Introduction
Gestational diabetes mellitus (GDM) is defined as
diabetes during pregnancy and is the most common
pregnancy-associated complication (1). Symptoms of GDM include hyperglycemia,
glucose intolerance, insulin resistance and fetal maldevelopment
(2). A previous study has indicated
that GDM may increase the risk of type II diabetes mellitus and
cardiovascular disease (3). Studies
also suggested that GDM poses a great threat for the fetal and
maternal safety during pregnancy (4–6). The
International Association of Diabetes in Pregnancy Study Group
criteria for GDM have been recommended by World Health Organization
(7). To date, various diagnostic
methods have been suggested based on the understanding of the
molecular mechanisms of the pathogenesis of this disease (8–10).
Therefore, investigation of potentially implicated cytokines may be
beneficial for diagnosing patients with GDM.
Fibroblast growth factor-21 (FGF-21) is an atypical
member of the family of FGFs, and is regarded as a multifunctional
cytokine (11). A study has
indicated that FGF-21 is also a multifunctional protein
predominantly secreted by adipose tissue, pancreas and liver, and
has been regarded as a polypeptide with efficacy in the treatment
of metabolic disorders (12,13). FGF21 is produced in the liver and has
a crucial role in regulating glucose and lipid metabolism, as well
as maintaining energy homeostasis; it has been implicated in the
regulation of the endocrine metabolism and various chronic diseases
occurs via regulated metabolic processes, including glucose and
lipid metabolism (14). Wang et
al (15) have indicated that the
serum concentration of FGF-19 and −21 in maternal patients with
gestational diabetes mellitus is associated with insulin
resistance, adiponectin and a history of polycystic ovary syndrome.
However, the diagnostic value of the serum levels of FGF-21 in
gestational diabetes mellitus patients has remained to be fully
elucidated.
The present study assessed the potential diagnostic
value of human FGF-21 for GDM and its possible association with
insulin resistance and glucose tolerance in affected patients. The
results demonstrated that the serum levels of FGF-21 were
significantly downregulated in GDM patients, which may be
associated with glucose and insulin metabolism, and suggest that
FGF-21 is a potential diagnostic factor for the diagnosis of
GDM.
Materials and methods
Ethical statement
The protocols were approved by Ethics Committee of
The Third Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China). Patients and healthy individuals were required to provide
written informed consent. The authors had no access to any
information that could identify the individual participants during
or after data collection.
Patients and healthy volunteers
A total of 50 patients with GDM and 50 age-matched
healthy individuals were enrolled in The Third Affiliated Hospital
of Sun Yat-sen University between May 2013 and June 2014 in the
present study. The mean age was 28.3 and 28.6 years in the GDM
group and healthy individuals, respectively. There was a difference
in mean BMI values between the GDM patients and healthy
individuals. The mean duration of follow-up in the GDM group was 6
months (Table I). The inclusion
criteria were as follows: i) Gestation period of 4–8 months; ii) no
history of diabetes mellitus, and no presence of renal failure,
intake of medications, ischemic heart disease, heart failure or
pancreatic disease.
 | Table I.Characteristics of GDM patients
compared with healthy volunteers. |
Table I.
Characteristics of GDM patients
compared with healthy volunteers.
| Parameter | GDM patients | Healthy
volunteers |
|---|
| Mean age (years) | 28.3 (22.5–35.2) | 28.6 (23.2–34.5) |
| No. of patients | 50 | 50 |
| Follow-up time
(months) | 6 | 6 |
| Blood glucose
(mmol/l) | 9.3±0.9 | 6.5±0.7a |
| Blood pressure
(mmHg) | 124±14 | 120±16 |
| Insulin concentration
(mmol/l) | 9.2±1.2 | 14.9±2.5a |
| Body mass index
(kg/m2) | 37.2±12 |
25.4±12.2a |
ELISA
The serum levels of FGF-21 in patients with GDM and
healthy volunteers were detected using an ELISA kit (cat. no.
