Introduction
Norcantharidin (NCTD; Fig. 1), the demethylated derivative of
cantharidin obtained from the dried body of the Chinese blister
beetle (Mylabris spp.) (1),
has been used as an anticancer drug in China (2,3). NCTD
acts by inducing cell death through mechanisms involving the
response to DNA damage and apoptosis by activation of the protein
kinase C signaling pathway (4). Like
sulfonamides for carbonic anhydrase or hydroxamic acids for
metalloproteinases, NCTD describes the archetypal small molecule
protein phosphatase inhibitor (5)
and has been used to inhibit proliferation and metastasis of
multiple types of carcinoma (6,7).
Previous studies indicated that NCTD has therapeutic value in the
treatment of various types of cancer, including liver cancer, when
administered orally or intravenously (6,8–11). However, the application of NCTD is
limited by numerous factors, including short half-life, high
systemic toxicity, high incidence of adverse effects and poor
bioavailability in the physiological environment (12,13). To
improve the safety and efficacy of NCTD, NCTD nanoscale drug
delivery systems (DDS) have been studied (14–16).
Liposomes are spheres with a lipid bilayer shell
prepared with a variety of phospholipids, which have been
extensively studied since the 1960s (17–19).
Cholesterol is an additional compound in the liposomal structure
that could regulate the fluidity of phospholipid membrane and may
control the retention of drugs (20). Therefore, liposomes may be used as
nanoscale vehicles for the administration of drugs. With good
biocompatibility and low toxicity, liposomes have been revealed to
enhance the therapeutic activity of numerous anticancer drugs
(21–23). However, conventional liposomes have
certain disadvantages, including the uptake by the
reticuloendothelial system (RES) (24,25), the
lack of tumor-specificity and insufficient uptake at tumor sites.
Ligand-targeted liposomes, where specific ligands are used to
modify liposomes, have demonstrated the potential to increase
therapeutic efficacy and reduce adverse effects through the
interaction between a specific ligand and the target molecule, and
facilitating the receptor mediated endocytosis of liposomes
(26).
Glycyrrhetinic acid (GA) may be obtained by
hydrolysis of glycyrrhicinate, extracted from the roots of
liquorice (Glycyrrhiza glabra) (27). GA possesses many pharmacological and
biological relevant activities, including anti-inflammatory,
antiallergic, antiulcer, antioxidant, antihepatotoxic,
antineoplasmic and antiviral activities (28–30).
Studies have reported that specific GA binding sites may be located
on the cell membrane of hepatocytes (31,32). GA
and its derivatives may be used as ligands targeting the liver
(33,34). Stearyl glycyrrhetinate (SG; Fig. 2), the stearyl ester of
18-β-glycyrrhetinic acid, has been demonstrated to improve
antiviral effects, reduce inflammation and suppress allergies in
the pharmaceutical and cosmetic industry (35,36).
Compared with GA, SG has an increased compatibility as its
hydrophobic tail may be adsorbed into the lipid layer of liposomes
while exposing the hydrophilic GA moiety at the surface.
The objective of the current study was to prepare
NCTD-loaded liposomes modified with SG (SG-NCTD-LIP) by ethanol
injection. Single factor test and orthogonal design were used to
optimize the formulation of SG-NCTD-LIP. The characterization of
the prepared liposomes included physical morphology, particle size
and encapsulation efficiency (EE). Equilibrium dialysis was used to
investigate in vitro release characteristics of SG-NCTD-LIP.
Furthermore, in vitro cytotoxicity of SG-NCTD-LIP in HepG2
cells was determined by MTT assay.
Materials and methods
Materials
NCTD (>99%) was purchased from Sunray
Pharmaceutical Co., Ltd. (Suzhou, China). SG (>98%) was obtained
from Xi'an Realin Biotechnology Co., Ltd. (Xi'an, China). Egg
phosphatidylcholine (EPC) was supplied by Lipoid GmbH
(Ludwigshafen, Germany). Cholesterol was purchased from Beijing
Solarbio Science & Technology Co., Ltd. (Beijing, China).
