Introduction
A special class of regulatory T (Treg) cells,
cluster of differentiation (CD)4+CD25+ Treg
cells, characterized by high expression of interleukin-2 (IL-2)
receptor α-chain, exists in the peripheral blood and spleen of
normal humans and mice (1,2). Treg cells are divided into two
categories: Natural regulatory T cells (nTreg), which are produced
in the thymus (3) and induced Treg
cells (iTreg), which are induced by inhibitive cytokines, drugs and
immature dendritic cells in vitro or in vivo
(4,5). Recently, studies have demonstrated that
CD4+CD25+ Treg cells with low reactivity and
immunosuppressive properties may serve an important role in
maintaining homeostasis within the internal environment, and
inducing transplantation tolerance, autoimmune diseases, the
response to infections and tumor immunity (6–8). The
proportion of Treg cells in normal peripheral blood, which has
immunosuppressive or tumor immunity abilities, is very small,
accounting for 1-3% of peripheral blood CD4+ T cells
(9,10).
Forkhead box protein 3 (Foxp3) belongs to the
forkhead/winged-helix transcription factor family and displays a
fork-like helical, a C2H2 zinc finger and a leucine zipper
structure (11,12). In humans, Foxp3 is located at
p11.23-q13.3 on the X chromosome, containing 11 exons and 10
introns. It encodes a 48 kDa protein, Scurfin, which is a key
factor in Treg cell development and immunosuppressive function
(13,14). Jiang et al (15) reported that Foxp3 protein was more
specific than CD4, CD25 and other surface markers, serving a
pivotal role in the inhibitive function of Treg cells. Schoenbrunn
et al (16) reported that in
mice, CD4+ cells could convert to Treg cells when Foxp3
was introduced via a retroviral vector.
CD4+CD25+ T cells displayed no immune
regulatory function in Foxp3-deficient mice (16). Chauhan et al (17) reported that Foxp3 expression
determined the regulatory ability of Treg cells and Foxp3
overexpression could lead to a low immune activity status in the
body, which illustrated that Foxp3 was the central regulator of
Treg cell activity.
Circulating tumor cells (CTCs) are a type of tumor
cell that enters the peripheral blood circulation from the primary
tumor or metastasis (18). Over the
course of a malignancy, tumors may spread from the local site to
the blood or lymph circulation. The clinical relevance of CTCs and
metastasis has been confirmed in metastatic breast cancer,
colorectal cancer and prostate cancer (19). There are numerous reports on the
correlation between non-small-cell lung cancer (NSCLC) metastasis
and CTCs (18,20). Additionally, the CTCs in NSCLC
metastasis were reported to cause immune responses, including both
proinflammatory and anti-inflammatory regulation (21,22).
However, the molecular mechanism of CD4+CD25+
Treg cell development, maturation and function in NSCLC development
remains unclear.
Duan et al (23) reported that NSCLC blood
CD4+CD25+ Treg cells could not inhibit
proliferation of reactive T cells activated by auto-antigens. Thus,
the authors proposed that functional maturation of human
CD4+CD25+ Treg cells occurred during
metastasis (23). Li et al
(24) reported that NSCLC blood
CD4+CD25+ Treg cells cultured with
anti-CD3/CD28 mAb in vitro could suppress 95% of allogeneic
mixed lymphocyte reaction and overexpress Foxp3 protein.
Furthermore, the authors indicated that NSCLC blood Treg cells
following treatment have stronger immune suppression ability
compared with normal blood Treg cells. Transforming growth factor
β1 (TGF-β1) serves an important role in the development and
maturation of T cells (4). However,
the role of TGF-β1 in proliferation of
CD4+CD25+ Treg cells and Foxp3 expression
regulation remains unclear.
To analyze the differences between NSCLC and normal
peripheral blood, the immune suppressive ability of
CD4+CD25+ Treg cells, the number of
CD4+CD25high Treg cells and Foxp3 expression
were measured. In addition, due to low immunogenicity and strong
regeneration ability, TGF-β1 was used to produce iTreg cells with
tumor inhibitive functions in the process of NSCLC blood
mononuclear cell (BMC) culturing. Furthermore, protein and mRNA
expression of Foxp3 were monitored by flow cytometry and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay. To explore the role of TGF-β1 in NSCLC blood iTreg cells,
proliferative response of iTreg cells induced by TGF-β1 was
analyzed by carboxyfluorescein succinimidyl ester (CFSE) staining.
The present study aimed to provide a potential new route for
clinical immunotherapy during NSCLC blood metastasis.
Materials and methods
The present study was approved by the Medical Ethics
Committee of Jiaxing No.1 Hospital (approval no. JY201513R;
Jiaxing, China). A total of 231 patients (age, 67±15 years old; 65%
male) who underwent clinical treatment in Jiaxing No. 1 Hospital
were enrolled from January 2016 to December 2017. All patients
provided written informed consent. The procedure of the study
strictly followed the Declaration of Helsinki. Blood samples were
collected according to normal clinical practice.