KA1849; Abnova, Taipei, Taiwan) according to the manufacturer's
protocols. Finally, the serum concentration levels of FGF-21 were
measured with an ELISA microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from adipocytes using by
using an RNAeasy Mini Kit (Qiagen, Hilden, Germany) according to
the manufacturer's protocols. Reverse transcription was performed
at 42°C for 2 h in a total of 50 µl containing 50 ng RNA, 2 µl
primers, 5 µl dNTP, 2 µl RT-buffer, and 1 µl reverse transcriptase.
All of the forward and reverse primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
the following sequences: FGF-21 forward, 5′-CTGCTGGGGGTCTACCAAG-3′
and reverse, 5′-CTGCGCCTACCACTGTTCC-3′; β-actin forward,
5′-CATCTCTTGCTCGAAGTCCA-3′ and reverse,
5′-ATCATGTTTGAGACCTTCAACA-3′. PCR amplification was performed using
the following program: Initial denaturation at 94°C for 2 min,
followed by 45 cycles of 95°C for 30 sec, annealing at 56°C for 30
sec and 72°C for 10 min. The reaction was performed in a volume of
20 µl containing 50 ng genomic cDNA, 200 µM deoxynucleoside
triphosphate, 2.5 units of Taq DNA polymerase (Takara Biotechnology
Co., Ltd., Dalian, China) and 200 µM primers using PCR (iQ5 Real
Time PCR System; Bio-Rad Laboratories, Inc.). Relative mRNA
expression levels were calculated via the 2−ΔΔCq method
(16). The results are expressed as
the ratio of the β-actin control.
Western blot analysis
Adipose cells were isolated from GDM and healthy
volunteers as described previously (17) and lysed in radioimmunoprecipitation
assay buffer [mammalian protein extraction reagent (PER) for the
cells and tissue PER for the tissues; Thermo Fisher Scientific,
Inc.] followed by homogenization at 4°C for 0 min. The protein
concentration was measured with a bicinchoninic protein assay kit
(Thermo Fisher Scientific, Inc.). A total of 10 µg protein extract
was electrophoresed on 12.5% SDS-PAGE and then transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were incubated in blocking buffer (5% BSA;
Sigma-Aldrich; Merck KGaA) prior to incubation with primary
antibodies at 4°C overnight. The following primary rabbit
anti-human antibodies were used in the immunoblotting assays:
FGF-21 (1:1,000 dilution; cat. no. ab171941) and GAPDH (1:1,500
dilution; cat. no. ab9485; both from Abcam, Cambridge, MA, USA).
After the incubation, the membrane was washed three times in
Tris-buffered saline containing Tween-20 (TBST) and incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin monoclonal antibody (cat. no. PV-6001; Zhongshan
Goldenbridge-Bio, Beijing, China) for 1 h at 37°C. After three
washes in TBST, the membrane was developed using a
chemiluminescence assay system (cat no. 17-677; Roche Diagnostics,
Basel, Switzerland) and exposed to Kodak film (Eastman-Kodak,
Rochester, NY, USA). Densitometric quantification of the immunoblot
data was performed by using Quantity-One software 1.20 (Bio-Rad
Laboratories, Inc.).
Immunohistochemistry staining
Immunohistochemical analysis was performed as
described previously (18).
Paraffin-embedded 4-µm in adipose tissue sections were prepared for
further analysis. The paraffin sections were incubated with
hydrogen peroxide (3%) for 15 min at 37°C and then hydrated in a
decreasing series of ethanols. Antigen retrieval was performed
using an antigen retrieval kit (cat. no. ab93684; Abcam).
Subsequently, 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA)
was used to block non-specific binding at 37°C for 2 h and tissue
sections were incubated with the abovementioned primary antibody to
FGF-21 (1:1,000 dilution) at 4°C for 12 h. All sections were washed
three times and incubated with the abovementioned HRP-conjugated
secondary antibody (1:10,000; cat. no. PV-6001; OriGene
Technologies, Inc., Beijing, China) for 12 h at 4°C.
Diaminobenzidine (Sigma-Aldrich; Merck KGaA) was incubated for 5
min at 37°C used to visualize the antibodies at room temperature
for 10 sec. Positive signals were detected in six random fields of
view under an inverted light microscope (Olympus Corporation,
Tokyo, Japan) and images were captured.
