Introduction
Gastric cancer (GC) is one of the most common
malignancies, and is considered as the second leading cause of
cancer-related mortality worldwide (1). Although the five-year post-operative
survival rate of GC has improved to some extent and concordantly
with advances made in specific treatment, the prognosis for GC
remains very poor. Further clarification of the mechanism
underlying GC development from the molecular level and seeking of
novel biological cancer markers and effective therapeutic targets
may aid in improving the prognosis of GC.
microRNAs (miRNAs) are a class of endogenous, small
non-coding regulatory RNAs that are 19-25 nucleotides in length,
and are known to be involved in the regulation of gene expression
by repressing translation or by decreasing the stability of mRNAs
(2). In total, >2,578 types of
miRNA have been discovered, and they regulate ~1/3 of human
protein-encoding genes (3).
Accumulating evidence has indicated that miRNAs are important in
numerous crucial biological processes, including cell
proliferation, apoptosis and energy metabolism (2,4,5). Moreover, it has been previously
reported that ~50% of miRNAs are located in the chromosomal regions
that are known to be frequently deleted or amplified in human
cancer cells (6). Previous studies
revealed that miRNAs may function as oncogenes or tumor suppressors
that are involved in tumorigenesis (7,8). In
addition, emerging evidence has confirmed that aberrant expression
of miRNAs in GC, including miR-21, miR-27a and miR-218, are
involved in tumor growth, invasion and metastasis (9–11). These
data have indicated the importance of miRNAs in GC development and
have provided insights into the mechanisms that underlie
tumorigenesis.
miR-187 has been revealed to be associated with
numerous neoplasms (12,13). However, mir-187 is differentially
expressed between normal and different cancer tissues, which has
revealed the various effects of prognostic evaluation (13). Mulrane et al (12) reported that miR-187 expression in
breast cancer leads to a more aggressive, rapidly progressing and
invasive phenotype, which serves as an independent predictor of the
outcome. However, Zhao et al (13) revealed that miR-187 was downregulated
in renal cell carcinoma tissues, and lower miR-187 expression
levels were associated with higher tumor grade and staging. To
date, the expression of miR-187 and its clinical significance in GC
remain poorly understood.
In the present study, the expression and clinical
significance of miR-187 in the tissues of GC were explored. Cell
proliferation in vitro and cell cycle phase analysis were
further investigated by the ectopic expression of miR-187 in a GC
cell-line. Furthermore, the potential target genes and their
expression levels were determined. The observations of the present
study may assist in the elucidation of the functions and
pathophysiological roles of miRNA-187 and provide a formal basis
for further research into the role of mir-187 in gastric
carcinoma.
Materials and methods
Patients and tumor tissues
In total, 32 pairs of human gastric tissue samples
were obtained from patients who underwent surgical resection in the
Department of Gastrointestinal Surgery at the First Hospital of
Wenzhou Medical College (Wenzhou, China) between 2008 and 2012.
Patients were aged between 42 and 80 years, with a mean age of 65
years, and were diagnosed with GC based on histopathological
evaluation. The matched normal adjacent tissue was obtained from a
segment of the resected specimens that was the farthest from the
tumor (i.e., >5 cm). The samples were snap-frozen in liquid
nitrogen and stored at −80°C. None of the patients received
chemotherapy or radiotherapy prior to surgical excision. In
addition, the tumor histological grade was staged using the TNM
staging of the International Union against Cancer/American Joint
Committee on Cancer (AJCC) system (2002) (14). The study was then approved by the
Research Ethics Committee of Wenzhou Medical College, and written
informed consent was obtained from all patients.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) quantification of miR-187
Total RNA was extracted from the specimens using
TRIzol reagent (cat. no. 15596026; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) following the manufacturer's
instructions. In order to remove potentially contaminated DNA, the
extracted RNA samples were further purified using DNase I reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. A NanoDrop2000 spectrophotometer (Thermo Fisher
Scientific, Inc.) detected RNA purity by measuring the
OD260/OD280 ratio and calculating the RNA
concentration and purity. Only an OD260/OD280
ratio >1.8 was considered suitable for subsequent experiments.
At last, the integrity of the RNA was checked by 1.0% denaturing
agarose gel electrophoresis (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Xue et al (15)
reported a method of detecting miRNA by stem-loop qPCR, where GAPDH
RNA was used as an endogenous control. Using this approach, the
expression of miR-187 was analyzed in tissue samples. The
first-strand cDNA synthesis was performed with the ReverTra
Ace® qPCR RT kit (cat. no. FSQ-101; Toyobo Co., Ltd.,
Osaka, Japan) using 0.5 µg total RNA as the template and specific
reverse primers at 16°C for 30 min, 42°C for 30 min and 95°C for 5
min of reverse transcription. The resulting cDNA was amplified by
PCR using miRNA specific primers with SYBR® Green
Real-Time PCR Master Mix (Toyobo Co., Ltd.) and was performed using
an Eppendorf Mastercycler Ep Realplex (SABiosciences; Qiagen
Sciences, Inc., Gaithersburg, MD, USA). The primers used for qPCR
are listed in Table I. PCR
parameters were as follows: 95°C for 5 min, followed by 40 cycles
of 95°C for 10 sec, 60°C for 20 sec and 72°C for 20 sec. At the end
of the PCR cycles, a melting curve analysis was performed, and PCR
products were analyzed using 2.5% PAGE electrophoresis.
 | Table I.RT-qPCR primer sequences for the
amplification of miR-187 and GAPDH. |
Table I.