Preparation of NSCLC BMCs and normal
peripheral BMCs (PBMCs)
NSCLC and normal peripheral blood were collected,
restored in heparin anticoagulation (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and diluted with an equal
amount of Roswell Park Memorial Institute (RPMI)-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
blood was treated with lymphocyte separation liquid (Beyotime
Institute of Biotechnology, Haimen, China). Mononuclear cells were
isolated by density gradient centrifugation (400 × g, 20 min,
20°C), washed twice with serum-free RPMI-1640 (400 × g, 10 min,
4°C, followed by 300 × g, 10 min, 4°C) and adjusted to
1-2×106 cells/ml with RPMI 1640 containing 10% human
autologous serum (Gibco; Thermo Fisher Scientific, Inc.) and 50
ml/l N-bromosuccinimide (NBS; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
NSCLC BMC labeling by CFSE
Cells were analyzed using a flow cytometer. Briefly,
CFSE (Molecular Probes; Thermo Fisher Scientific, Inc.) was
dissolved in dimethyl sulfoxide (2 mmol/l) and stored at −80°C
following aliquoting. Serum-free RPMI-1640 was used to adjust BMCs
to 1×107 cells/ml. Fluorescence intensity of the parent
generation cells labeled by CFSE (FL1) at
104−106 cells per 200 µl well was determined
in a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). CFSE stock solution was added to the cell suspension to
achieve a final concentration of 1.6 µmol/l. Following staining at
37°C for 10 min, the cells were washed three times with RPMI-1640
containing 10% human autologous serum and 100 ml/l NBS to remove
excess dye and adjusted to 1×106 cells/ml with RPMI 1640
containing 10% human autologous serum and 50 ml/l NBS. The cells
were assessed using a flow cytometer and evaluated through an
auto-analyze method using ModFit software (version 4.1; Verity
Software House, Topsham, ME, USA).
T cell culturing and
proliferation
A total of 104−106 BMCs were
added to 24-well cell culture plates at 1 ml per well and incubated
with anti-CD3 monoclonal antibodies (3 µg/ml; cat. no. ab11089;
Abcam, Cambridge, MA, USA) and IL-2 (100 U/ml; PeproTech, Inc.,
Rocky Hill, NJ, USA). Experimental groups were defined by the
addition of varying concentrations (1, 5 and 25 ng/ml) of
recombinant human TGF-β1 (PeproTech, Inc.). Cells were cultured at
37°C, 5% CO2 and harvested on day 4. Experiments were
performed in triplicate.
Proliferation of T cell subsets
analyzed by flow cytometry
Flow cytometry was performed according to a previous
study (19). T cell subsets were
collected. The following antibodies were applied to stain for CD3,
CD4, CD8 on the cell surface with different colored fluorophores,
following previous reports (23):
Anti-CD3-PE/Cy5.5® (cat. no. ab190285) and
anti-CD8-PE/Cy7® (cat. no. ab39853) or anti-CD3-APC
(1:5,000; cat. no. 17-0036-42; Invitrogen; Thermo Fisher
Scientific, Inc), anti-CD4-PerCP/Cy5.5 (cat. no. ab210324) and
anti-CD25-PE/Cy7 (cat. no. ab210335; all 1:5,000; Abcam).
Proliferation of T cell subsets was analyzed by flow cytometry.
CFSE was considered as the first fluorescence (FL1), PE-labeled
antibody as the second fluorescence (FL2), PeCy5.5 as the third
fluorescence (FL3), APC as the fourth fluorescence (FL4). Flow
cytometry was assessed using FACSCalibur (BD Biosciences, Franklin
Lakes, NJ, USA). Cell Quest software (version 5.1; BD Biosciences)
was used for analyzing the data. The cell proliferation ability and
the proportions of CD4+CD25+ and
CD8+ T cells were calculated with the Cell Quest
software.
