Introduction
Sepsis is a series of complex disease processes that
results from infections (1).
Approximately 750,000 patients are infected with sepsis each year
and over 225,000 of these patients die from the condition in the
United States (2). However, the
prevalence of sepsis has increased by 5% annually (3,4). To
date, anti-infective therapeutic agents and supportive care have
been considered effective therapeutic strategies against sepsis.
However, sepsis and septic shock remain to be primary causes of
fatality in intensive care units (1). Subsequently, innovative therapeutic
schedules that are different from traditional strategies are
required. With a breakthrough in immunology and a clearer
pathophysiology of sepsis, immunotherapy is considered to become a
promising therapeutic strategy (5).
However, the clinical applications of immunostimulatory agents such
as interferon (INF)-γ (6) and
anti-inflammatory components such as interleukin (IL)-1 receptor
antagonist (7) have not yet been
used successfully utilized. Researchers have recently identified
that an antimicrobial combined with low-dose cyclophosphamide (CTX)
can improve the survival of mice in a sepsis murine model (8). Notably, the use of CTX to treat
infectious diseases is not novel. Wei et al (9) suggested that low-dose CTX elicits a
protective effect on acute lung injury in sepsis rats and Waymack
and Alexander (10) demonstrated
that low-dose CTX extends the survival time of burned and fatal
virus-infected guinea pigs. As an immunosuppressor, CTX has been
widely used in tumor and autoimmunity disease therapy. However, CTX
produces various complications, including neutrophil
granulocytopenia and end-organ damage (8,11).
Notably, intestinal barrier dysfunction is a common complication of
antimicrobial agents and high-dose CTX (12–15).
The intestinal tract serves a pivotal role in the
progression of diseases, including inflammatory bowel disease
(16) and hepatitis (17), and contains large quantities of
endogenous and exogenous bacteria and toxins (18). The intestinal mucosal barrier between
the gut and the body is influenced by intestinal cells and tight
junction structures (19). The role
of the intestinal mucosal barrier is to prevent intestinal flora
and toxins from entering the blood circulation. Under unfavorable
conditions, including cytotoxic conditions, the intestinal mucosal
barrier may become dysfunctional, thus causing the translocation of
intestinal bacterial microflora or toxins, which can therefore
infect other organs; such occurrences may then promote sepsis and
result in multiple organ dysfunction and failure (20–22).
However, the treatment of intestinal barrier function may yield
improved outcomes. It is not known whether imipenem combined with
low-dose CTX is related to intestinal barrier in sepsis.
In the present study, it was hypothesized that
imipenem combined with low-dose cyclophosphamide could improve the
sepsis survival rate compared with imipenem alone. In addition, the
intestinal barrier function was examined and the possible
mechanisms influencing intestinal barrier function were
explored.
Materials and methods
Animals
All animal experiments were approved and supervised
by the Experimental Animal Centre Committee of Shihezi University
(Shihezi, China). A total of 116 healthy male Sprague-Dawley (SD)
rats aged 8–10 weeks and weighing 150–250 g (experimental animal
production license no. XJYK0011, 2011; Laboratory Animal Centre of
Xinjiang Medical University, Urumqi, China) were prepared for the
experiments. Rats had free access to food and water for 2 days
under experimental conditions (12-h light/dark cycle; 22±1°C;
humidity, 45%) and were subsequently subjected to 12 h of fasting.
All rats used in experiments were maintained in a special cage with
no more than 8 rats per cage. The rats were randomly divided into
sham, cecal ligation and puncture (CLP), imipenem and combination
treatment (imipenem with CTX) groups using the number table method
(23) prior to the surgery.
Sepsis model establishment
According to the previously published method, the
rat model of sepsis was used with CLP (20,24).
After weighing, the rats were intraperitoneally injected with 1%
pentobarbital (30 mg/kg; Merck KGaA, Darmstadt, Germany) for
anaesthetization. The animals were placed in the supine position on
a workstation and along the white line of the abdomen an ~2-cm
longitudinal incision was made under full anesthesia after routine
disinfection. Approximately 2/3 of the caecum was ligated using a
4-0 silk suture and the caecum was punctured twice in different
places using 21 needles (24,25). A
small amount of stool was extruded and the abdomen was close using
a 4-0 suture. Pre-warmed saline (37°C) was injected into the
subcutaneous tissue with fluid resuscitation following surgery. The
rats under sham operation were exposed and the caecum was removed
following laparotomy without ligation and puncture. The animals
were monitored every 30 min post-surgery. The rats that
demonstrated unimproved lethargy or moribund behavior were
euthanized.
Imipenem and CTX administration
Imipenem was obtained from the Merck KGaA. For
survival studies, imipenem (120 mg/kg) was intraperitoneally
injected 6 h post-CLP and every 12 h for a total of 7 days. For
intestinal barrier function analysis, imipenem (120 mg/kg) was
intraperitoneally injected 6 h post-CLP and twice every 12 h
thereafter. CTX was purchased from the Sigma-Aldrich (Merck KGaA).
For survival studies, CTX (10 mg/kg, dissolved in saline) was
intraperitoneally injected 6 h post-CLP and every 12 h for a total
of 7 days into animals treated with or without imipenem. For
intestinal barrier function analysis CTX (10 mg/kg, dissolved in
saline) was intraperitoneally injected 6 h post-CLP and twice every
12 h thereafter into animals treated with or without imipenem.