RT-qPCR primer sequences for the
amplification of miR-187 and GAPDH.
| Gene | Primer | Sequence | Product (bp) |
|---|
| miR-187 | RT stem-loop |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCGGCTGC-3′ | 61 |
|
| Forward |
5′-TCGTGGGTCGTGTCTTGTGTTGC-3′ |
|
|
| Reverse |
5′-GCAGGGTCCGAGGTATTC-3′ |
|
| GAPDH | Forward |
5′-CAGGGCTGCTTTTAACTCTGGTAA-3′ | 101 |
|
| Reverse |
5′-GGGTGGAATCATATTGGAACATGT-3′ |
|
The relative expression level of miR-187 in tissues
was compared to the endogenous control or paired normal tissues.
Thus, the 2−ΔCq and 2−ΔΔCq methods were used
to evaluate the relative expression levels of the target (16). In these analyses;
ΔCq=CqmiR-187-CqGAPDH, and
ΔΔCq=[(CqmiR-187-CqGAPDH)tumor]-[(CqmiR-187-CqGAPDH)control].
When the data were calculated by the ΔΔCq method, the value of the
relative expression ratio <1.0 was considered to be low
expression in cancer relative to the control, and a ratio >1.0
was considered to indicate a high level of expression. Moreover,
all qPCR assays were run in triplicate.
Cell line and culture conditions
MGC-803 is a poorly differentiated GC cell-line and
was purchased from the Institute of Biochemistry and Cell Biology
at the Chinese Academy of Sciences (Shanghai, China). MGC-803 cells
were cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life
Sciences, Chalfont, UK) and were all supplemented with 10% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences), 50 U/ml
penicillin-G (Invitrogen; Thermo Fisher Scientific, Inc.) and 50
µg/ml streptomycin sulfate (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C and 5% CO2 in the air.
RNA oligoribonucleotides and cell
transfection
The miRNA mimics were designed and synthesized by
the GenePharma Company (Shanghai, China). miR-187 mimics (Table II) were an RNA duplex and negative
control (NC) RNA duplex and were non-homologous to any human
genomic sequences. All the oligonucleotide sequences used in this
experiment are listed in Table II.
The mimic transfection was performed with Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The GC cells were seeded into 6-well
plates and were grown to 60-70% confluence before transfection.
Moreover, transfection efficiency was then monitored using
qPCR.
 | Table II.Sequence of miR-187 mimics and
negative control. |
Table II.
Sequence of miR-187 mimics and
negative control.
| RNA oligo | Sequence |
|---|
| miR-187 mimic |
5′-UCGUGUCUUGUGUUGCAGCCGG-3′ |
|
|
3′-UUAGCACAGAACACAACGUCGG-5′ |
| Negative
control |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
|
3′-TTAAGAGGCUUGCACAGUGCA-5′ |
Cell Counting Kit-8 (CCK-8)
proliferation assay
The effect of miR-187 on the proliferation of GC
cells was evaluated using the CCK-8 assay kit (Dojindo Molecular
technologies, Inc., Kumamoto, Japan), according to the
manufacturers instructions. MGC-803 cells were plated into 96-well
plates at a density of 8×103 cells/well in three parts.
After 24 h of static culture, the cells were transfected with
miR-187 mimics and the negative control (NC) using Lipofectamine
2000. The cellular proliferation capacity for the untransfected
blank control (Blank), cells transfected with miR-187 mimic NCs and
cells transfected with miRNA-187 mimics were measured using the
CCK-8 assay. Moreover, the transfected miRNA-187 mimics group was
set to final concentrations of 20, 40 and 80 nM. The cells were
then incubated for 24, 48 and 72 h at 37°C in an atmosphere of 5%
CO2 in the air. Next, a total 10 µl of the CCK-8
solution was added to each well, and the cells were incubated for a
further 4 h. The absorbance (A) values were determined using a
spectrophotometer (EPOCH2; BioTeK Instruments, Inc., Winooski, VT,
USA) at a wavelength of 450 nm, and the experiment was repeated
three times. The inhibition rate of cell proliferation=1-(A450
nm of the study group/A450 nm of the control
group) × 100%. In addition, the relative activity of the
cells=A450 nm of the study group/A450 nm of
the control group.
Cell cycle analysis
For the cell cycle analysis, cells were seeded into
6-well plates at a density of 2×105 cells/well, and
transfected according to the protocol described above. The
experimental groups and time points were set as described for the
proliferation assay above. The cells were obtained after being
cultured for an appropriate time by trypsinization, and were then
pooled with the floating cells, centrifuged at 1,000 × g for 5 min
at 4°C and stored at −20°C overnight. DNA staining solution that
contained (PI) iodide and RNaseA reagent [MultiSciences (Lianke)
Biotech Co., Ltd., Hangzhou, China] were added to the cells.
Moreover, cell cycle analysis was performed by
fluorescence-activated cell sorting flow cytometry using CellQuest
software 1.0 (BD Biosciences, San Jose, CA, USA).