Glucose tolerance and insulin
resistance test
GDM patients and healthy individuals were fasted for
6 h and orally given glucose at a dose of 2.0 g/kg for the glucose
tolerance test. The blood glucose concentration was analyzed with
an ACCU-CHEK Advantage glucometer (Roche Diagnostics). The glucose
tolerance test results were recorded at baseline and after glucose
injection (0, 15, 45, 75 and 105 min). For the insulin tolerance
test, all participants were intraperitoneally injected insulin at
0.75 U/kg body weight. All participants were intraperitoneally
injected with insulin (1 mU/kg) after a 0, 15, 30, 45, 60, 75 and
90 min fast and the blood glucose concentration was measured at
baseline and after insulin injection (15, 30, 60, 90 and 120
min).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean or median (interquartile range) Data were analyzed by
Student's t-test or analysis of variance, followed by Dunnett's
post-hoc test using GraphPad Prism software 5.0 (GraphPad Inc., La
Jolla, Ca, USA). The differences in regression coefficients between
models were compared by Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Serum levels of FGF-21 in GDM
patients
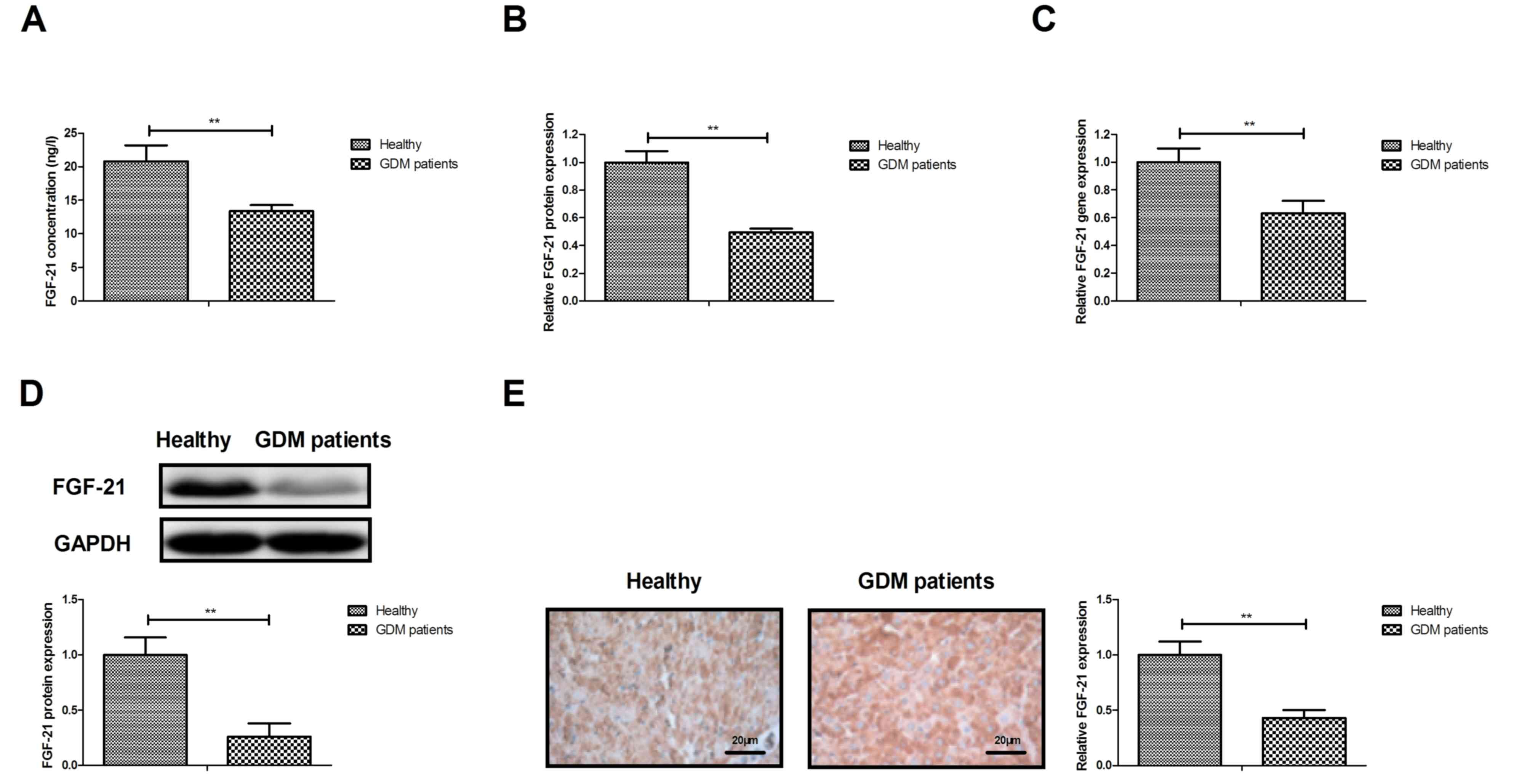
First, FGF-21 expression was detected in the serum
and cells of GDM patients. As presented in Fig. 1A, the serum levels of FGF-21 were
downregulated in patients with GDM compared with those in healthy
individuals. Furthermore, FGF-21 protein was downregulated in
adipocytes isolated from GDM patients compared with those of
healthy volunteers (Fig. 1B). Gene
expression analysis also indicated that FGF-21 mRNA levels were
downregulated in adipocytes isolated from GDM patients compared
with those in healthy volunteers (Fig.
1C). As presented in Fig. 1D,