Analysis of Foxp3 expression in
CD4+CD25+ Treg cells by flow cytometry
Following the collection of 3×105 BMCs
and PBMCs cultured at different TGF-β1 concentration (~300
µl/tube), cells were washed with eBioscience™ Flow Cytometry
Staining Buffer (Invitrogen; Thermo Fisher Scientific, Inc.) and
centrifuged at 300 × g for 5 min at 4°C. The supernatant was
discarded; cells were re-suspended in PBS and incubated with
anti-human CD3-APC, anti-human CD25-PE/Cy7 and anti-human
CD4-PerCP/Cy5.5 antibodies for cell surface staining at 4°C for 30
min. Cells were then washed twice with staining buffer,
re-suspended, centrifuged at 300 × g for 5 min at 4°C and fixed
with paraformaldehyde (PFA; cat. no. A500684; 20 g/l; ~500 µl/tube;
Sangon Biotech Co., Ltd., Shanghai, China) at 4°C for 30 min. The
supernatant was discarded after centrifugation (300 × g for 5 min
at 4°C) and the cells were washed twice with staining buffer. The
cells were incubated with a permeable membrane buffer,
Fixation/Permeabilization Solution kit with BD GolgiStop™ (BD
Biosciences) at 4°C for 15 min. Cells were centrifuged at 400 × g
for 15 min at 4°C and the supernatant was discarded. Staining was
performed with anti-human Foxp3-fluorescein isothiocyanate (FITC; 5
µl (0.5 µg)/test; cat. no. 11-4776-41; Invitrogen; Thermo Fisher
Scientific, Inc.) at 4°C for 30 min. Cells were washed with
permeable membrane buffer and with staining buffer. PFA (10 g/l;
~300 µl/tube) was added and Foxp3 expression in
CD4+CD25+ Treg cells was analyzed by flow
cytometry. The control group was prepared without intracellular
Foxp3 stain and mouse Immunoglobulin G1-FITC (5 µl (0.5 µg)/test;
cat. no. 11-4015-80; Invitrogen; Thermo Fisher Scientific, Inc.)
intracellular staining was used as isotype control. Anti-CD3-FITC
(cat. no. ab34275; Abcam), anti-CD4-PE (cat. no. ab134354; Abcam),
anti-CD3-PE/Cy5.5 or anti-CD3-APC antibodies were used for quality
control of the flow cytometer prior to the experiments, as
recommended by a previous report (23).
mRNA expression of Foxp3 is detected
by RT-qPCR
Total RNA from NSCLC BMCs and normal peripheral BMCs
was isolated using TRIzol® reagent (Invitrogen)
according to the manufacturer's protocol. NanoDrop-1000 (Thermo
Fisher Scientific, Inc.) was used to determine the concentration
and purity of total RNA. A GoScript™ reverse transcription kit
(Promega, Madison, WI, USA) was used to synthesize the first strand
of cDNA with 1 µg total RNA. RT-qPCR assay was used to detect the
mRNA expression of transcription factor Foxp3 (forward primer:
5′-CTGGCGAAGGGCTCGGTAGTCCT-3′, reverse primer:
5′-CTCCCAGAGCCCATGGCAGAAGT-3′) using a PowerUp™ SYBR®
Green kit (cat. no. A25778; Thermo Fisher Scientific, Inc). GAPDH
(cat. no. B661104-0001; Sangon Biotech Co., Ltd.) mRNA served as a
loading controls (forward primer: 5′-AATGCATCCTGCACCACCAA-3′ and
reverse primer: 5′-GTAGCCATATTCATTGTCATA-3′). The thermocycling
protocol was performed as previously described (25).
Statistical analysis
SPSS (version 22.0; IBM Corp., Armonk, NY, USA)
statistical software was used for data processing. Data are
presented as the mean ± standard deviation. Student's t-test was
applied to compare the CD4+ T cell subpopulation ratio
and Foxp3 expression in NSCLC blood and normal peripheral blood. A
one-way analysis of variance was performed, followed by a Tukey's
post hoc test, to analyze the effects of different concentrations
of TGF-β1 on Foxp3 expression in CD4+CD25+ T
cells. P<0.05 was considered to indicate a statistically
significant difference.
Results
Proportion of
CD4+CD25high Treg cells in NSCLC blood is
significantly higher compared with normal peripheral blood
The present study demonstrated that CD25+
and CD25− cells could form two different subsets with
different proportions in NSCLC blood CD4+ T cells
(Fig. 1A). No boundary between
CD25+ and CD25− cells in normal peripheral
blood was observed, displaying a transitional distribution.
CD25+ T cells may be divided into high expression
(CD25high) and low/moderate expression
(CD25int), according to the expression intensity of CD25
analyzed by flow cytometry. In general,
CD4+CD25high T cells are regarded as Treg
cells (24). In the current study,
the data demonstrated that the proportion of
CD4+CD25high T cells in CD4+ T
cells in NSCLC blood (6.89±1.78%) was significantly increased
compared with normal peripheral blood (2.75±1.34%; P<0.05;
Fig. 1A).
Foxp3 expression in CD4+ T
cells in NSCLC blood is significantly decreased compared with
normal peripheral blood
To detect the Foxp3 expression in
CD4+CD25high Treg cells, flow cytometry was
used. Data revealed that the amount of
CD4+CD25high Foxp3+ Treg cells and
CD4+CD25int T cells in NSCLC blood
(23.21±3.56 and 9.14±2.89%, respectively) was significantly lower
compared with normal peripheral blood (72.3±7.89 and 17.11±4.28%,
respectively; both P<0.05; Figs.
1B). The expression of Foxp3 in
CD4+CD25high Treg cells in NSCLC blood
(32.11±3.21%) was significantly lower compared with normal
peripheral blood (68.02±5.59%; P<0.05; Fig. 1C). These results indicate that
CD4+CD25high Treg cells in NSCLC blood were
functionally immature.