Western blot analysis
miR-187 mimics (5′-UCGUGUCUUGUGUUGCAGCCGG-3′;
Applied Biological Materials, Inc., Richmond, BC, Canada) or
negative control oligonucleotides (5′-UUCUCCGAACGUGUCACGUTT-3′;
Applied Biological Materials, Inc.) at a concentration of 40 nM
were transfected into GC MGC-803 cells using the Lipofectamine™
2000 Transfection Reagent kit (cat. no. 11668019; Invitrogen;
Thermo Fisher Scientific, Inc.) and cells were harvested 48 h after
transfection. Proteins were extracted and separated on a 12%
SDS-PAGE gel and transferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.). The membrane was then blocked with 5% non-fat
milk proteins and incubated with anti-MAD2L2 (cat. no. ab180579;
1:2,000), anti-STOML2 (cat. no. ab37531; 1:2,000; each Abcam,
Cambridge, UK) or anti-β-actin antibody (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). After being washed extensively, a goat
anti-mouse secondary antibody (Pierce Biotechnology, Inc.,
Rockford, IL, USA) was added to the system. The proteins were
detected using enhanced chemiluminescence reagents (cat. no. 32106;
Pierce Biotechnology, Inc.). Protein expression levels were
quantified using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Luciferase reporter assays
In order to verify the direct interaction of miR-187
to the target genes MAD2L2 and STOML2, human mRNA sequence were
cloned into the pMIR-reporter construct (Ambion; Thermo Fisher
Scientific, Inc.) in order to synthesize the luciferase reporter
construct. Wild-type and mutant MAD2L2 and STOML2 mRNA fragments
were amplified and sub-cloned into HindIII sites of the luciferase
reporter, and luciferase reporter assays were performed as
previously described (17). MGC803
cells were co-transfected with 50 nM single-stranded miRNA mimics
or internal control oligonucleotides, then plated into 24-well
plates with 10 ng pRL-TK (Promega Corporation, Madison, WI, USA)
and 50 ng firefly luciferase reporter using the JetPRIME reagent
(Polyplus-transfection) according to the manufacturer's
instructions. Cells were collected at 48 h after transfection
(performed as aforementioned) and analyzed using the
Dual-Luciferase Reporter Assay System (Promega Corporation).
Immunohistochemistry
Paired paraffin-embedded tissue sections (n=20) were
cut into 5-µm sections, deparaffinized in xylene and rehydrated in
graded series of ethanols followed by heat-induced epitope
retrieval in citrate buffer (pH 6.0). The expression levels of
MAD2L2 and STOML2 were detected using polyclonal antibodies
targeted to MAD2L2 (cat. no. ab180579; 1:50) and STOML2 (cat. no.
ab37531; 1:50; each Abcam). Next, samples were incubated with
horseradish peroxidase-conjugated secondary goat anti-mouse
antibodies (cat. no. SPKIT-C7; Fuzhou Maixin Biotech Co., Ltd.,
Fuzhou, China) and visualized with 3,3′-diaminodbenzidine (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
Images obtained were processed using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The expression of miR-187 was calculated for paired
groups (i.e., the tumor and paired non-tumor) were compared using
non-parametric Wilcoxon tests. The statistical significance of
correlation between the expression of miR-187 and the
clinicopathological parameters was calculated using non-parametric
tests (Mann-Whitney U test between two groups and Kruskall-Wallis
test for three or more groups). The association between miR-187
expression and prognosis was analyzed using the Kaplan-Meier
survival curve statistics. In the Kaplan-Meier survival curve, high
expression was defined as the fold-change of >1 and low
expression levels as <1 compared to the probability of survival
by the Log-rank test. Statistical analysis was performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Differences were
considered statistically significant at a value of P<0.05.
Results
Expression of miR-187 in GC tissues
and matched non-tumor tissues
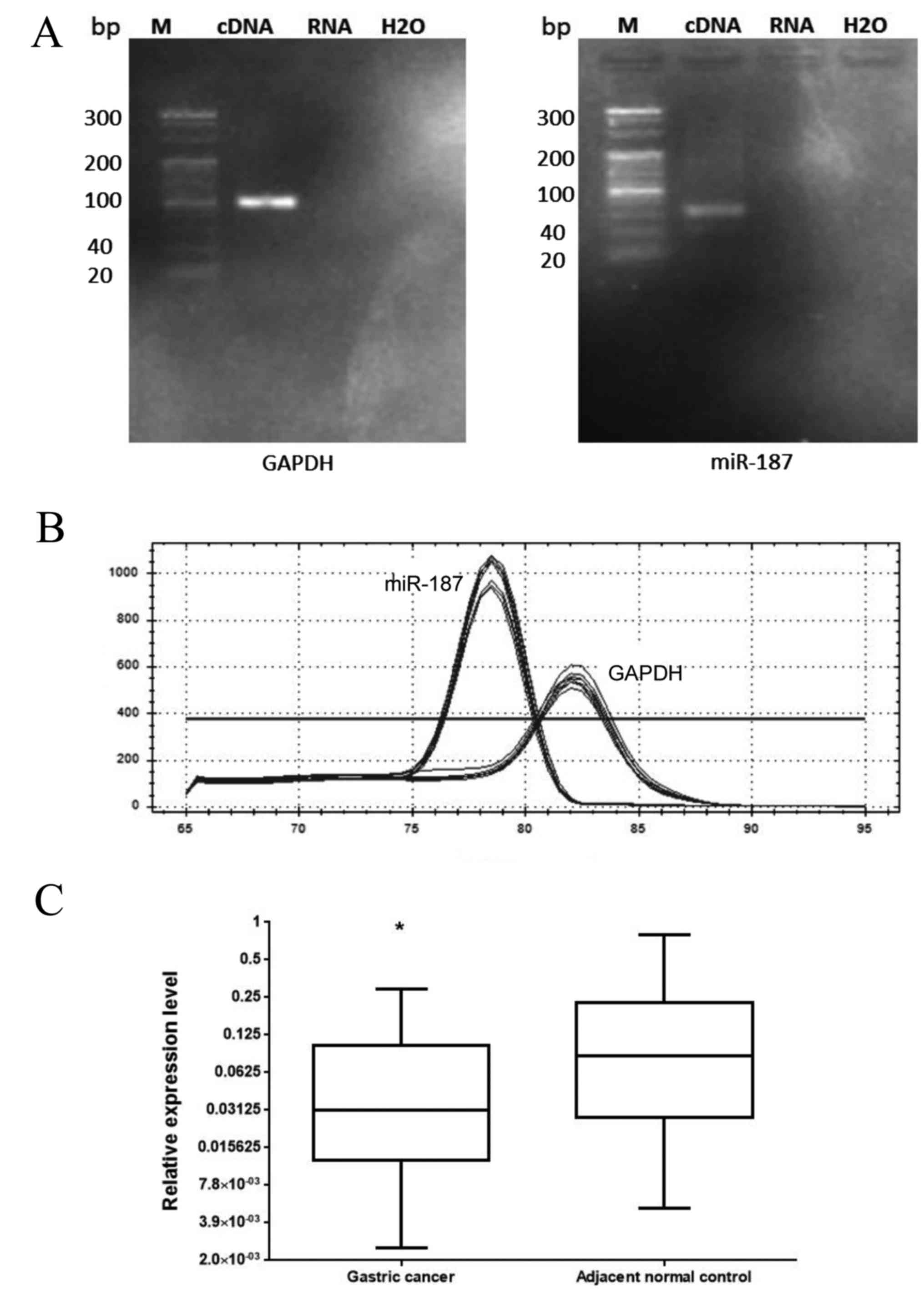
PCR products of miR-187 and GAPDH cDNA revealed a
single band at the appropriate position (67 bp for miR-187 and 101
bp for GAPDH) on the electrophoretic gel (Fig. 1A). In addition, the melting curves of
the products were sharply defined curves with a narrow peak
(Fig. 1B). The combination of
melting curves and gel electrophoresis confirmed PCR specificity.
