detected by enhanced
chemiluminescent (ECL) kit (Merck KGaA) and the band density were
quantified by Image J software (version 1.48; National Institutes
of Health, Bethesda, MD, USA). The quantities of samples were
normalized based on the expression of GAPDH.
Statistical analysis
All values are expressed as the mean ± standard
deviation of ≥3 results. Student's t test and one-way analysis of
variance with Dunnett's multiple comparisons test were used to
assess differences between groups using SPSS 21.0 (IBM Corp.,
Armonk, NY, USA). Logistic regression analysis was used to assess
the correlation between plasma miR-423-5p expression and tumor
grade. P<0.05 was determined to indicate a statistically
significant difference.
Results
Downregulation of miR-423-5p in colon
cancer tissues and cell lines
The expression of miR-423-5p in colon cancer tissues
and cell lines was investigated using RT-qPCR. miR-423-5p
expression was significantly lower in colon tumor tissues
(0.55±0.08) compared with normal colon tissues (1.87±0.24;
P<0.0001; Fig. 1A). Logistic
regression analysis indicated that miR-423-5p expression was lower
in patients with high grade tumors (P<0.05; Fig. 1B). The expression of miR-423-5p in
the plasma of 25 patients with colon cancer compared with healthy
controls was also determined. The results indicated that the
expression of miR-423-5p in plasma was significantly lower in
patients with colon cancer (0.88±0.20) compared with healthy
controls (3.85±0.57; P<0.0001; Fig.
1C). The expression of miR-423-5p in plasma was also decreased
as the tumor grade increased (P<0.05; Fig. 1D). miR-423-5p expression was