the protein expression levels of FGF-21 in adipocytes were markedly
downregulated in patients with GDM compared with those in healthy
individuals. Immunohistochemistry indicated that the expression
levels of FGF-21 were also decreased in adipose tissue from GDM
patients compared with those in healthy individuals (Fig. 1E). These results suggest that FGF-21
is downregulated in serum and adipocytes of patients with GDM.
Analysis of glucose and insulin
metabolism in GDM patients
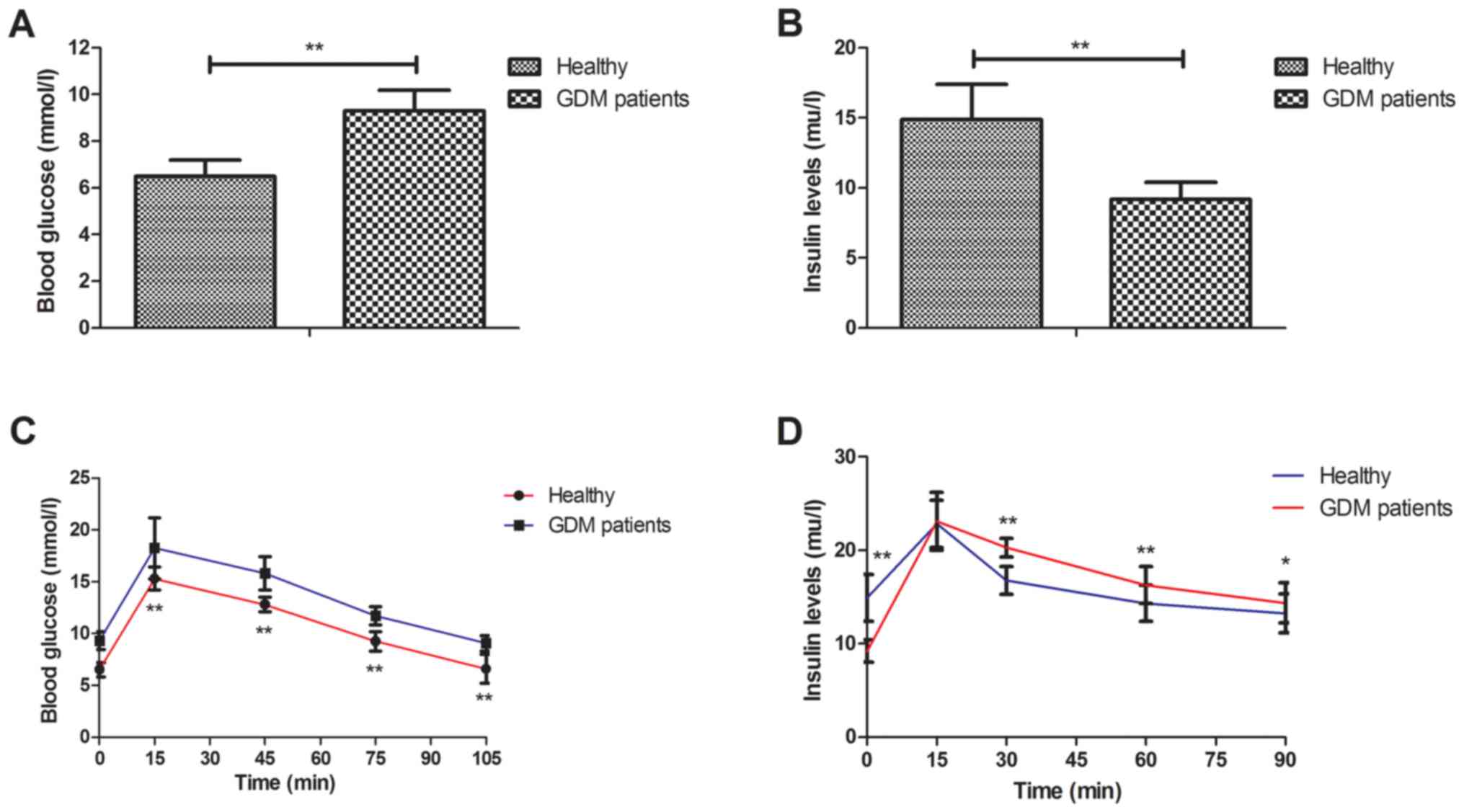
Next, the glucose and insulin metabolism was
assessed in GDM patients and healthy volunteers. The results
indicated that GDM patients presented with a higher blood glucose
concentration than healthy volunteers (Fig. 2A). Furthermore, it was observed that
insulin levels were slightly decreased in GDM patients (Fig. 2B). It was also revealed that GDM
patients had significantly higher glucose intolerance and insulin
resistance than healthy individuals (Fig. 2C and D). These results indicate that
compared to healthy individuals, GDM patients exhibited disorders
in glucose and insulin metabolism.
Association of serum levels of FGF-21
with glucose and insulin metabolism in GDM patients
The correlation of the levels of FGF-21 with
parameters of GDM was then assessed. As presented in Fig. 3A, the plasma levels of FGF-21 were
positively correlated with the blood glucose levels in GDM
patients. Furthermore, the plasma levels of FGF-21 were positively
correlated with the concentration of insulin in GDM patients
(Fig. 3B). These results indicate
that FGF-21 is associated with the serum levels of glucose as well
as with the insulin levels in GDM patients.
Association between serum levels of
FGF-21 and prognosis of GDM patients