Intestinal histopathology
At 24 h following imipenem or CTX administration,
small-intestine tissue (~10 cm) above the ileocecal valves was
collected, repeatedly flushed with PBS and placed in 10% formalin
(40°C; 24–48 h). The intestinal tissue was sliced, fixed,
deparaffinized by soaking in xylene for 15 min at room temperature,
dehydrated in 100% alcohol for 10 min, 95% alcohol for 10 min, 85%
alcohol for 3 min, 75% alcohol 3 min at room temperature and
stained with hematoxylin and eosin (20% Harris for 10 min and 0.5%
eosin for 1 min) at room temperature prior to observation under a
light microscope (magnification, ×100).
Intestinal permeability
Intravenous injection of 120 mg/ml fluorescein
isothiocyanate-conjugated-dextran (FD-70; 0.5 ml, molecular mass 70
kDa, Sigma-Aldrich; Merck KGaA) was administered approximately ~6 h
prior to the sacrifice. Blood samples were centrifuged at 12,000 ×
g for 4 min at 4°C, and the plasma was diluted with an equal volume
of PBS (pH 7.4). The excitation wavelength of 480 nm and the
emission wavelength of 520 nm were used to analyze fluorescence in
a microplate reader.
Intestinal epithelial apoptosis
Apoptosis of epithelial cells was analyzed using the
Terminal deoxynucleotidyl-transferase-mediated dUTP nick
end-labeling (TUNEL) assay and the number of apoptotic cells was
quantified. Intestinal tissues were fixed, embedded in paraffin
wax, and cut into 5-µm sections. The TUNEL apoptosis assay kit
(Sigma-Aldrich; Merck KGaA) was used for the experiments according
to the manufacturer's instructions. The apoptotic cells stained
with brown nuclei were analyzed under an optical microscope
(magnification, ×400).
Cytokine measurement
Rats were anesthetized and fixed on the operating
table. The abdominal cavity was opened to expose the abdominal
aorta. Approximately 10 ml of blood was extracted from the
abdominal aorta and placed into 2-ml EP tubes. Blood samples were
centrifuged at 2,000 × g for 15 min and the plasma was removed into
2-ml EP tube. Interleukin (IL)-6 (cat. no. F15870), IL-10 (cat. no.
F15900) and tumor necrosis factor (TNF)-α (cat. no. F3768; all
Shanghai Westang Bio-tech Co., Ltd.) serum levels were detected
using an ELISA according to the manufacturer's instructions.
Tight junction protein expression
analysis
Approximately 120 mg of intestinal tissue was
extracted and placed in liquid nitrogen for 2 min. Total protein
was extracted using radioimmunoprecipitation assay buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at a
ratio of 10 mg tissue to 100 µl buffer. The turbid liquid was
placed in an ultra-high-speed centrifuge with at 12,000 × g for 20
min at 4°C and the resulting solution was transferred to the 2-ml
EP tubes. This procedure was repeated three times. The protein
content was determined using the bicinchoninic acid method
(Beyotime Institute of Biotechnology, Haimen, China). Western
blotting was performed as previously described (13,26).
Samples were incubated with 4× SDS-PAGE loading buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) in a
boiling water bath for 5 min. Equal amounts of total proteins (30
µg/lane) were separated using 10% SDS-PAGE gels. Proteins were
transferred to polyvinylidene difluoride membranes and blocked
using 5% skimmed milk powder for 2 h at room temperature. Membranes
were subsequently incubated with primary antibodies occludin
(1:5,000; cat. no. ab167161), claudin-2 (1:500; cat. no. ab53032;
both Abcam, Cambridge, UK), ZO-1 (1:500; cat. no. sc-33725; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin (1:100; cat.
no. ab8226) at 4°C overnight, followed by horseradish
peroxidase-linked anti-rabbit IgG (1:10,000; cat. no. ab6734; both
Abcam) secondary antibody for 2 h at room temperature. The films
were then stored in a dark room and a chemiluminescent peroxidase
substrate (cat. no. 34094; Thermo Fisher Scientific, Inc.) was
applied according to the manufacturer's instructions. Proteins were
detected using a chemiluminescent system and visualized using a gel
imaging system (ChemiDoc Touch, Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The results were analyzed using intensity
quantification software (ImageLab 5.2, Bio-Rad Laboratories,
Inc.).