decreased in malignant colon tissues and plasma from patients with
colon cancer; therefore, the expression of miR-423-5p was
subsequently assessed in colon cancer cell lines. The results
indicated that it was significantly downregulated in all colon
cancer cell lines compared with HCoEpiCs (all P<0.01; Fig. 1E). These results suggest that the
expression of miR-423-5p is downregulated in colon cancer and
decreases as tumor grade increases.
Overexpression of miR-423-5p inhibits
colon cancer growth and colony formation
miR-423-5p was cloned into a plasmid expression
system and transfected into cells to validate its effects on colon
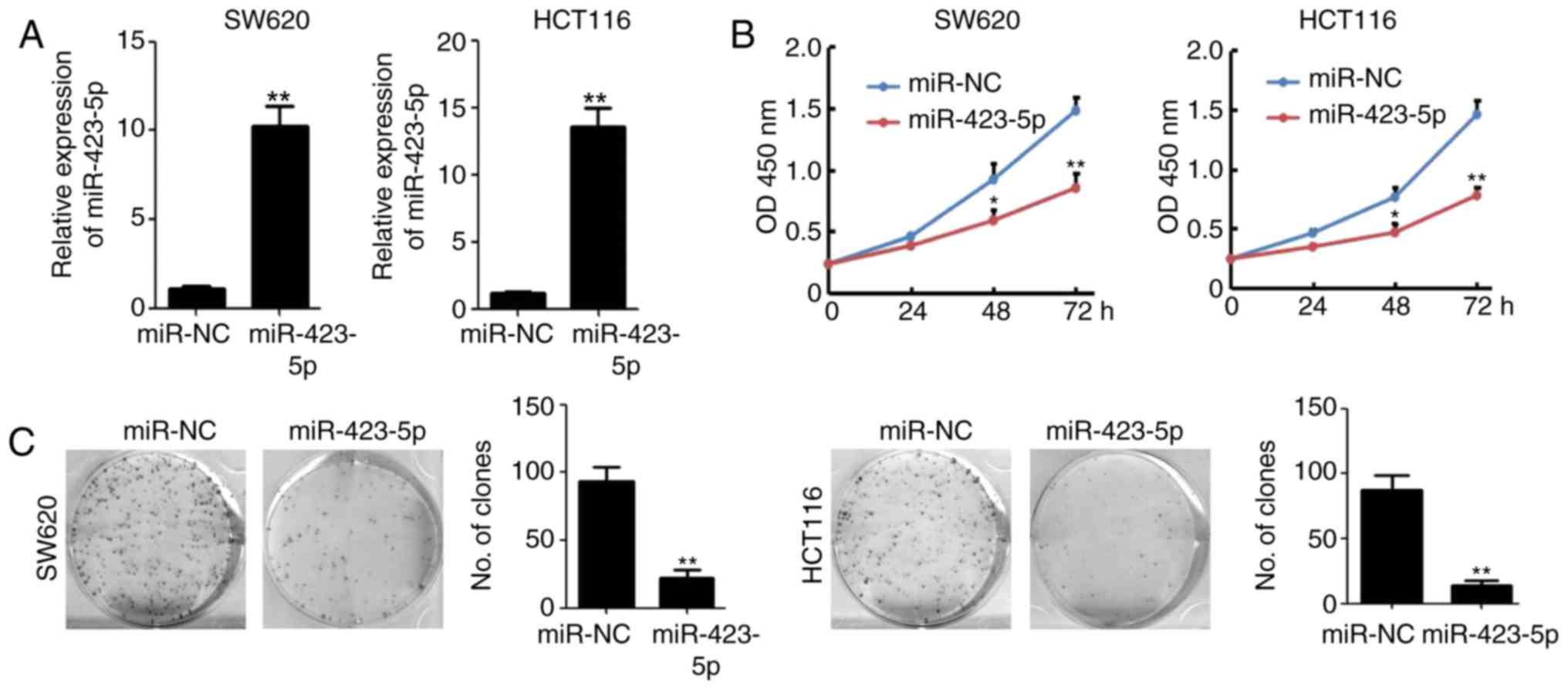
cancer. miR-423-5p transfection significantly upregulated
miR-423-5p levels in SW620 and HCT116 cells (Fig. 2A). The results of the CCK8 assay
indicated that the overexpression of miR-423-5p in SW620 and HCT116
cells significantly reduced cell viability by 42.5 and 46.6%,
respectively, compared with that of the miR-NC-transfected groups
at 72 h (n=4; P<0.01; Fig. 2B).
Colony formation was also significantly decreased in HCT116
(87.3±11.3 vs. 13.7±3.7, miR-NC vs. miR-423-5p) and SW620
(93.3±10.6 vs. 22.3±5.9, miR-NC vs. miR-423-5p) cells transfected
with miR-423-5p (both P<0.01; Fig.
2C). These results suggest that the overexpression of
miR-423-5p inhibits colon cancer cell proliferation and colony
formation.
Overexpression of miR-423-5p promotes
apoptosis and caspase protein expression in colon cancer cells
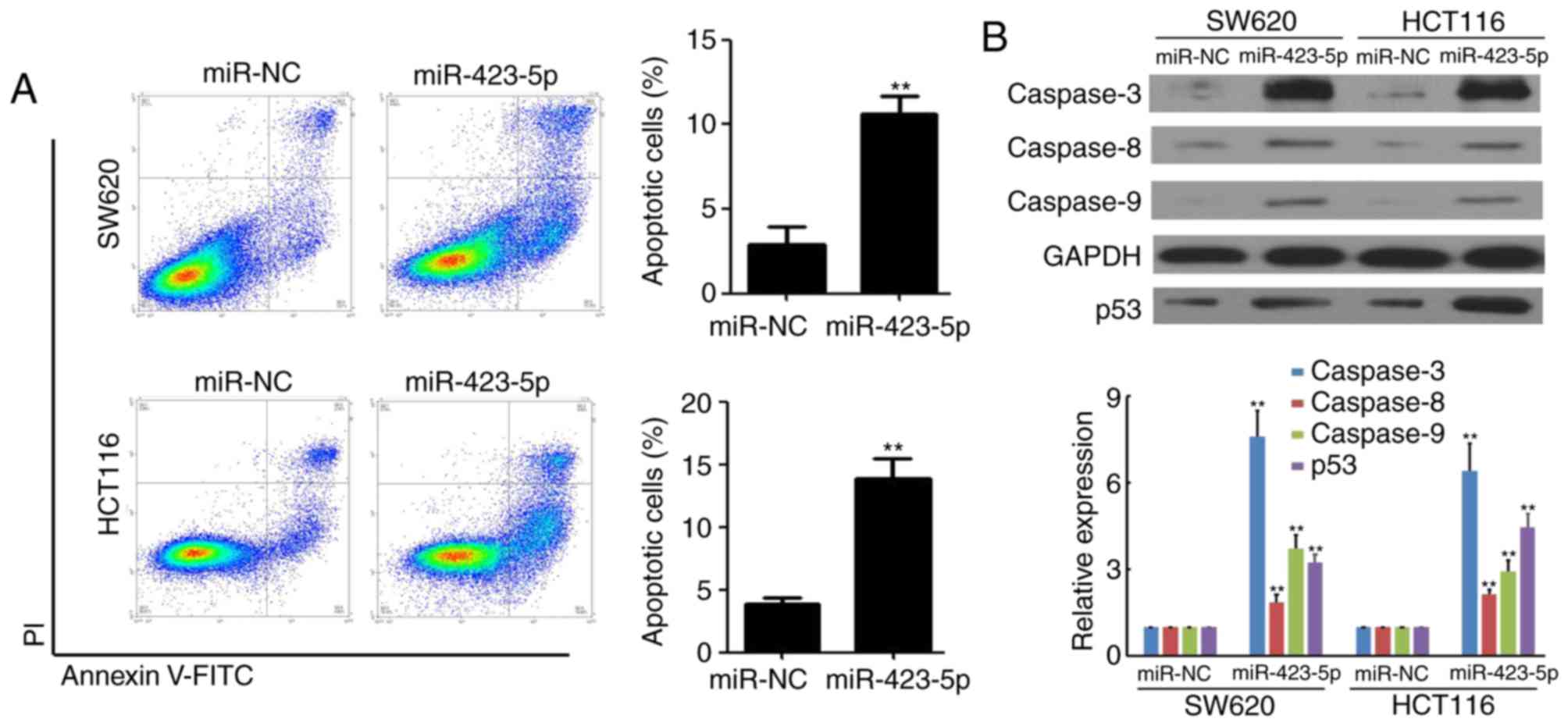
The mechanism underlying the miR-423-5p-mediated