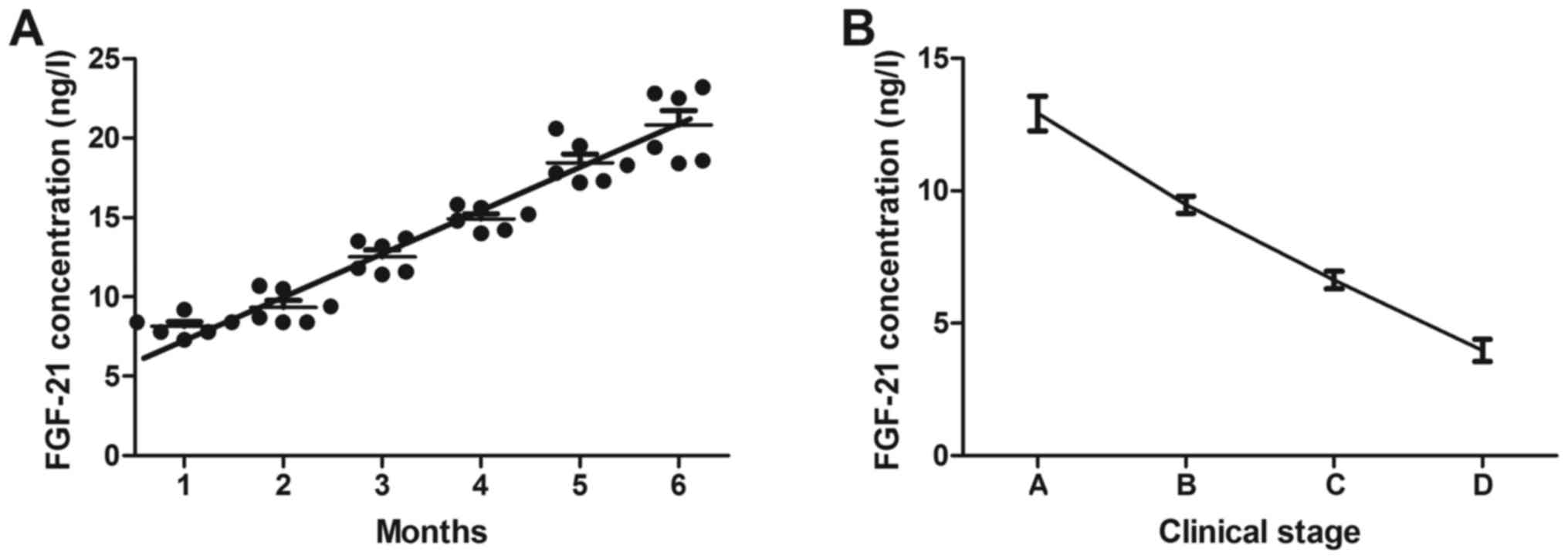
Finally, the association between serum levels of
FGF-21 and the prognosis of GDM patients was analyzed. The results
indicated that the serum levels of FGF-21 were upregulated in GDM
patients after the end of pregnancy (Fig. 4A). The serum levels of FGF-21 were
also associated with the clinical stage of GDM patients (Fig. 4B). These results indicate that FGF-21
may be a prognostic indicator in patients with GDM.
Discussion
FGF-21 has been reported as a novel hepatoprotective
substance (19), and has been
identified as a momentous controller and regulator of glucose and
lipid metabolism, as well as long-term energy balance (20,21). A
study has indicated that attenuation of FGF signaling in mouse
β-cells leads to diabetes (22).
However, the role of FGF-21 in the diagnosis of GDM has remained to
be fully elucidated. The present study investigated the diagnostic
value of FGF-21 in patients with suspected GDM. The results
indicated that the serum levels of FGF-21 are downregulated in GDM
patients. It was demonstrated that the serum levels of FGF-21 are
correlated with insulin resistance and glucose tolerance in
clinical GDM patients.
Studies have suggested that the effects of FGF-21 on
metabolic hormones to regulate energy metabolism are essential for
human vascular endothelial cells (23,24).
Wang et al (25) have
indicated that FGF-21 is positively associated with atrial fibrosis
in atrial fibrillation patients with rheumatic heart disease. The
present study reported that GDM patients exhibit a disorder in
glucose and insulin metabolism compared to healthy individuals.
Chen et al (26) have
indicated that plasma insulin and Helicobacter pylori outer
membrane protein A are independent factors influencing plasma