Proliferation ability of
CD4+CD25+ T cells and CD8+ T cells
is significantly enhanced following TGF-β1 treatment
To explore changes to the proliferative dynamics of
T cells treated with TGF-β1, the proliferation changes of
CD4+CD25+ T cells and CD8+ T cells
following TGF-β1 treatment were measured by flow cytometry. Results
demonstrated that on day 4 following treatment, the first four
generations [from parental cells (G0) to the 4th generation (G4)]
of CD4+CD25+ Treg cells accounted for 73.77%
(treated with 5 ng/ml TGF-β1) and 83.70% (untreated), while the
proportion G5-6 accounted for 25.28% (treated) and 13.70%
(untreated) of all CD4+CD25+ Treg cells at
that time point (Fig. 2A and B). For
CD8+ T cells, G0-4 accounted for 94.18% (treated) and
95.62% (untreated), and the G5-6 accounted for 5.70% (treated) and
4.83% (untreated) on day 4 of all CD8+ T cells at that
time point (Fig. 2A and B). On day
6, the amount of CD4+CD25+ Treg cells from
G0-4 was 18.65% (treated) and 45.01% (untreated) of all
CD4+CD25+ Treg cells at that time point, and
for G5-8, the proportion of CD4+CD25+ Treg
cells was determined to be 81.29% (treated) and 55.20% (untreated)
(Fig. 2C and D). For CD8+
T cells on day 6, the proportion from G0-4 were 55.92% (treated)
and 77.73% (untreated), while the proportion for G5-8 were 44.04%
(treated) and 23.20% (untreated), respectively (Fig. 2C and D). On day 8,
CD4+CD25+ Treg cells for G0-4 accounted for
8.54% (treated) and 9.54% (untreated), and for G8-10 accounted for
54.35% (treated) and 34.79% (untreated) of all
CD4+CD25+ Treg cells at that time point
(Fig. 2E and F). For CD8+
T cells, the proportion for G0-4 was 42.43% (treated) and 66.20%
(untreated), while the proportion for G8-10 and 13.86% (treated)
and 7.46% (untreated) were determined, respectively (Fig. 2E and F). Blue and orange arrows
reveal increased proliferation (or proliferation trend) of
CD4+CD25+ Treg and CD8+ T cells.
The arrows shift from the prior generations to later generations in
Fig. 2A, C and E. In addition,
proportions of CD4+CD25+ Treg and
CD8+ T cells were significantly increased following
treatment with TGF-β1 on G6-8 at day 6 and G8-9 at day 8 (Fig. 2C and E) compared with untreated cells
(Fig. 2D and F; Student's t-test;
all P<0.05). In summary, TGF-β1 treatment enhanced the
proliferation ability of CD4+CD25+ Treg and
CD8+ T cells.
Foxp3 mRNA expression in
CD4+CD25+ T cells treated with TGF-β1
To examine the effect of TGF-β1 on Foxp3 expression,
varying concentrations of TGF-β1 were used to treat
CD4+CD25+ T cells and Foxp3 mRNA expression
was detected by RT-qPCR. Data indicated that Foxp3 mRNA expression
was significantly increased in cells treated with TGF-β1 (5 ng/ml)
compared with the control group on day 4 and 6 of culturing
(P<0.05; Fig. 3A). In addition,
Foxp3 mRNA expression was examined in TGF-β1 treated
CD4+, CD4+CD25+,
CD4+CD25high CD3+ T cells,
CD4+CD25− and
CD4+CD25high Foxp3+ T cells. The
results indicated that Foxp3 mRNA expression in
CD4+CD25+ and
CD4+CD25high T cells was not significantly
different in CD3+ T cells following TGF-β1 treatment,
while Foxp3 mRNA was significantly upregulated in
CD4+CD25high Foxp3+ T cells
compared with CD4+CD25− Foxp3+ T
cells (P<0.05; Fig. 3B),
suggesting that these cells may have transformed into
CD4+CD25+ Treg cells.