Statistical analysis
All values are represented as mean ± standard
deviation of at least three independent experiments. The log-rank
test was used to statistically analyze differences in survival
between the experimental groups. Multiple group comparisons were
performed with using one-way analysis of variance followed by an
LSD post hoc test to compare differences between the two groups. If
data were not normally distributed, the Kruskal-Wallis
non-parametric test was used to compare differences among multiple
groups followed by the Dunn-Bonferroni post hoc test to compare
differences between two groups. The test level was α=0.05, and
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using GraphPad
Prism software (Version 5; GraphPad Software, Inc., La Jolla, CA,
USA) and SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
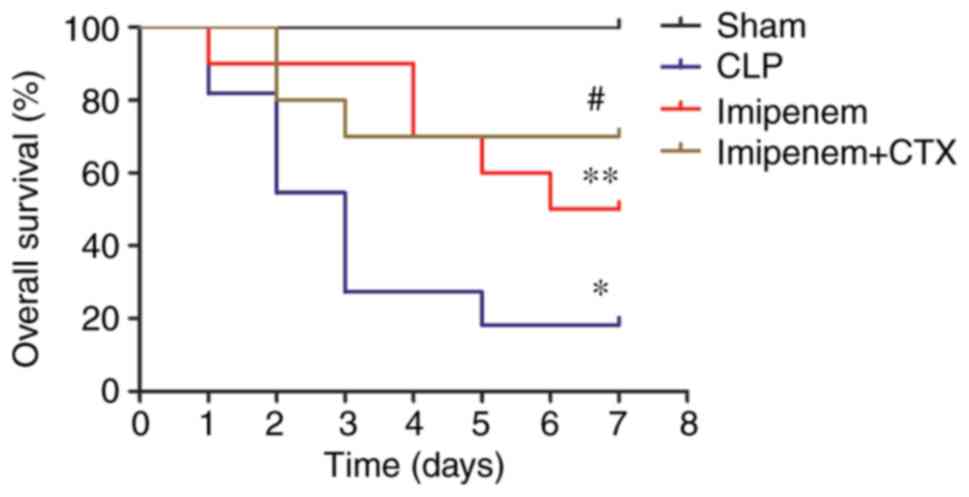
Effect of imipenem combined with CTX
on survival
To evaluate the effect of CTX combined with
antimicrobial drugs, the survival rates for all the groups were
assessed following treatment. The CLP group had an 81.18% survival
rate on day 1 and an 18.18% survival rate on day 7. Notably, on day
2 the mortality was the highest. As previously reported (8), septic rats treated with imipenem
exhibited a significantly higher 7-day survival rate (50%) compared
with the CLP group (P<0.05). The combination treatment group
exhibited a 100% survival rate on day 1 and a 70% survival rate on
day 7, which was significantly higher than that of imipenem group
(P<0.05; Fig. 1).
Effect of imipenem combined with CTX
on intestinal permeability
To assess whether CTX causes intestinal barrier
dysfunction, the intestinal permeability to FD-70 was examined.
Results indicated that the septic rats achieved significantly
higher serum levels of FD-70 compared with the sham group
(P<0.05; Fig. 2). Compared with
the CLP group, the expression of FD-70 in the imipenem group and
combination treatment group was significantly reduced (P<0.05).
Notably, the serum FD-70 levels in the combination treatment group
were increased compared with that of the imipenem group
(P>0.05).
Effect of imipenem combined with CTX
on intestinal tissue integrity
Histopathologically stained sections were used to
measure the effect of imipenem combined with CTX on intestinal
mucosal integrity (Fig. 3). No
notable inflammatory cells were observed in the sham group.
Furthermore, the intestinal goblet cells were not damaged and no
intestinal villi were ruptured in the sham operation group
(Fig. 3A). The CLP group
demonstrated clear inflammatory cell infiltration and locally
necrotic areas compared with the sham group (Fig. 3B). In the combination treatment and
imipenem groups, regular epithelial appearance with intestinal
epithelial infiltration and mild alteration was observed (Fig. 3C and D). However, no distinction
between the combination treatment and the imipenem groups was
observed with regard to intestinal tissue pathology.
Effect of imipenem combined with CTX
on intestinal epithelial apoptosis
Apoptotic cells were stained brown and analyzed
under a light microscope. Few brown-stained cells were observed in
the sham operation group (Fig. 4A).
The number of apoptotic cells was markedly increased in the CLP
group (Fig. 4B). Imipenem and
combination treatment markedly reduced the number of intestinal
apoptotic epithelial cells (Fig. 4C and
D). No marked difference in the number of apoptotic epithelial
cells was noted between the imipenem group and the combination
treatment group.
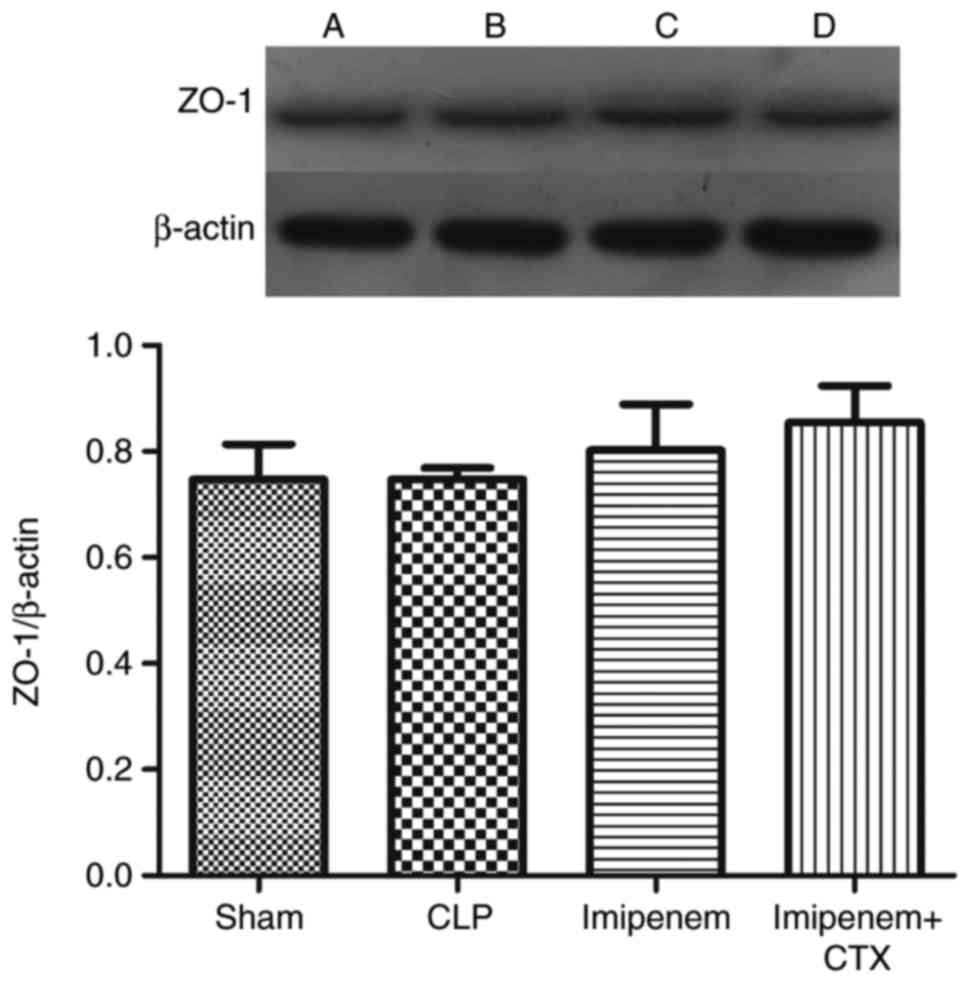
Effect of imipenem combined with CTX
on tight junction protein expression
Results indicated that there was no significant
difference in the expression level of ZO-1 protein between groups
(Fig. 5). Notably, the protein
expression levels of occludin and claudin-2 in intestinal tissue
were significantly decreased in the CLP group compared with the
sham group (P<0.05; Figs. 6 and
7). Compared with the CLP group, the
expression of occludin in the imipenem group was significantly
decreased (P<0.05). By contrast, its expression was
significantly increased in the combination treatment group
(P<0.05; Fig. 6). As indicated in
Table I, a significant increase in
occludin protein expression was indicated in the combination
treatment group compared with the imipenem group (P<0.05).