FGF-21 levels, and due to its role in the pathogenesis of insulin
resistance and type 2 diabetes mellitus, FGF-21 is a potential
diagnostic factor. The present results indicate that the serum
levels of FGF-21 are positively correlated with the concentration
of blood glucose and insulin metabolism in GDM patients. The
results also demonstrated that FGF-21 expression is significantly
different between the GDM and healthy group. However, the
association between FGF-21 and BMI should be further established in
future studies.
FGF-21 alleviates diabetes-associated vascular
complications by inhibiting nuclear factor-κB/NACHT, LRR and PYD
domains-containing protein 3 inflammasome-mediated inflammation
(27). A previous study also
suggested that a high dose of polyethylene glycol-conjugated FGF-21
(500 mg/kg) at the start, followed by a low maintenance dose
results has favorable effects by controlling the glycolipid
metabolic balance, providing a novel method for the management of
diabetes (28). The results of the
present study indicated that the serum levels of FGF-21 are
associated with the prognosis of GDM patients, suggesting that
FGF-21 is a potential prognostic marker in patients with GDM. Chen
et al (26) have demonstrated
that FGF-21 not only increases insulin secretion and insulin
content in diabetic islets, but also protects β-cells from
apoptosis via the activation of extracellular signal-regulated
kinase 1/2 and Akt signaling pathways. The present study reported
that the insulin concentration in GDM patients returns to normal
levels after the end of the pregnancy (within 3 months). However,
the control group was not pregnant, which is a limitation of the
present study. Further studies should thus incorporate a pregnant
control.