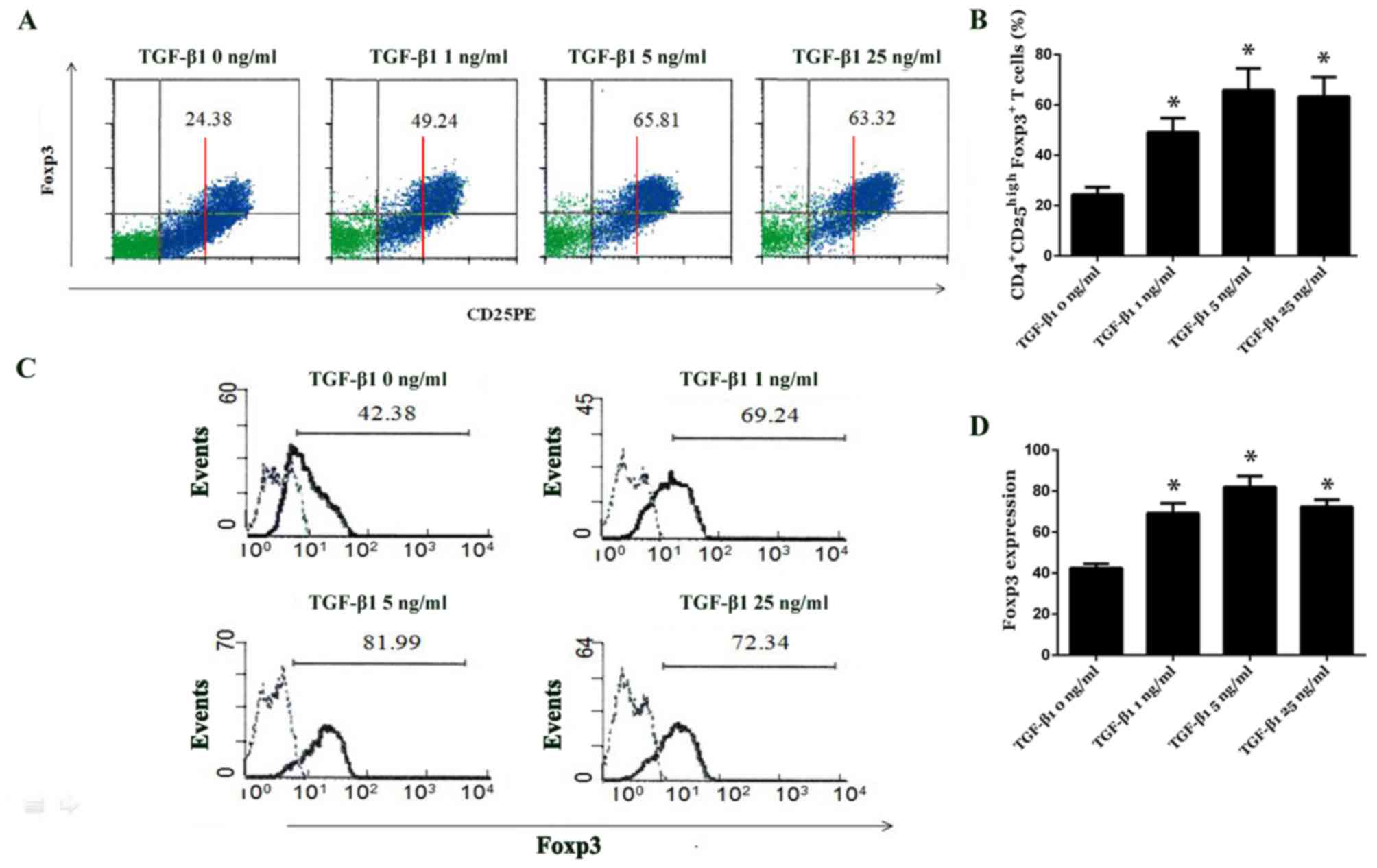
Proportion of
CD4+CD25high Foxp3+ T cells is
significantly increased following TGF-β1 treatment
The effect of TGF-β1 treatment on the induction of
CD4+CD25+ T cell proliferation and Foxp3
expression regulation was determined by analyzing the proportions
of CD4+CD25+,
CD4+CD25high and
CD4+CD25high Foxp3+ T cells by
flow cytometry following TGF-β1 treatment at varying
concentrations. Data revealed that on day 4 following TGF-β1
treatment, the proportion of CD4+CD25+ and
CD4+CD25high T cells was not significantly
different among the different TGF-β1-treated groups (Fig. 4A). However, the proportion of
CD4+CD25high Foxp3+ T cells was
significantly increased in the treatment groups compared with
control group (all P<0.05; Fig. 4A
and B). Foxp3 expression in CD4+CD25high
T cells treated with 1, 5 and 25 ng/ml TGF-β1 was 69.24±4.91%,
81.99±5.29% and 72.34±3.41%, respectively; it was significantly
increased compared with the control group (42.38±2.13%; P<0.05;
Fig. 4C and D). It was demonstrated
that CD4+CD25− T cells displayed negligible
Foxp3 expression (data not shown).