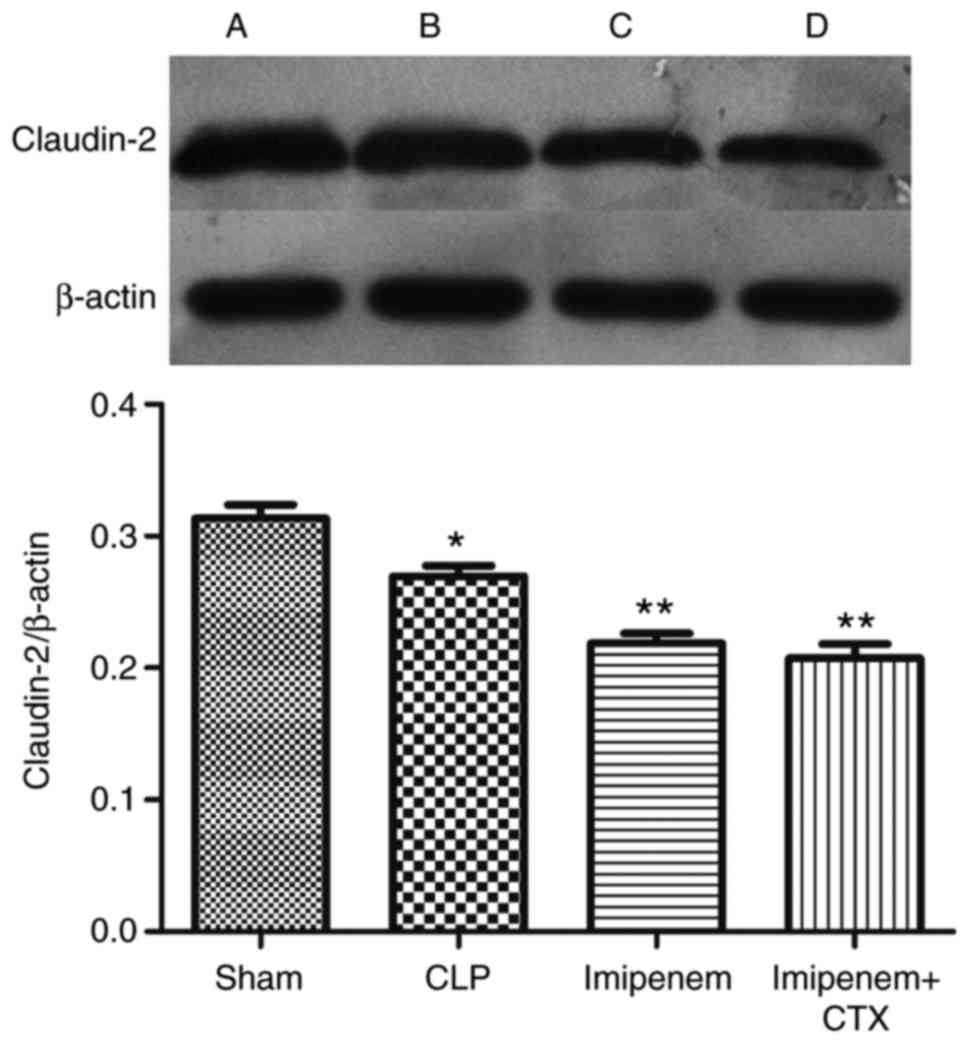
Furthermore, results indicated that claudin-2 expression was
significantly decreased in the imipenem and combination treatment
groups compared with the CLP group (P<0.05; Fig. 7). However, there was no significant
difference between the imipenem group and the combination treatment
group (Table I; Fig. 7).
 | Table I.Expression of the tight junction
proteins in the intestine of each group. |
Table I.