Furthermore, the expression of miR-187 in all 32 pairs of GC
tissues and their matched non-cancerous tissues was detected using
qPCR. As shown in Fig. 1C, the
expression levels of miR-187 were lower in gastric tumors than in
normal control tissues. The median relative expression levels of
miR-187 was 0.031 (25-75th percentile, 0.013-0.102) in tumor
samples, and was compared to that in non-tumor control samples,
which was set at 0.086 (25-75th percentile, 0.028-0.192). Moreover,
the difference in expression of miR-21 between the tumor and the
control samples was statistically significant (P=0.037, Wilcoxon
test).
Association between the expression of
miR-187 and clinicopathological features of GC
Τhe association between miR-187 expression and the
clinical and pathological characteristics of GC were explored
further (Table III). The
downregulated expression levels of miR-187 were associated with
cell differentiation (P=0.042) and TNM stage (P=0.034) in GC
patients. However, no significant association was identified
between the expression of miR-187 and the demographic or clinical
variables, including gender, age, tumor location, tumor size, depth
of tumor invasion and lymph node metastasis (P>0.05). According
to the relative expression levels of miR-187 of paired tumor and
normal tissues, specimens with a relative expression level <1
were set as Group One, and specimens with a relative value ≥1 were
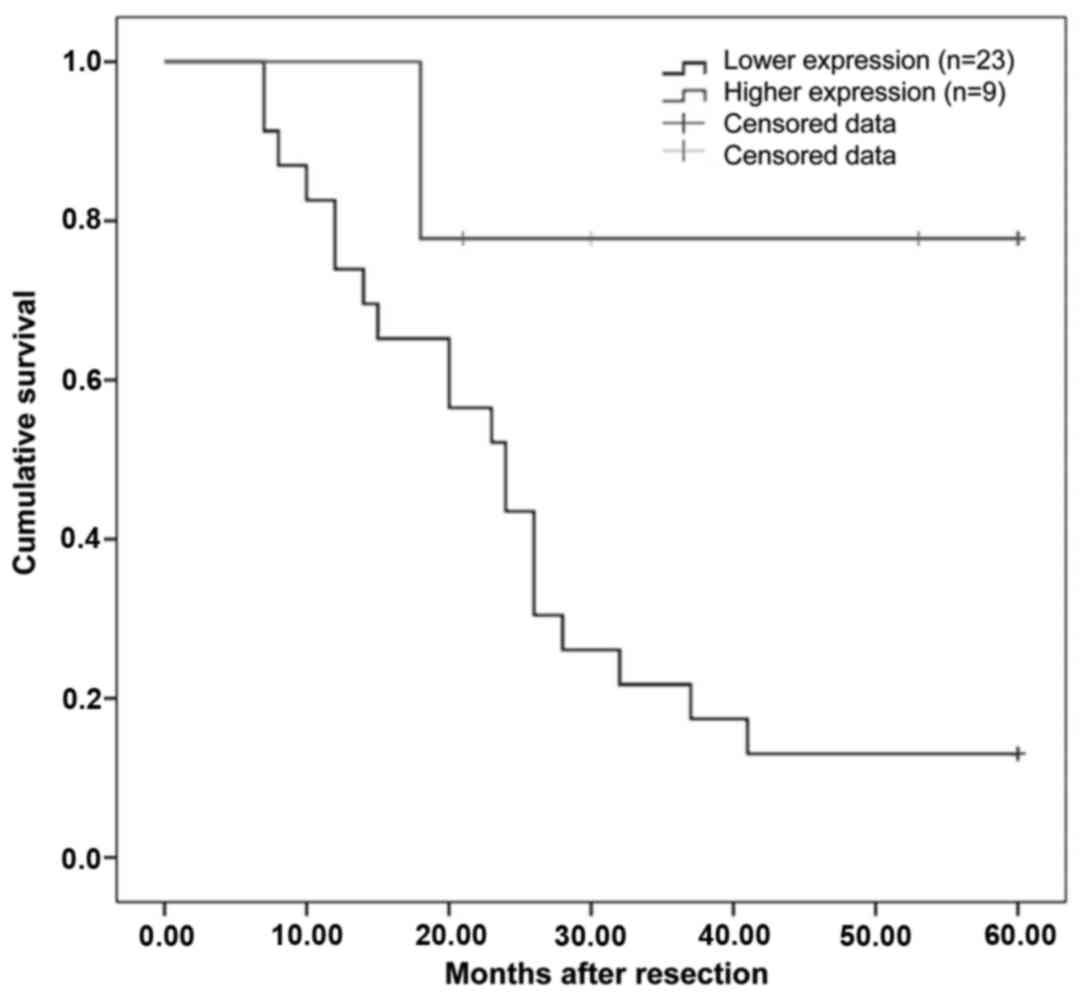
set as Group Two. A Kaplan-Meier survival analysis illustrated that
the cohort with higher expression levels of miR-187 demonstrated
greater rates of survival than those with lower levels of
expression of miR-187 (Fig. 2).