Discussion
CD4+CD25+ Treg cells are a
subset of T cells serving a central role in maintaining the body's
peripheral immune tolerance, including tumor surveillance progress
(6–8). Previous reports have demonstrated that
abnormal numbers of CD4+CD25+ Treg cells or
CD4+CD25+ Treg cell dysfunction are closely
associated with autoimmune diseases, cancer, infectious diseases,
organ transplant rejection, allergic diseases and other diseases
(25,26). CD4+CD25+ Treg
cells overexpress the IL-2 receptor α chain, the cytotoxic T
lymphocyte associated antigen-4, the IL-2 receptor β chain,
neuropilin-1, the glucocorticoid-induced tumor necrosis factor
receptor, integrin αE, the chemokine receptor (CCR)4 and CCR8
(27,28). However, these molecules are all
expressed on activated CD4+ effector T cells, with a
lack of specificity. In addition to these molecules, Guo et
al (29) reported increased
Foxp3 expression on CD4+CD25+ Treg cells, but
decreased expression on CD8+ T and
CD4+CD25− T cells. Although the proposal that
Foxp3 is a specific marker for Treg cells remains controversial,
numerous studies have confirmed that Foxp3 serves a crucial role in
CD4+CD25+ T cell development and its immune
suppression (30). In the current
study, the results indicated high Foxp3 expression in
CD25high T cells, low expression in CD25int T
cells and further decreased expression in CD25− T cells.
Therefore, it was postulated that CD25high T cells may
be leading contributors in the immune regulation of the
CD4+ nTreg cell cluster.
Additionally, the data revealed that CD25 positive
and negative cells formed two distinct groups in NSCLC blood
CD4+ T cell clusters, while CD25 positive and negative
cells formed a continuous cell population in normal peripheral
blood CD4+ T cell clusters. Furthermore, it was
demonstrated that NSCLC blood CD4+CD25high T
cells have clear distinctions from
CD4+CD25int T cells. The number of
CD4+CD25high T cells was significantly
increased in NSCLC blood compared with normal peripheral blood (6
vs. 3%), which is consistent with previous reports (31). The level of intracellular Foxp3
expression in NSCLC blood CD4+CD25high T
cells was significantly decreased compared with normal peripheral
blood CD4+CD25high T cells. This phenomenon
suggests that CD4+CD25high T cells in NSCLC
blood were rarely stimulated by antigens, therefore only a small
number of CD4+CD25high T cells became mature.
Therefore, it is considered that Foxp3 may be a specific marker of
CD4+ Treg cell functional maturation, where low
expression of Foxp3 in NSCLC blood
CD4+CD25high Treg cells suggests its
immaturity.
CD4+CD25+ Treg cells can be
divided into two categories, nTreg and iTreg cells. iTreg cells
used as specific immunotherapy have become a hotpot in
immunologists (32). Either immature
dendritic cells (33) or drugs
(34) have been used to induce Treg
cells in vitro. Baecher-Allan et al (35) established that
CD4+CD25− T cells could be converted to
CD4+CD25+ Treg cells with low suppressive
ability, when dexamethasone and IL-7 were added to purified
CD4+CD25− T cells. Furthermore,
CD4+CD25+ Treg cells may be induced by IL-10,
IL-2, TGF-β and other cytokines (36). In the current study, the number,
proliferation effect and Foxp3 expression of NSCLC blood
CD4+CD25+ Treg cells were detected following
TGF-β1 treatment. The results demonstrated that the proportion of
CD4+ and CD4+CD25+ T cells was
increased in TGF-β1 treated cells compared with control groups.
Foxp3 protein and mRNA expression was significantly increased in
the control group in NSCLC blood CD4+CD25+ T
cells following TGF-β1 treatment, suggesting that TGF-β1 may
promote proliferation of CD4+ Foxp3+ Treg
cells. The proliferation of CD4+CD25+ Treg
cells induced by TGF-β1 surpassed that of CD8+ T cells.
TGF-β1 inducing CD4+CD25high Treg cells has
been reported previously (37,38). Fu
et al (37) reported that
TGF-β1 induced Foxp3+ Treg cells from
CD4+CD25− precursors during mice
transplantation. Ethan et al (38) reviewed studies of TGF-β1 induced
Foxp3+ Treg cells in both mouse and human. However, the
molecular mechanism underlying the upregulation of Foxp3 expression
induced by TGF-β1 in CD4+CD25+ T cells
requires further exploration.
Immune regulation in tumor metastasis demands a
complex balance (39). Enhancing or
suppressing immunity may cause totally different effects. This
preliminary study indicates that NSCLC blood
CD4+CD25high Treg cells may be functionally
immature. The induction of the immune naive precursor cells in
NSCLC blood by Treg cells with strong immunosuppressive ability may
provide a new basis for the prognosis and treatment of NSCLC blood
metastasis in the future.
Acknowledgements
The authors sincerely acknowledge for the
cooperation and help from Dr. Guojia Zhao and Dr. Mengxu Liu in the
Department of Cardiothoracic Surgery and the Clinical Laboratory,
Jiaxing No.1 Hospital (Zhejiang, China).