inhibition of colon cancer cell growth was determined using flow
cytometry in SW620 and HCT116 cells following transfection with
miR-423-5p and miR-NC. The results indicated that the rate of
apoptosis was increased in SW620 (2.9±0.9% vs. 10.6±1.1%, miR-NC
vs. miR-423-5p) and HCT116 (3.8±0.5% vs. 13.8±1.6%, miR-NC vs.
miR-423-5p) cells following miR-423-5p transfection (both
P<0.01; Fig. 3A). Furthermore,
the expression of caspases 3, 8 and 9 in miR-NC and SW620 and
HCT116 cells transfected with miR-423-5p was measured using western
blotting. The expression of caspases 3, 8, 9 and p53 were increased
in SW620 and HCT116 cells transfected with miR-423-5p compared with
the respective cells transfected with miR-NC (all P<0.01;
Fig. 3B). These results suggest that
the overexpression of miR-423-5p promotes apoptosis and the
expression of caspases in colon cancer cells.
miR-423-5p induces caspase-dependent
apoptosis in colon cancer cells
z-VAD is an inhibitor of caspase activity and was
used to treat miR-423-5p-transfected colon cancer cells. z-VAD
treatment attenuated the miR-423-5p-mediated upregulation of
apoptosis in SW620 cells (11.5±1.5% vs. 4.5±0.9%, miR-423-5p vs.
miR-423-5p+z-VAD; P<0.05) and HCT116 cells (14.9±0.9% vs.
5.8±0.5%, miR-423-5p vs. miR-423-5p+z-VAD; P<0.01; Fig. 4). There were no significant
differences in the rates of apoptosis between the
miR-NC-transfected and miR-423-5p+z-VAD groups in SW620 cells
(2.9±0.7% vs. 4.5±0.9%, miR-NC vs. miR-423-5p+z-VAD) and HCT116
cells (3.9±0.6% vs. 5.8±0.5%, miR-NC vs. miR-423-5p+z-VAD). These
results indicate that miR-423-5p induces caspase-dependent
apoptosis in colon cancer cells.