Moreover, the 5-year survival rate for Group One was 66.7%, while
that for Group Two was 13.5%.
 | Table III.Association between the expression of
miR-187 with clinicopathological features of gastric cancer. |
Table III.
Association between the expression of
miR-187 with clinicopathological features of gastric cancer.
| Clinical
characteristics | Cases (n) | miR-187
expressiona | P-value |
|---|
| Age (years) |
|
| 0.258 |
|
<60 | 8 | 0.60±0.63 |
|
|
≥60 | 24 | 0.97±0.89 |
|
| Sex |
|
| 0.630 |
|
Male | 23 | 0.86±0.87 |
|
|
Female | 9 | 0.91±0.82 |
|
| Tumor size
(cm) |
|
| 0.440 |
|
<5 | 26 | 0.88±0.91 |
|
| ≥5 | 6 | 0.87±0.53 |
|
| Location |
|
| 0.967 |
| Upper
area | 4 | 0.59±0.17 |
|
| Middle
area | 7 | 0.77±0.87 |
|
| Lower
area | 21 | 0.96±0.92 |
|
| T stage |
|
| 0.500 |
|
T1/T2 | 7 | 1.21±1.00 |
|
| T3 | 10 | 0.72±0.63 |
|
| T4 | 15 | 0.82±0.89 |
|
| Cell
differentiation |
|
| 0.042 |
| Well or
Moderately-differentiated | 9 | 1.45±0.92 |
|
|
Poorly-differentiated | 23 | 0.65±0.71 |
|
| Lymph node
metastasis |
|
| 0.090 |
| No | 9 | 1.21±0.86 |
|
|
Yes | 23 | 0.74±0.82 |
|
| N stage |
|
| 0.270 |
| N1 | 3 | 1.65±1.18 |
|
| N2 | 12 | 0.65±0.85 |
|
| N3 | 8 | 0.52±0.34 |
|
| TNM stage |
|
| 0.034 |
|
I/II | 11 | 1.23±0.84 |
|
|
III/IV | 21 | 0.69±0.80 |
|
miR-187 inhibited cell proliferation
and induced cell cycle arrest at the G0/G1
phase in vitro
The significant reduction in miR-187 expression in
GC samples prompted us to explore the possible biological roles of
miR-187 in the mechanism of tumorigenesis. Thus, the effect of
miR-187 expressing again was investigated on the proliferative
capacity of the GC cell-line. Compared with that of the cells
transfected with either the negative or blank control, MGC-803
cells that were transiently transfected with miR-187 mimics had a
significant growth inhibition to different degrees (P<0.05;
Fig. 3A). Moreover, the cell
vitality of GC cells was markedly associated with different
transfected concentrations and was time-dependent (P<0.05;
Fig. 3A). In order to investigate
further whether inhibition of MGC-803 proliferation reflected cell
cycle arrest, the progression of cell cycle phases was analyzed by
PI staining and flow cytometry. The results revealed that MGC-803
cells that were dose-dependently transfected with miR-187 mimics
had an evident effect on cell cycle arrest at the
G0/G1 phase (Fig.
3B and C) as compared with the blank and the negative control
group (P<0.05). However, the proportion of cells that were
transfected with different doses of miR-187 mimics had little
effect on the cell cycle phases (P>0.05).
miR-187 targets MAD2L2 and STOML2
As miRNAs function mainly through the inhibition of
target genes, the target of miR-187 that functions in the
pathogenesis of GC was further analyzed. Based on previously
published CLASH data, which provided direct experimental data of
miRNA-targeted pairs (18), MAD2L2,
STOML2 and tubulin, γ 1 (TUBG1) were identified as potential
targets of miR-187. In order to confirm whether these genes that
had been regulated by miR-187 were involved in the pathogenesis of
GC, MGC-803 cells were transfected with miR-187 mimics or negative
control oligonucleotides, and the protein expression levels of the
genes were examined by western blotting. Data indicated that the
expression of MAD2L2 and STOML2 were consistently and substantially
downregulated by the ectopic expression of miR-187, whereas TUBG1
expression was not significantly affected by miR-187 (Fig. 4A).