In conclusion, the present study suggests that
downregulation of FGF-21 may be associated with the risk of GDM. Of
note, the results indicate that FGF-21 may be a diagnostic and
prognostic indicator in patients with GDM. However, further studies
should be performed in large populations to assess the association
between FGF-21 with impaired glucose metabolism.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Development of Science and Technology of the Department of Science
and Technology of Guangzhou, China (grant no. 201704020170).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CX designed the present study. ZH, PL and XL
performed all experiments and analyzed the data in the present
study.
Ethical approval and consent to
participate
The protocols were approved by the Ethics Committee
of The Third Affiliated Hospital of Sun Yat-sen University
(Guangzhou, China). All patients and healthy individuals were
required to provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Popova P, Castorino K, Grineva EN and Kerr
D: Gestational diabetes mellitus diagnosis and treatment goals:
measurement and measures. Minerva Endocrinol. 41:421–432. 2016.
|
|
2
|
Khalafallah A, Phuah E, Al-Barazan AM,
Nikakis I, Radford A, Clarkson W, Trevett C, Brain T, Gebski V and
Corbould A: Glycosylated haemoglobin for screening and diagnosis of
gestational diabetes mellitus. BMJ Open. 6:e0110592016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hopmans TE, van Houten C, Kasius A,
Kouznetsova OI, Nguyen LA, Rooijmans SV, Voormolen DN, van Vliet
EO, Franx A and Koster MP: Increased risk of type II diabetes
mellitus and cardiovascular disease after gestational diabetes
mellitus: A systematic review. Ned Tijdschr Geneeskd.
159:A80432015.(In Dutch). PubMed/NCBI
|
|
4
|
Wei Y, Yang H, Zhu W, Li H, Yan J and
Zhang C: International association of diabetes and pregnancy study
group criteria is suitable for gestational diabetes mellitus
diagnosis: Further evidence from China. Chin Med J (Engl).
127:3553–3556. 2014.PubMed/NCBI
|
|
5
|
Kalter-Leibovici O, Freedman LS, Olmer L,
Liebermann N, Heymann A, Tal O, Lerner-Geva L, Melamed N and Hod M:
Screening and diagnosis of gestational diabetes mellitus: Critical
appraisal of the new International Association of Diabetes in
pregnancy study group recommendations on a national level. Diabetes
Care. 35:1894–1896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan PC, Aziz AZ, Ismail IS and Omar SZ:
Gamma-glutamyltransferase, alanine transaminase and aspartate
transaminase levels and the diagnosis of gestational diabetes
mellitus. Clin Biochem. 45:1192–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sagili H, Kamalanathan S, Sahoo J,
Lakshminarayanan S, Rani R, Jayalakshmi D and Kumar KT: Comparison
of different criteria for diagnosis of gestational diabetes
mellitus. Indian J Endocrinol Metab. 19:824–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Askari G, Iraj B, Salehi-Abargouei A,
Fallah AA and Jafari T: The association between serum selenium and
gestational diabetes mellitus: A systematic review and
meta-analysis. J Trace Elem Med Biol. 29:195–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amin M, Suksomboon N, Poolsup N and Malik
O: Comparison of glyburide with metformin in treating gestational
diabetes mellitus: A systematic review and meta-analysis. Clin Drug
Investig. 35:343–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuo Z, Wang A and Yu H: Effect of
metformin intervention during pregnancy on the gestational diabetes
mellitus in women with polycystic ovary syndrome: a systematic
review and meta-analysis. J Diabetes Res. 2014:3812312014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Durovcová V, Marek J, Hána V, Matoulek M,
Zikán V, Haluzíková D, Kaválková P, Lacinová Z, Krsek M and Haluzík
M: Plasma concentrations of fibroblast growth factors 21 and 19 in
patients with Cushing's syndrome. Physiol Res. 59:415–422.