Expression of the tight junction
proteins in the intestine of each group.
| Group | ZO-1 | Occludin | Claudin-2 |
|---|
| Sham | 0.7469±0.0385 | 1.9370±0.0172 | 0.3135±0.0103 |
| CLP | 0.7470±0.0123 |
1.5816±0.0140a |
0.2696±0.0078a |
| Imipenem | 0.8022±0.0500 |
1.3698±0.0123b |
0.2187±0.0075b |
| Imipenem+CTX | 0.8548±0.0397 |
1.9117±0.0156c |
0.2075±0.0107b |
Effect of imipenem combined with CTX
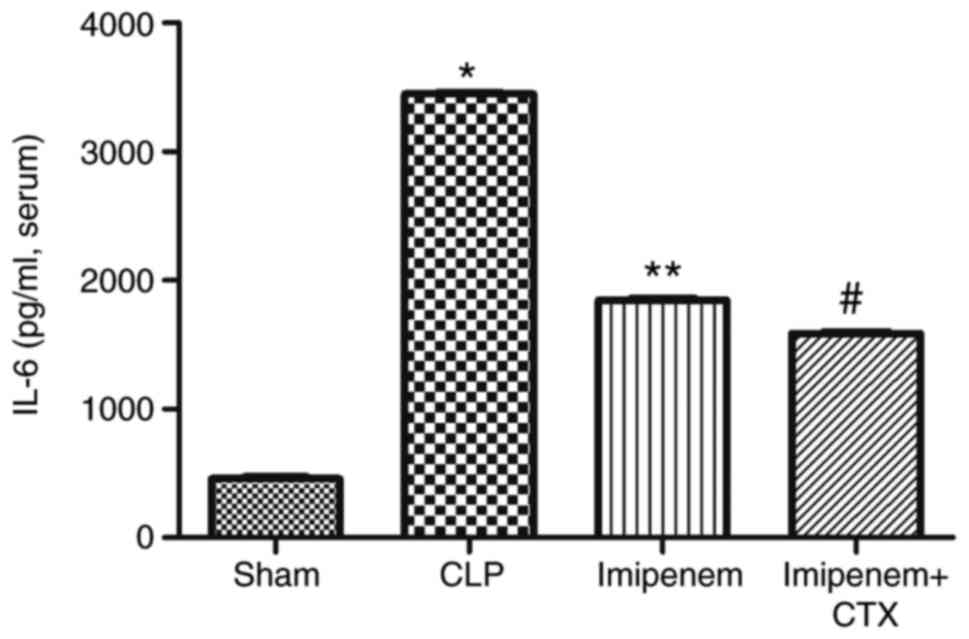
on the expression of plasma cytokines
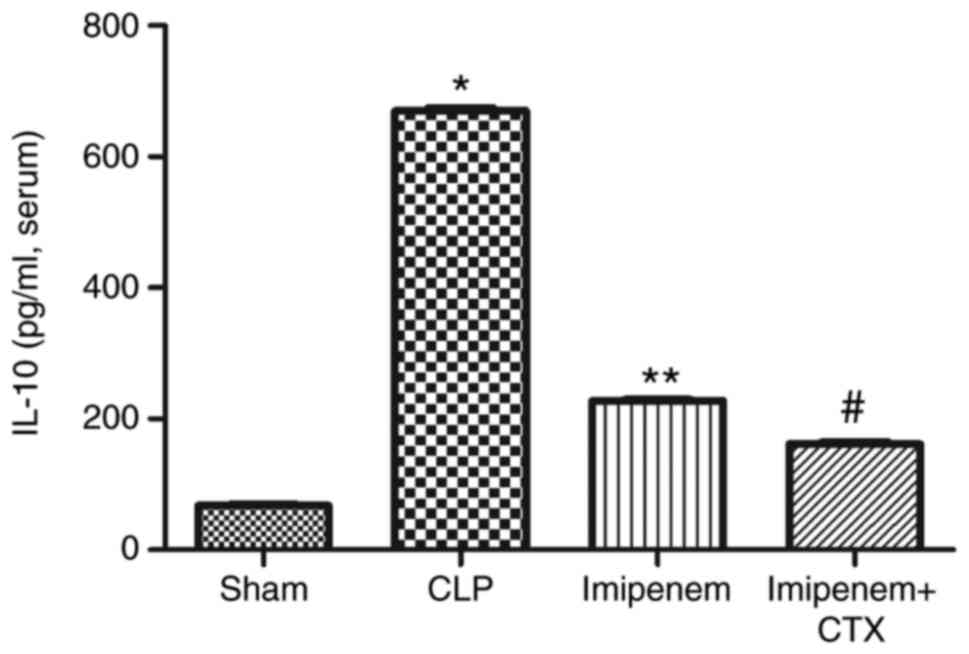
IL-6, IL-10 and TNF-α expression levels were
significantly increased in the CLP group compared with the sham
group (P<0.05; Figs. 8–10). Compared with the CLP group, the
expression levels of IL-6, IL-10 and TNF-α in the imipenem and
combination treatment groups were decreased significantly
(P<0.05; Figs. 8–10). Combination treatment significantly
decreased the expression levels of IL-6 (P<0.05; Fig. 8) and IL-10 (P<0.05; Fig. 9) and increased the expression level
of TNF-α (P>0.05; Fig. 10)
compared with imipenem alone.
Discussion
With the exception of antibiotics and fluid
resuscitation, there are still no effective treatments for sepsis.
The concept of treating sepsis with immunosuppressant has recently
become a promising approach. Some researchers revealed that
imipenem combined with low-dose CTX improved the survival rate of
mice with sepsis (8,9). In their study, the early excessive
inflammatory response to sepsis caused an excessive utilization of
inflammatory factors, which resulted in immunosuppression. CTX is
an immunosuppressant that inhibits the innate immune response and
reduces the hyperinflammatory response of sepsis, which reduces the
damage to the body itself (8). In
the present study, a sepsis rat model was successfully replicated
based on a previous report (24).