Luciferase reporter assay
In order to verify the interaction between miR-187,
MAD2L2 and STOML2, luciferase reporter assays were performed in
MGC803 cells. The luciferase reporter plasmid with sequences of
MAD2L2 and STOML2 mRNA or mutant sequences were co-transfected into
MGC803 cells for 48 h with miR-187 or NC, respectively. Moreover,
the luciferase activity was measured in transfected cells. The
results demonstrated that the reporter plasmid of MAD2L2 and STOML2
mRNA caused a significant decrease in the luciferase activity in
cells that were transfected with miR-187. By contrast, the
luciferase activity of the reporter plasmid with mutant sequences
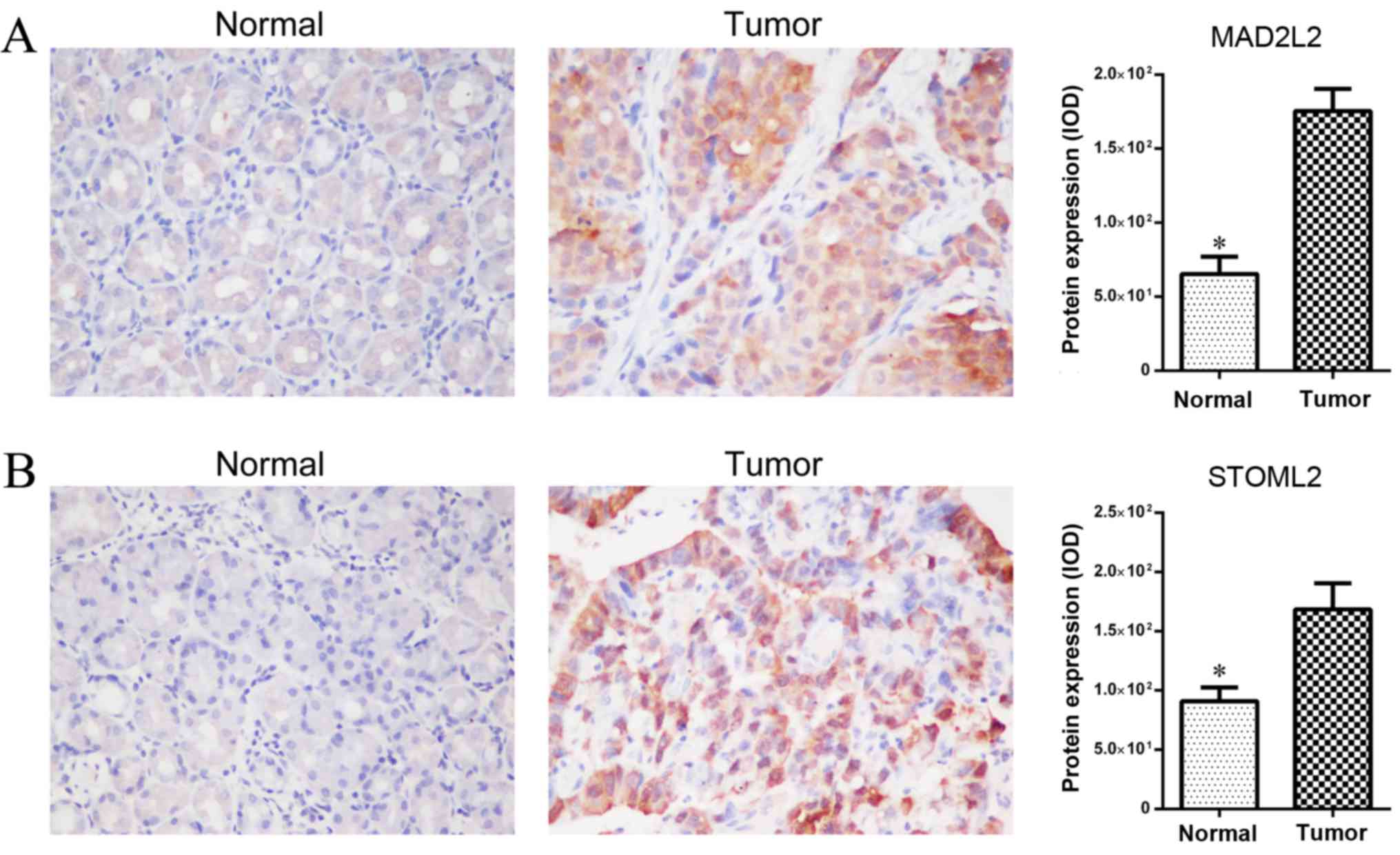
of MAD2L2 and STOML2 did not change (Fig. 4B). Furthermore, GC tissues with a low
miR-187 demonstrated much higher MAD2L2 and STOML2 expression when
compared with paired normal gastric tissues (Fig. 5). Altogether, the results suggested
that MAD2L2 and STOML2 was the target of miR-187 in GC cells.
Discussion
Accumulating evidence has indicated that the
abnormal expression of miR-187 is closely associated with cancer
cell proliferation and apoptosis, and that they may serve as tumor
suppressors or oncogenes (19,20).
Previously, numerous studies have shown that miR-187 is anomalously
expressed in various tumor types, including thyroid (21), nasopharyngeal carcinoma (22), esophageal (23) and pancreatic cancer (24) and neuroblastoma cell tumors (25). However, in different cancers, the
changes in expression of miR-187 varied greatly, indicating that it
may be involved in carcinogenesis and progression in a specific
way.
A previous study demonstrated that miR-187 that was
downregulated in tumor tissue was involved in ovarian cancer
progression (26). Mulrane et
al (12) revealed that the
expression levels of miR-187 in breast cancer tissue was correlated
with breast cancer invasion, and could be used as an independent
prognostic factor. However, the expression level and the biological
impact of miR-187 in GC remain unclear. In order to investigate the
association of miR-187 and GC pathogenesis, qPCR was used to
profile the expression of miR-187 in 32 matched gastric tumor
tissues, and clarified the association between miR-187 and the
clinicopathological characteristics of GC.
In the present study, the expression of miR-187 was
found to be downregulated in gastric tumors compared to non-tumor
tissues. Besides, miR-187 expression was associated with cell
differentiation and TNM staging in GC patients. In addition, the
Kaplan-Meier survival analysis illustrated that the cohort with a
higher expression of miR-187 demonstrated higher survival rates
than those cells with lower levels. These results indicated that
miR-187 is important in the development and progression of GC. In
view of the above, miR-187 is speculated to function as a tumor
suppressor in GC, and the downregulation of miR-187 may promote its
occurrence and development of GC.
To further clarify this point, the biological
function of miR-187 was investigated. Deregulated cell
proliferation is a key biological characteristic of neoplastic
progression (27). In the present
study, miR-187 mimics were transfected into MGC-803 cells, then the
cell proliferation ability was evaluated. The CCK-8 assay data
indicated that the overexpression of miR-187 could suppress the
proliferation of GC cells. However, the reason for miR-187 induced
inhibition of cell proliferation remains to be investigated.