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH analyzed and interpreted the patient data. LS
and HZ performed the experiments, including the flow cytometry. WQ
and BZ were responsible for the patient sample collection. YH
performed the statistical analysis and drafted the manuscript. ZY
designed this study and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent. The
present study was approved by the Medical Ethics Committee of the
Jiaxing No. 1 Hospital (Zhejiang, China; approval no. JY201513R),
and experiments were performed in accordance with institutional
guidelines and strictly followed the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Madelung CF, Falk MK and Sørensen TL: The
association between neovascular age-related macular degeneration
and regulatory T cells in peripheral blood. Clin Ophthalmol.
9:1147–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Attridge K and Walker LS: Homeostasis and
function of regulatory T cells (Tregs) in vivo: Lessons from
TCR-transgenic Tregs. Immunol Rev. 259:23–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Käser T, Mair KH, Hammer SE, Gerner W and
Saalmüller A: Natural and inducible Tregs in swine: Helios
expression and functional properties. Dev Comp Immunol. 49:323–331.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Kong H, Zeng X, Guo L, Sun X and
He S: Subsets of regulatory T cells and their roles in allergy. J
Transl Med. 12:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu Y, Wang X, Gu J, Lu H, Zhang F, Li X,
Qian X, Wang X and Lu L: iTreg induced from CD39(+) naive T cells
demonstrate enhanced proliferate and suppressive ability. Int
Immunopharmacol. 28:925–930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biswas M, Sarkar D, Kumar SR, Nayak S and
Rogers GL: Synergy between rapamycin and FLT3 ligand enhances
plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+
Treg. Blood. 125:2937–2947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shigematsu Y, Hanagiri T, Shiota H, Kuroda
K, Baba T, Ichiki Y, Yasuda M, Uramoto H, Takenoyama M, Yasumoto K
and Tanaka F: Immunosuppressive effect of regulatory T lymphocytes
in lung cancer, with special reference to their effects on the
induction of autologous tumor-specific cytotoxic T lymphocytes.
Oncol Lett. 4:625–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demir N, Ilhan F, Demir T and Godekmerdan
A: sCTLA-4, CD4+CD25+Foxp3+ regulatory T cells in Behçet's disease
patients. Clin Exp Rheumatol. 30(3): Suppl 72:S116–S117.
2012.PubMed/NCBI
|
|
9
|
Daniel V, Sadeghi M, Wang H and Opelz G:
CD4 (+)CD25 (+)Foxp3(+)IFNγ(+) Treg are immunosuppressive in vitro
and increase with intensity of the alloresponse in pretransplant
MLC. Transpl Immunol. 27:114–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tselios K, Sarantopoulos A, Gkougkourelas
I and Boura P: The influence of therapy on CD4+CD25 (high)FOXP3+
regulatory T cells in systemic lupus erythematosus patients: A
prospective study. Scand J Rheumatol. 44:29–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng H and Chi H: The interplay between
regulatory T cells and metabolism in immune regulation.
Oncoimmunology. 2:e265862013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun YL, Lin GG, Zhang K and Wang LN:
Application and effects of mouse Foxp3 antibody and
fixation/permeabilization buffer on the detection of CD4+
regulatory T cells in various mammal species. Genet Mol Res.
12:6535–6545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MH, Koo JS and S L: FOXP3 expression
is related to high Ki-67 index and poor prognosis in lymph
node-positive breast cancer patients. Oncology. 85:128–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vent-Schmidt J, Han JM, MacDonald KG and
Levings MK: The role of FOXP3 in regulating immune responses. Int
Rev Immunol. 33:110–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang N, Li M and Zeng X: Correlation of
Th17 cells and CD4+CD25+ regulatory T cells
with clinical parameters in patients with systemic sclerosis. Chin
Med J (Engl). 127:3557–3561. 2014.PubMed/NCBI
|
|
16
|
Schoenbrunn A, Frentsch M, Kohler S, Keye
J, Dooms H, Moewes B, Dong J, Loddenkemper C, Sieper J, Wu P, et
al: A converse 4-1BB and CD40 ligand expression pattern delineates
activated regulatory T cells (Treg) and conventional T cells
enabling direct isolation of alloantigen-reactive natural Foxp3+
Treg. J Immunol. 189:5985–5994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chauhan SK, Saban DR, Dohlman TH and Dana
R: CCL-21 conditioned regulatory T cells induce allotolerance
through enhanced homing to lymphoid tissue. J Immunol. 192:817–823.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tognela A, Spring KJ, Becker T, Caixeiro
NJ, Bray VJ, Yip PY, Chua W, Lim SH and de Souza P: Predictive and
prognostic value of circulating tumor cell detection in lung
cancer: A clinician's perspective. Crit Rev Oncol Hematol.