The survival rate was significantly increased following imipenem
treatment and combination treatment groups. Consistent with
previous reports, the present results suggested that imipenem
combined with low-dose CTX may improve the mortality of sepsis
compared with imipenem alone (70 vs. 50%). Notably, combination
treatment improved the 7-day survival rate of sepsis rats.
It is not clear whether the exacerbation of
intestinal barrier dysfunction cause serious complications,
including multiple organ dysfunction syndrome (27,28). To
investigate the effect of imipenem combined with low-dose CTX on
the intestinal barrier function, the quantitative and qualitative
markers that affected the function of the intestinal barrier were
evaluated. Consistent with our previous findings (unpublished
data), the present results suggested that the intestinal
permeability increased significantly in septic rats and this was
improved in the rats treated with imipenem. Compared with the CLP
group, intestinal permeability was also improved in the combination
treatment group. However, the intestinal permeability of rats was
higher in the combination treatment group compared with that in the
imipenem monotherapy group, which suggested that the combination
treatment may further damage intestinal barrier function. In our
previous study, a combination treatment of imipenem, normal saline
and CTX significantly improved the survival rate of septic rats
(unpublished data). Conversely, the results of previous study
revealed that the combination therapy of imipenem, normal saline
and CTX is beneficial for intestinal barrier. Notably, previous
studies have identified many factors can affect intestinal
permeability (29–31). The present study further analyzed the
possible mechanisms involved in changes in intestinal barrier
function.
Intestinal mucosal permeability includes
transcellular and paracellular permeability (32). Transcellular permeability is
primarily impacted by mucosal cells and intrinsic layer cells in
the intestine whereas paracellular permeability is impacted by the
tight junction structure of intestinal endothelial cells (33). Consistent with our previous study
(unpublished data), intestinal injury influencing transcellular
permeability was evident in the rats with sepsis. However,
treatment with imipenem markedly improved intestinal injury.
Furthermore, intestinal epithelial apoptosis analysis indicated the
number of apoptotic cells was increased in the CLP group in the
present study. Notably, the number of apoptotic cells was
diminished in the imipenem group compared with CLP group. However,
no significant differences in intestinal tissue injury or apoptosis
of intestinal epithelium cells were identified between the imipenem
combined with CTX compared with the imipenem group.
Intestinal tight junctions regulate paracellular
permeability. Notably the intestinal tight junction proteins
comprise of the ZO family, the claudin family and occluding
(13). The combination therapy of
imipenem, normal saline and CTX significantly increased expression
of occludin and reduced expression of FD-70 compare with
combination therapy of imipenem and CTX (unpublished data).
However, in the present study, no significant differences the
expression levels of ZO-1 protein were observed between groups.
Consistent with previous results (34), the present findings revealed that the
expression of occludin decreased in the intestines of the rats with
sepsis. It is interesting to note that imipenem treatment resulted
in decreased occludin expression and that combined treatment of
imipenem with CTX increased occludin expression. Therefore,
according to the present results, there is no reason to believe
that occludin is responsible for the increased permeability
observed in the combination treatment group. Claudin-2, which is
primarily present in intestinal tissue, is member of the claudin
family. In the present study, results indicated that the expression
of claudin-2 was reduced in the imipenem and combination treatment
groups. However, there was no significant difference between the
imipenem and combination treatment groups. Notably, the combination
treatment group exhibited increased intestinal permeability
compared with the imipenem group. It was therefore inferred that
intestinal permeability may not be independently affected by the
occludin tight junction. Therefore, these findings suggest that
combination treatment can increase intestinal permeability, which
may be associated with factors other than occludin.
Cytokines are also a factor involved in intestinal
barrier function, particularly IL-6, IL-10 and TNF-α. Previous
studies have indicated that TNF-α (35) and IL-6 (36) increase intestinal permeability and
IL-10 (37) exerts a protective
effect on the function of the intestinal barrier. In the present
study, imipenem treatment decreased IL-6, IL-10 and TNF-α
expression levels. Inconsistent with our previous study
(unpublished data), the combined treatment of imipenem and CTX
further reduced IL-6 and IL-10 expression. Therefore, it was
inferred that the increase in intestinal permeability in the
combination treatment group may be due to the decrease in IL-6 and
IL-10. In addition, various studies have identified that mice
lacking IL-10 exhibit a significant increase in intestinal
permeability but the exact mechanisms remain clear (38–40).
Taken together, the present findings suggest a decrease in IL-10
may increase intestinal permeability. It is interesting that the
expression of IL-6 in the combination treatment group is also
reduced, which is not consistent with previous studies (36,41).
Therefore, the possible mechanisms of this change were we further
explored. Wang indicated that increased intestinal permeability
during sepsis was regulated by an interaction between IL-6 and
IL-10 and that treatment with IL-10 may prevent the increase in
mucosal permeability during sepsis in IL-6-/- mice (42). They found that IL-6 did not directly
affect the intestinal mucosa but did suppress the expression of
IL-10. Therefore, a reduction in IL-6 may be associated with the
increase in intestinal permeability in present study. In the
present study, imipenem combined with low-dose CTX significantly
reduced the expression of IL-10. It was suggested that there was a
slight increase in intestinal permeability in the
combined-treatment group due to the decrease in IL-10 expression
level. In light of this, combination therapy may have a potential
effect on increased intestinal permeability by inhibiting the
expression of IL-10.
The present study exhibited some limitations.