2010.PubMed/NCBI
|
|
12
|
Eto K: FGF-21, a newcomer in the field of
hypertension research. J Hum Hypertens. 27:343–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reinehr T, Woelfle J, Wunsch R and Roth
CL: Fibroblast growth factor 21 (FGF-21) and its relation to
obesity, metabolic syndrome, and nonalcoholic fatty liver in
children: A longitudinal analysis. J Clin Endocrinol Metab.
97:2143–2150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F, Yu L, Lin X, Cheng P, He L, Li X,
Lu X, Tan Y, Yang H, Cai L and Zhang C: Minireview: Roles of
fibroblast growth factors 19 and 21 in metabolic regulation and
chronic diseases. Mol Endocrinol. 29:1400–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Zhu W, Li J, An C and Wang Z:
Serum concentrations of fibroblast growth factors 19 and 21 in
women with gestational diabetes mellitus: Association with insulin
resistance, adiponectin, and polycystic ovary syndrome history.
PloS One. 8:e811902013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams KJ, Picou AA, Kish SL, Giraldo
AM, Godke RA and Bondioli KR: Isolation and characterization of
porcine adipose tissue-derived adult stem cells. Cells Tissues
Organs. 188:251–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Selinger CI, Li BT, Pavlakis N, Links M,
Gill AJ, Lee A, Clarke S, Tran TN, Lum T, Yip PY, et al: Screening
for ROS1 gene rearrangements in non-small-cell lung cancers using
immunohistochemistry with FISH confirmation is an effective method
to identify this rare target. Histopathology. 70:402–411. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cariello M and Moschetta A: Fibroblast
growth factor 21: A new liver safeguard. Hepatology. 60:792–794.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suomalainen A, Elo JM, Pietiläinen KH,
Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK,
Tyni T, Kiuru-Enari S, et al: FGF-21 as a biomarker for
muscle-manifesting mitochondrial respiratory chain deficiencies: A
diagnostic study. Lancet Neurol. 10:806–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S,
Xiao J, Wang X, Feng W and Li X: Serum levels of FGF-21 are
increased in coronary heart disease patients and are independently
associated with adverse lipid profile. PloS One. 5:e155342010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hart AW, Baeza N, Apelqvist A and Edlund
H: Attenuation of FGF signalling in mouse beta-cells leads to
diabetes. Nature. 408:864–868. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dushay J, Chui PC, Gopalakrishnan GS,
Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML
and Maratos-Flier E: Increased fibroblast growth factor 21 in
obesity and nonalcoholic fatty liver disease. Gastroenterology.
139:456–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hotta Y, Nakamura H, Konishi M, Murata Y,
Takagi H, Matsumura S, Inoue K, Fushiki T and Itoh N: Fibroblast
growth factor 21 regulates lipolysis in white adipose tissue but is
not required for ketogenesis and triglyceride clearance in liver.
Endocrinology. 150:4625–4633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang R, Yi X, Li X and Jiang X: Fibroblast
growth factor-21 is positively associated with atrial fibrosis in
atrial fibrillation patients with rheumatic heart disease. Int J
Clin Exp Pathol. 8:14901–14908. 2015.PubMed/NCBI
|
|
26
|
Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu
W, Tang Y, Liu H and Boden G: Circulating FGF-21 levels in normal
subjects and in newly diagnose patients with Type 2 diabetes
mellitus. Exp Clin Endocrinol Diabetes. 116:65–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu MH: FGF-21 alleviates
diabetes-associated vascular complications: Inhibiting NF-kB/NLRP3
inflammasome-mediated inflammation? Int J Cardiol. 185:320–321.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu P, Zhang Y, Song L, Khoso MH, Li J,
Jiang X, He J, Li J, Ma X, Ren G and Li D: Efficacy of a
combination of high and low dosage of PEGylated FGF-21 in treatment
of diabetes in db/db mice. Biomed Pharmacother. 84:97–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|