Overall, cell cycle arrest was likely to be an important factor
(28,29). Indeed, the subsequent cell cycle
analyses of the present study revealed that MGC-803 cells that were
transfected with miR-187 mimics had an evident cell cycle arrest at
the G0/G1 phase. Nevertheless, the proportion
of cells of the different transfected groups demonstrated no
difference. It is likely that the process of cell cycle arrest
induced by miR-187 was influenced by another molecular mechanism
since indirect regulation and miRNA may have complex roles that may
formally involve multiple aspects and factors in cell cycle
regulation.
The main function of miRNA is to regulate target
gene expression by direct cleavage of the mRNA or by inhibition of
protein synthesis according to the degree of complementarities with
the 3′untranslated region of target genes (30,31).
Perfect or nearly perfect base-pairing induced targeted mRNA
cleavage whereas imperfect base pairing induced mainly
translational silencing of the target (32). Chao et al (26) demonstrated that miR-187 inhibited the
epithelial-mesenchymal transition process and migration by
targeting Dab2 in ovarian cancer cells. Furthermore, Mulrane et
al (12) reported that miR-187
regulated collagenase (matrix metallopeptidase 13) expression by
either direct or indirect pathways in breast cancer, and that this
pathway may have also been involved in the migration of tumor
cells. Moreover, Sirotkin et al (33) revealed that overexpression of miR-187
could reduce the expression of the apoptosis-related B-cell
lymphoma 2-associated X protein and the proliferation marker
proliferating cell nuclear antigen in human ovarian granulosa
cells. However, whether or not other target genes are responsible
for the effect in GC is currently unknown.
A recent advance in high-throughput direct mapping
of miRNA-mRNA binding sites from CLASH experiments directly
identified the miRNA-mRNA target pairs that were associated with
the human AGO1 protein, and presents an improved determination of
the miRNA target (18). Based on the
CLASH experiment data and combined with the analysis of miR-187
interference, MAD2L2 and STOML2 were consistently and substantially
downregulated by the ectopic expression of miR-187.
In the present study, luciferase reporter assays
further confirmed the interaction between miR-187, MAD2L2 and
STOML2. Thus, MAD2L2 and STOML2 could be considered to be potential
targets that were involved in the development of GC. It has been
demonstrated that MAD2L2, which is a member of a family of genes
involved in mitotic checkpoint control mechanisms, were
significantly upregulated in colon cancer as compared to matched
normal tissue (34). In addition,
tumors that show upregulated MAD2L2 expression had significantly
higher numbers of aberrant mitotic figures (anaphase bridges), an
indication of chromosomal instability and a poor prognosis in
colorectal cancer (34). STOML2 is a
member of the stomatin superfamily, and has been identified as an
oncogenic-related protein, whose functional expression is enhanced
in numerous cancer types (35–38).
Moreover, overexpression of STOML2 has been reported in the tissues
of GC compared with the adjacent normal gastric epithelium. In
addition, a high-level STOML2 expression was significantly
correlated with the depth of invasion, lymph node and distant
metastasis, and staging according to the AJCC and poor prognosis in
GC (39).
The present data revealed that the expression of
MAD2L2 and STOML2 were significantly inhibited in MGC-803 cells
transfected with miR-187 mimics. Furthermore, high levels of
expression of MAD2L2 and STOML2 were also confirmed in GC tissues
compared with adjacent normal tissues. These results provided
insights into the regulatory mechanism of miR-187 in GC.
Downregulation of miR-187 in GC cells resulted in enhanced
expression of its target genes, which may consequently favor tumor
progression. However, miRNA displays multi-target characteristics,
and has complex regulatory networks that are involved in numerous
aspects of biological functions (2,40,41).
Therefore, the consequent molecular mechanism of miR-187 remains to
be further investigated.
In conclusion, the present study demonstrated that
there was a significantly reduced expression of miR-187 in GC
tissues compared with their non-tumor counterparts. Moreover, the
expression of miR-187 was associated with the degree of cell
differentiation, pathological staging and prognosis in GC patients.
The data of the present study also indicated that miR-187 could
inhibit cell proliferation in vitro, and could induce cell
cycle arrest at the G0/G1 phase. To the best
of our knowledge, the present study confirmed the tumor suppressive
roles of miR-187 in GC for the first time, and provided evidence
for the potential utility of miR-187 as a biomarker and therapeutic
target against GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81472308
and31470891), the Zhejiang Provincial Natural Science Foundation of
China (grant nos. Y2100909 and LY12H05003), the Jinhua Science and
Technology Bureau (grant no. 2012-3-005) and the Zhejiang Science
and Technology Bureau (grant nos. 2012C33126, 2012C37080 and
2010C33094). These sponsors provided funding for the experiments
and the collection of specimens.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have approved this manuscript and agreed
to be accountable for all aspects of this work. WC, JC and SS
contributed in the conception and design and revision of the
manuscript, the acquisition, analysis and interpretation of data.
XS and LZ prepared the initial draft of the manuscript, contributed
in the acquisition, analysis and interpretation of data, and helped
perform the cell biology experiments. YC, JW and YY made
substantial contributions to conception and design, acquisition of
data, and analysis of data, and performed the western blot
experiments.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Wenzhou Medical College and written informed
consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Care A, Catalucci D, Felicetti F, Bonci D,
Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al:
MicroRNA-133 controls cardiac hypertrophy. Nat Med. 13:613–618.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan HY, Guo LM, Liu T, Liu M, Li X and
Tang H: Regulation of the transcription factor NF-kappaB1 by
microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 9:162010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu
W, Xiao S and Lu H: miR-21 plays a pivotal role in gastric cancer
pathogenesis and progression. Lab Invest. 88:1358–1366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mulrane L, Madden SF, Brennan DJ, Gremel
G, McGee SF, McNally S, Martin F, Crown JP, Jirström K, Higgins DG,
et al: miR-187 is an independent prognostic factor in breast cancer
and confers increased invasive potential in vitro. Clin Cancer Res.