Firstly, converse to previous studies, male SD rats were used as
the experimental animals; different species or sexes may elicit
different effects on study results. Additionally, the present study
focused on the CTX-induced intestinal mucosal injury without
further investigating its effects on cellular immune function.
Furthermore, the present study consisted of a 24-h investigation
without the dynamic monitoring of the relevant indicators,
including intestinal permeability, intestinal epithelial apoptosis,
IL-6, IL-10, TNF-α, and tight junction proteins, which will be
further explored in subsequent studies. In the present study, the
tight junction protein expression levels were implicated in
intestinal permeability; however, other mechanisms could be
involved that were not discussed. Finally, previous studies
suggested a dose-dependent damaging effect of CTX on intestinal
mucosa (13). The present findings
also implied that low-dose CTX has a potential effect on the
intestinal barrier dysfunction. However, these results should be
investigated further to determine whether long-term low-dose CTX
treatment causes intestinal injury.
In conclusion, a rat model of sepsis was
successfully replicated in the present study. Imipenem combined
with low-dose CTX improved the survival rate of rats with sepsis
compared with treatment with imipenem alone. Taken together, the
present findings suggest that imipenem combined with low-dose CTX
has the potential to damage to the intestinal mucosal barrier and
the mechanism may be associated with reduced IL-10.
Acknowledgements
The authors would like to thank Professor Qing-Hong
Cheng (Peking Union Medical College, Tsinghua University, Beijing,
China) for his assistance with the experimental research. The
authors were further supported by the Laboratory of Xinjiang
Endemic and Ethnic Diseases, which they would like to
acknowledge.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PG performed all animal experiments and revised the
manuscript. S-WZ was a major contributor in writing the manuscript
and performed the statistical analysis W-JZ and FW jointly designed
the study. JZ and J-TD performed and guided animal experiments.
J-DW revised the statistical analysis. STT and J-TY performed
survival experiments. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Shihezi University (Shihezi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have competing
interests.
References
|
1
|
Rhodes A, Evans LE, Alhazzani W, Levy MM,
Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally
ME, et al: Surviving sepsis campaign: International guidelines for
management of sepsis and septic shock: 2016. Crit Care Med.
45:486–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K:
International Forum of Acute Care Trialists: Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stoller J, Halpin L, Weis M, Aplin B, Qu
W, Georgescu C and Nazzal M: Epidemiology of severe sepsis:
2008–2012. J Crit Care. 31:58–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hotchkiss RS and Opal S: Immunotherapy for
sepsis-a new approach against an ancient foe. N Engl J Med.
363:87–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weighardt H, Heidecke CD, Emmanuilidis K,
Maier S, Bartels H, Siewert JR and Holzmann B: Sepsis after major
visceral surgery is associated with sustained and
interferon-gamma-resistant defects of monocyte cytokine production.
Surgery. 127:309–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeni F, Freeman B and Natanson C:
Anti-inflammatory therapies to treat sepsis and septic shock: A
reassessment. Crit Care Med. 25:1095–1100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown I, Bellevue O, Shawo A, Woldesemayat
H, Lyo V, Rayikanti B, Lee M, Uzosike ED, Kasravi S and Harris HW:
Low-dose cyclophosphamide improves survival in a murine treatment
model of sepsis. Shock. 43:92–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei ZR, Wang DL and Liu Q: Effects of low
dose of cyclophosphamide on acute lung injury in sepsis sats. Chin
J Crit Care Med. 26:124–126. 2006.
|
|
10
|
Waymack JP and Alexander JW:
Immunomodulators for the prevention of infections in burned guinea
pigs. J Burn Care Rehabil. 8:363–365. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fibbe WE, van der Meer JW, Falkenburg JH,
Hamilton MS, Kluin PM and Dinarello CA: A single low dose of human
recombinant interleukin 1 accelerates the recovery of neutrophils
in mice with cyclophosphamide-induced neutropenia. Exp Hematol.
17:805–808. 1989.PubMed/NCBI
|
|
12
|
van Vliet MJ, Tissing WJ, Dun CA, Meessen
NE, Kamps WA, de Bont ES and Harmsen HJ: Chemotherapy treatment in
pediatric patients with acute myeloid leukemia receiving
antimicrobial prophylaxis leads to a relative increase of
colonization with potentially pathogenic bacteria in the gut. Clin
Infect Dis. 49:262–270. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Liu KX, Qu JM and Wang XD: The
changes induced by cyclophosphamide in intestinal barrier and
microflora in mice. Eur J Pharmacol. 714:120–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nord CE and Edlund C: Impact of
antimicrobial agents on human intestinal microflora. J Chemother.
2:218–237. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto T, Ishikawa H, Tateda K,
Yaeshima T, Ishibashi N and Yamaguchi K: Oral administration of
Bifidobacterium longum prevents gut-derived Pseudomonas aeruginosa
sepsis in mice. J Appl Microbiol. 104:672–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dancygier H and Frick B: Crohn's disease
of the upper gastrointestinal tract. Endoscopy. 24:555–558. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fahal AH, el Razig SA, Suliman SH, Ibrahim
SZ and Tigani AE: Gastrointestinal tract cancer in association with
hepatitis and HIV infection. East Afr Med J. 72:424–426.