Discussion
The results of the current study demonstrated that
miR-423-5p expression was significantly downregulated in colon
malignant tissues, as well as in colon cancer cell lines. It was
demonstrated that miR-423-5p inhibits colon cancer cell
proliferation and colony formation, thereby promoting cell
apoptosis. Furthermore, the effects of z-VAD demonstrated that
miR-423-5p-mediated colon cancer cell apoptosis is
caspase-dependent. These results suggest that miR-423-5p is
downregulated in colon cancer and functions as a tumor
suppressor.
miR-423-5p is upregulated in the myocardium
following heart failure and is associated with the prohormone brain
natriuretic peptide and ejection fraction (13). However, it is not an effective
biomarker of systemic ventricular and left ventricular remodeling
(14,15). Furthermore, it has been demonstrated
that miR-423-5p significantly downregulates the expression of
β-linked N-acetylglucosamine transferase and its associated
downstream targets, and also induces apoptosis in cardiomyocytes
(16). Furthermore, miR-423-5p
expression is increased in the serum of patients with
hepatocarinoma following treatment with sorafenib and miR-423-5p
promotes autophagy (17). It has
been determined that miR-423-5p knockdown enhances the sensitivity
of glioma stem cells to apigenin via the mitochondrial pathway
(18). In addition, plasma
miR-423-5p may be a biomarker for colon cancer (9,10). The
current study demonstrated that miR-423-5p is downregulated in
colon malignant tissues and colon cancer cell lines. Overexpression
of miR-423-5p impaired colon cancer cell proliferation and colony
formation in vitro. These results improve understanding of
the role miR-423-5p serves in colon cancer.
Environmental factors and accumulation of mutations
serve a role in the development of colon cancer, resulting in
increased cell proliferation, uncontrolled angiogenesis, inhibition
of apoptosis and immune system evasion (19–22).
Evasion of apoptosis is one of the hallmarks of human cancer
(23). Damaged or unnecessary cells
are normally eliminated by apoptosis via the extrinsic and
intrinsic apoptotic pathways (23).
Thus, promoting the apoptosis of tumor cells may be an effective
method of treating colon cancer. Agents that induce cellular DNA
damage and cause cell apoptosis, including irinotecan and
cisplatin, are commonly used to treat patients with colon cancer
(24,25). In the current study, the role of
miR-423-5p in promoting cell apoptosis and caspase expression in
colon cancer was determined. The results indicated that
miR-423-5p-mediated cell apoptosis is caspase-dependent, thus
improving the understanding of the mechanisms by which miR-423-5p
regulates apoptosis.
In conclusion, the results of the current study
demonstrated that miR-423-5p is downregulated in colon malignant
tissues and colon cancer cell lines. Overexpression of miR-423-5p
induces caspase-dependent apoptosis, resulting in inhibition of
cell proliferation and colony formation. Taken together, the
results of the current study suggest that miR-423-5p may serve be
an effective target for the detection and treatment of colon
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data and materials supporting the results of the
current study are available within the article.
Authors' contributions
WZJ and TY were involved in the acquisition of the
data. QA was involved in the analysis and interpretation of the
data. XLC were involved in the collection of human tissues. HDP was
involved in the conception and design of the present study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Hospital (Beijing, China). Written informed
consent was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonas S, Thelen A, Benckert C, Spinelli A,
Sammain S, Neumann U, Rudolph B and Neuhaus P: Extended resections
of liver metastases from colorectal cancer. World J Surg.
31:511–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith JJ, Deane NG, Dhawan P and Beauchamp
RD: Regulation of metastasis in colorectal adenocarcinoma: A
collision between development and tumor biology. Surgery.