18:6702–6713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao J, Lei T, Xu C, Li H, Ma W, Yang Y,
Fan S and Liu Y: MicroRNA-187, down-regulated in clear cell renal
cell carcinoma and associated with lower survival, inhibits cell
growth and migration though targeting B7-H3. Biochem Biophys Res
Commun. 438:439–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC Cancer staging manual: TNM
classification of malignant tumors. 6th. New York: Springer-Verlag;
2002, View Article : Google Scholar
|
|
15
|
Xue X, Sun J, Zhang Q, Wang Z, Huang Y and
Pan W: Identification and characterization of novel microRNAs from
Schistosoma japonicum. PLoS One. 3:e40342008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L and Eisenman RN: Regulation of c-Myc
protein abundance by a protein phosphatase 2A-Glycogen synthase
kinase 3β-Negative feedback pathway. Genes Cancer. 3:23–36. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helwak A, Kudla G, Dudnakova T and
Tollervey D: Mapping the human miRNA interactome by CLASH reveals
frequent noncanonical binding. Cell. 153:654–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SY, Lee JH, Ha M, Nam JW and Kim VN:
miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct
Mol Biol. 16:23–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nikiforova MN, Tseng GC, Steward D, Diorio
D and Nikiforov YE: MicroRNA expression profiling of thyroid
tumors: Biological significance and diagnostic utility. J Clin
Endocrinol Metab. 93:1600–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen HC, Chen GH, Chen YH, Liao WL, Liu
CY, Chang KP, Chang YS and Chen SJ: MicroRNA deregulation and
pathway alterations in nasopharyngeal carcinoma. Br J Cancer.
100:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wijnhoven B, Hussey DJ, Watson DI, Tsykin
A, Smith C and Michael MZ: South Australian Oesophageal Research
Group; MicroRNA profiling of Barrett's oesophagus and oesophageal
adenocarcinoma. Br J Surg. 97:853–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bloomston M, Frankel WL, Petrocca F,
Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C and Croce
CM: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y and Stallings RL: Differential
patterns of microRNA expression in neuroblastoma are correlated
with prognosis, differentiation, and apoptosis. Cancer Res.
67:976–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao A, Lin CY, Lee YS, Tsai CL, Wei PC,
Hsueh S, Wu TI, Tsai CN, Wang CJ, Chao AS, et al: Regulation of
ovarian cancer progression by microRNA-187 through targeting
Disabled homolog-2. Oncogene. 31:764–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sánchez CA, Rodríguez E, Varela E, Zapata
E, Paez A, Massó FA, Montaño LF and Lóopez-Marure R: Statin-induced
inhibition of MCF-7 breast cancer cell proliferation is related to
cell cycle arrest and apoptotic and necrotic cell death mediated by
an enhanced oxidative stress. Cancer Invest. 26:698–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park WH, Lee YY, Kim ES, Seol JG, Jung CW,
Lee CC and Kim BK: Lovastatin-induced inhibition of HL-60 cell
proliferation via cell cycle arrest and apoptosis. Anticancer Res.
19:3133–3140. 1999.PubMed/NCBI
|
|
30
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sirotkin AV, Lauková M, Ovcharenko D,
Brenaut P and Mlynček M: Identification of microRNAs controlling
human ovarian cell proliferation and apoptosis. J Cell Physiol.
223:49–56. 2010.PubMed/NCBI
|
|
34
|
Rimkus C, Friederichs J, Rosenberg R,
Holzmann B, Siewert JR and Janssen KP: Expression of the mitotic
checkpoint gene MAD2L2 has prognostic significance in colon cancer.
Int J Cancer. 120:207–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao W, Zhang B, Liu Y, Li H, Zhang S, Fu
L, Niu Y, Ning L, Cao X, Liu Z and Sun B: High-level SLP-2
expression and HER-2/neu protein expression are associated with
decreased breast cancer patient survival. Am J Clin Pathol.
128:430–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao WF, Zhang LY, Liu MB, Tang PZ, Liu ZH
and Sun BC: Prognostic significance of stomatin-like protein 2
overexpression in laryngeal squamous cell carcinoma: Clinical,
histologic, and immunohistochemistry analyses with tissue
microarray. Hum Pathol. 38:747–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Cao W, Yu Z and Liu Z:
Downregulation of a mitochondria associated protein SLP-2 inhibits
tumor cell motility, proliferation and enhances cell sensitivity to
chemotherapeutic reagents. Cancer Biol Ther. 8:1651–1658. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Ding F, Cao W and Liu Z, Liu W,
Yu Z, Wu Y, Li W, Li Y and Liu Z: Stomatin-like protein 2 is
overexpressed in cancer and involved in regulating cell growth and
cell adhesion in human esophageal squamous cell carcinoma. Clin
Cancer Res. 12:1639–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu D, Zhang L, Shen Z, Tan F, Hu Y, Yu J
and Li G: Increased levels of SLP-2 correlate with poor prognosis
in gastric cancer. Gastric Cancer. 16:498–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brennecke J, Stark A, Russell RB and Cohen
SM: Principles of microRNA-target recognition. PLoS Biol.
3:e852005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brodersen P and Voinnet O: Revisiting the
principles of microRNA target recognition and mode of action. Nat
Rev Mol Cell Biol. 10:141–148. 2009. View Article : Google Scholar : PubMed/NCBI
|