1995.PubMed/NCBI
|
|
18
|
Wells CL: Relationship between intestinal
microecology and the translocation of intestinal bacteria. Antonie
Van Leeuwenhoek. 58:87–93. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Udall JN, Pang K, Fritze L, Kleinman R and
Walker WA: Development of gastrointestinal mucosal barrier. I. The
effect of age on intestinal permeability to macromolecules. Pediatr
Res. 15:241–244. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Liu Z, Zhao S, Sun C and Yang M:
Effect of BML-111 on the intestinal mucosal barrier in sepsis and
its mechanism of action. Mol Med Rep. 12:3101–3106. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berg RD and Garlington AW: Translocation
of certain indigenous bacteria from the gastrointestinal tract to
the mesenteric lymph nodes and other organs in a gnotobiotic mouse
model. Infect Immun. 23:403–411. 1979.PubMed/NCBI
|
|
22
|
Gao M, Jiang Y, Xiao X, Peng Y, Xiao X and
Yang M: Protective effect of pioglitazone on sepsis-induced
intestinal injury in a rodent model. J Surg Res. 195:550–558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao W and Sun GZ: Random grouping of
experimental animals is often used. Xu Mu Shou Yi Ke Ji Xin Xi.
2009:61–62. 2009.(In Chinese).
|
|
24
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma LQ, Chen DC and Liu SZ: Influence of
broad-spectrum antibiotics on the gut microflora in sepsis in rats.
Zhongguo Wei Zhong Bing Ji Jiu Yi. 20:520–522. 2008.(In
Chinese).
|
|
26
|
Ulluwishewa D, Anderson RC, McNabb WC,
Moughan PJ, Wells JM and Roy NC: Regulation of tight junction
permeability by intestinal bacteria and dietary components. J Nutr.
141:769–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deitch EA: Gut lymph and lymphatics: A
source of factors leading to organ injury and dysfunction. Ann N Y
Acad Sci. 1207 Suppl 1:E103–E111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma R, Tepas JJ III, Hudak ML, Mollitt
DL, Wludyka PS, Teng RJ and Premachandra BR: Neonatal gut barrier
and multiple organ failure: Role of endotoxin and proinflammatory
cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg.
42:454–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Costantini TW, Loomis WH, Putnam JG,
Drusinsky D, Deree J, Choi S, Wolf P, Baird A, Eliceiri B, Bansal V
and Coimbra R: Burn-induced gut barrier injury is attenuated by
phosphodiesterase inhibition: Effects on tight junction structural
proteins. Shock. 31:416–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song D, Shi B, Xue H, Li Y, Yang X, Yu B,
Xu Z, Liu F and Li J: Confirmation and prevention of intestinal
barrier dysfunction and bacterial translocation caused by
methotrexate. Dig Dis Sci. 51:1549–1556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thakre-Nighot M and Blikslager AT:
Indomethacin induces increase in gastric epithelial tight junction
permeability via redistribution of occludin and activation of p38
MAPK in MKN-28 Cells. Tissue Barriers. 4:e11873252016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munjal C, Tyagi N, Lominadze D and Tyagi
SC: Matrix metalloproteinase-9 in homocysteine-induced intestinal
microvascular endothelial paracellular and transcellular
permeability. J Cell Biochem. 113:1159–1169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu L, Li N, Gong J, Li Q, Zhu W and Li J:
Berberine ameliorates intestinal epithelial tight-junction damage
and down-regulates myosin light chain kinase pathways in a mouse
model of endotoxinemia. J Infect Dis. 203:1602–1612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Costantini TW, Bansal V, Peterson CY,
Loomis WH, Putnam JG, Rankin F, Wolf P, Eliceiri BP, Baird A and
Coimbra R: Efferent vagal nerve stimulation attenuates gut barrier
injury after burn: Modulation of intestinal occludin expression. J
Trauma. 68:1349–1356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Sadi R, Guo S, Ye D and Ma TY: TNF-α
modulation of intestinal epithelial tight junction barrier is
regulated by ERK1/2 activation of Elk-1. Am J Pathol.
183:1871–1884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang R, Han X, Uchiyama T, Watkins SK,
Yaguchi A, Delude RL and Fink MP: IL-6 is essential for development
of gut barrier dysfunction after hemorrhagic shock and
resuscitation in mice. Am J Physiol Gastrointest Liver Physiol.
285:G621–G629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun X, Yang H, Nose K, Nose S, Haxhija EQ,
Koga H, Feng Y and Teitelbaum DH: Decline in intestinal mucosal
IL-10 expression and decreased intestinal barrier function in a
mouse model of total parenteral nutrition. Am J Physiol
Gastrointest Liver Physiol. 294:G139–G147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arrieta MC, Madsen K, Doyle J and Meddings
J: Reducing small intestinal permeability attenuates colitis in the
IL10 gene-deficient mouse. Gut. 58:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Z, Zhang P, Ma Y, Chen H, Zhou Y,
Zhang M, Chu Z and Qin H: Lactobacillus plantarum prevents the
development of colitis in IL-10-deficient mouse by reducing the
intestinal permeability. Mol Biol Rep. 38:1353–1361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arrieta MC, Madsen KL, Field CJ and
Meddings JB: Increasing small intestinal permeability worsens
colitis in the IL-10-/- mouse and prevents the induction of oral
tolerance to ovalbumin. Inflamm Bowel Dis. 21:8–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zahs A, Bird MD, Ramirez L, Choudhry MA
and Kovacs EJ: Anti-IL-6 antibody treatment but not IL-6 knockout
improves intestinal barrier function and reduces inflammation after
binge ethanol exposure and burn injury. Shock. 39:373–379. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Q, Fang CH and Hasselgren PO:
Intestinal permeability is reduced and IL-10 levels are increased
in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp
Physiol. 281:R1013–R1023. 2001. View Article : Google Scholar : PubMed/NCBI
|