93:90–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greystoke A, Ayub M, Rothwell DG, Morris
D, Burt D, Hodgkinson CL, Morrow CJ, Smith N, Aung K, Valle J, et
al: Development of a circulating miRNA assay to monitor tumor
burden: From mouse to man. Mol Oncol. 10:282–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Y, Chen Z, Dong J, Wei P, Hu R, Zhou C,
Sun N, Luo M, Yang W, Yao R, et al: Folate receptor-positive
circulating tumor cells as a novel diagnostic biomarker in
non-small cell lung cancer. Transl Oncol. 6:697–702. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li G, Liu D, Cooper TK, Kimchi ET, Qi X,
Avella DM, Li N, Yang QX, Kester M, Rountree CB, et al: Successful
chemoimmunotherapy against hepatocellular cancer in a novel murine
model. J Hepatol. 66:75–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Y, Wang B, Wu J, Zhang C, Zhou Y,
Yang X, Zhou J, Guo W and Fan J: Association of preoperative EpCAM
Circulating Tumor Cells and peripheral Treg cell levels with early
recurrence of hepatocellular carcinoma following radical hepatic
resection. BMC Cancer. 16:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan MC, Zhong XN, Liu GN and Wei JR: The
Treg/Th17 paradigm in lung cancer. J Immunol Res. 2014:7303802014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Li Y, Qu X, Liu X and Liang J:
Detection and significance of TregFoxP3(+) and Th17 cells in
peripheral blood of non-small cell lung cancer patients. Arch Med
Sci. 10:232–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Wu J and Yi S: Foxp3 is critical
for human natural CD4+CD25+ regulatory T cells to suppress
alloimmune response. Transpl Immunol. 26:71–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sela U, Olds P, Park A, Schlesinger SJ and
Steinman RM: Dendritic cells induce antigen-specific regulatory T
cells that prevent graft versus host disease and persist in mice. J
Exp Med. 208:2489–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasegawa H, Lei J, Matsumoto T, Onishi S,
Suemori K and Yasukawa M: Lysophosphatidylcholine enhances the
suppressive function of human naturally occurring regulatory T
cells through TGF-β production. Biochem Biophys Res Commun.
415:526–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Almeida AS, Fiske CT, Sterling TR and
Kalams SA: Increased frequency of regulatory T cells and T
lymphocyte activation in persons with previously treated
extrapulmonary tuberculosis. Clin Vaccine Immunol. 19:45–52. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo Y, Wu CZ, Liao Y and Zhang QY: The
expression and significance of CD4+CD25+CD127low/-regulatory T
cells and Foxp3 in patients with portal hypertension and
hypersplenism. Hepatogastroenterology. 60:581–584. 2013.PubMed/NCBI
|
|
30
|
Kumar S, Naqvi RA, Ali R, Rani R, Khanna N
and Rao DN: CD4+CD25+ T regs with acetylated FoxP3 are associated
with immune suppression in human leprosy. Mol Immunol. 56:513–520.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamza E, Akdis CA, Wagner B, Steinbach F
and Marti E: In vitro induction of functional allergen-specific
CD4+ CD25high Treg cells in horses affected with insect bite
hypersensitivity. Clin Exp Allergy Aug. 43:889–901. 2013.
View Article : Google Scholar
|
|
32
|
Avalos-Martínez CE, Rodríguez-Alba JC,
Berrón-Ruiz L, Romero-Ramírez H, Santos-Argumedo L, Jiménez-Zamudio
LA, Domínguez-López ML, Vega-López A and García-Latorre E:
Measurement of suppressor activity of T
CD4+CD25+ T reg cells using bromodeoxyuridine
incorporation assay. Immunol Invest. 42:369–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turner MS, Kane LP and Morel PA: Dominant
role of antigen dose in CD4+Foxp3+ regulatory T cell induction and
expansion. J Immunol. 183:4895–4903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen JH, Huang PH, Lee CC and Chen PY: A
bovine whey protein extract can induce the generation of regulatory
T cells and shows potential to alleviate asthma symptoms in a
murine asthma model. Br J Nutr. 109:1813–1820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baecher-Allan CM, Costantino CM,
Cvetanovich GL, Ashley CW, Beriou G, Dominguez-Villar M and Hafler
DA: CD2 costimulation reveals defective activity by human
CD4+CD25(hi) regulatory cells in patients with multiple sclerosis.
J Immunol. 186:3317–3326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamza E, Gerber V, Steinbach F and Marti
E: Equine CD4(+) CD25(high) T cells exhibit regulatory activity by
close contact and cytokine-dependent mechanisms in vitro.
Immunology. 134:292–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu S, Zhang N, Yopp AC, Chen D, Mao M,
Chen D, Zhang H, Ding Y and Bromberg JS: TGF-beta induces Foxp3 +
T-regulatory cells from CD4 + CD25-precursors. Am J Transplant.
4:1614–1627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ethan S, Dat T, Todd D and John A: The
critical contribution of TGF-β to the induction of foxp3 expression
and treg function. Eur J Immunol. 38:915–917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goubran HA, Kotb RR, Stakiw J, Emara ME
and Burnouf T: Regulation of tumor growth and metastasis: The role
of tumor microenvironment. Cancer Growth Metastasis. 7:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|