144:353–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore MJ, Scheel TK, Luna JM, Park CY, Fak
JJ, Nishiuchi E, Rice CM and Darnell RB: miRNA-target chimeras
reveal miRNA 3′-end pairing as a major determinant of Argonaute
target specificity. Nat Commun. 6:88642015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slezak-Prochazka I, Durmus S, Kroesen BJ
and van den Berg A: MicroRNAs, macrocontrol: Regulation of miRNA
processing. RNA. 16:1087–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rupaimoole R, Wu SY, Pradeep S, Ivan C,
Pecot CV, Gharpure KM, Nagaraja AS, Armaiz-Pena GN, McGuire M, Zand
B, et al: Hypoxia-mediated downregulation of miRNA biogenesis
promotes tumour progression. Nat Commun. 5:52022014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu SY, Rupaimoole R, Shen F, Pradeep S,
Pecot CV, Ivan C, Nagaraja AS, Gharpure KM, Pham E, Hatakeyama H,
et al: A miR-192-EGR1-HOXB9 regulatory network controls the
angiogenic switch in cancer. Nat Commun. 7:111692016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tajima K, Yae T, Javaid S, Tam O, Comaills
V, Morris R, Wittner BS, Liu M, Engstrom A, Takahashi F, et al:
SETD1A modulates cell cycle progression through a miRNA network
that regulates p53 target genes. Nat Commun. 6:82572015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang Z, Tang J, Bai Y, Lin H, You H, Jin
H, Lin L, You P, Li J, Dai Z, et al: Plasma levels of microRNA-24,
microRNA-320a, and microRNA-423-5p are potential biomarkers for
colorectal carcinoma. J Exp Clin Cancer Res. 34:862015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X and Lu J: The significance of
detection of serum miR-423-5p and miR-484 for diagnosis of
colorectal cancer. Clin Lab. 61:187–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumarswamy R, Anker SD and Thum T:
MicroRNAs as circulating biomarkers for heart failure: Questions
about MiR-423-5p. Circ Res. 106:e8author reply e9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nabiałek E, Wańha W, Kula D, Jadczyk T,
Krajewska M, Kowalówka A, Dworowy S, Hrycek E, Wludarczyk W, Parma
Z, et al: Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in
acute myocardial infarction and stable coronary heart disease.
Minerva Cardioangiol. 61:627–637. 2013.PubMed/NCBI
|
|
15
|
Bauters C, Kumarswamy R, Holzmann A,
Bretthauer J, Anker SD, Pinet F and Thum T: Circulating miR-133a
and miR-423-5p fail as biomarkers for left ventricular remodeling
after myocardial infarction. Int J Cardiol. 168:1837–1840. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo P, He T, Jiang R and Li G:
MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in
cardiomyocytes. Mol Med Rep. 12:1163–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stiuso P, Potenza N, Lombardi A,
Ferrandino I, Monaco A, Zappavigna S, Vanacore D, Mosca N,
Castiello F, Porto S, et al: MicroRNA-423-5p promotes autophagy in
cancer cells and is increased in serum from hepatocarcinoma
patients treated with sorafenib. Mol Ther Nucleic Acids.
4:e2332015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan Y, Fei X, Wang Z, Jiang D, Chen H,
Wang M and Zhou S: miR-423-5p knockdown enhances the sensitivity of
glioma stem cells to apigenin through the mitochondrial pathway.
Tumour Biol. 39:10104283176955262017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Offit K, Kohut K, Clagett B, Wadsworth EA,
Lafaro KJ, Cummings S, White M, Sagi M, Bernstein D and Davis JG:
Cancer genetic testing and assisted reproduction. J Clin Oncol.
24:4775–4782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calvert PM and Frucht H: The genetics of
colorectal cancer. Ann Intern Med. 137:603–612. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei EK, Wolin KY and Colditz GA: Time
course of risk factors in cancer etiology and progression. J Clin
Oncol. 28:4052–4057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Connell MJ: Oxaliplatin or irinotecan as
adjuvant therapy for colon cancer: The results are in. J Clin
Oncol. 27:3082–3084. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dienstmann R, Salazar R and Tabernero J:
Personalizing colon cancer adjuvant therapy: Selecting optimal
treatments for individual patients. J Clin Oncol. 33:1787–1796.
2015. View Article : Google Scholar : PubMed/NCBI
|