HPLC-grade acetonitrile was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). All other chemicals used in this study
were of analytical grade.
Preparation of SG-NCTD-LIP
Liposomes were prepared by ethanol injection method
(Fig. 3) (37,38). EPC
(0.1–0.4%), cholesterol (0.03–0.20%) and SG (0.04%) were dissolved
in 2 ml absolute ethanol. The aqueous phase was prepared by
dissolving NCTD (0.02–0.08%) in 15 ml PBS (pH 7.0) and heating in a
water bath at 37°C. The 2 ml ethanol phase was immediately injected
into the heated aqueous solution through a fine needle under
magnetic stirring. The liposome dispersions were incubated at
between 50°C for 30 min with gentle stirring.
Determination of NCTD content and
EE
SG-NCTD-LIP was separated from the free drug by
equilibrium dialysis (the dialysis membrane bag molecular weight
cut-off of 8,000–14,000). The liposome fraction was obtained and
the liposomes were ruptured in methanol for drug solubilisation.
The resulting solution was sonicated (150 W at 25°C) for 5 min and
filtered through polytetrafluorethylene membranes (0.22 µm). The
drug concentration was determined using a reversed-phase
high-performance liquid chromatography (RP-HPLC) system (LC-20AT;
Shimadzu Corporation, Kyoto, Japan) at 25°C and 98% NCTD was used
as a quantification standard. The mobile phase was
acetonitrile/water (10:90, v/v; pH 3.1) and an isocratic elution
was performed using a WondaCract ODS-2 column (5 µm, 4.6×250 mm;
Shimadzu Corporation) with a flow rate of 0.8 ml/min. NCTD was
detected at 220 nm. The EE was determined by dividing the amount of
drug in the liposome fraction by the amount of drug in the total
fractions.
Optimization of SG-NCTD-LIP
formulation
Using the ethanol injection method, several factors
were trialed to achieve optimal formulation, including
NCTD-phospholipid mass ratio (factor A), phospholipid concentration
(factor B), incubation temperature (factor C) and
cholesterol-phospholipid mass ratio (factor D) during the
fabrication process. Only one factor was replaced in each series of
experiments. When changing the amount of phospholipids, the
NCTD-phospholipid mass ratio was 1:5, cholesterol-phospholipid mass
ratio was 1:7 and incubation temperature was 50°C. When changing
the amount of NCTD, the phospholipid concentration was 0.24%,
cholesterol-phospholipid mass ratio was 1:7 and incubation
temperature was 50°C. When changing cholesterol-phospholipid mass
ratio, the phospholipid concentration was 0.24%, NCTD-phospholipid
mass ratio was 1:20 and incubation temperature was 50°C. When
changing incubation temperature, the phospholipid concentration was
0.24%, NCTD-phospholipid mass ratio was 1:20 and
cholesterol-phospholipid mass ratio was 1:5. Based on the
investigation of factors, the four aforementioned factors were
selected, and three levels of each factor were designated for the
orthogonal design, with the EE as the investigating indicator to
screen the formulation.
SG-NCTD-LIP size and polydispersity
index (PDI)
The particle size and PDI of SG-NCTD-LIP were
determined at 25°C by dynamic light scattering (Nano-ZS; Malvern
Instruments, Ltd., Malvern, UK). Prior to measurement, the liposome
dispersions were diluted 10 times with distilled water.
Measurements were performed in triplicate on independent
formulations at a detection angle of 90°.
Transmission electron microscopy (TEM)
measurement of SG-NCTD-LIP
A diluted SG-NCTD-LIP sample (0.018 mg/ml; 10 µl)
was placed on a copper grid and air-dried at room temperature.
Subsequently, a drop of 1% (w/v) aqueous solution of
phosphotungstic acid was added for negative staining; the sample
was dried at room temperature for 20 min. The analyses of the
vesicle shape were carried out on a JEM-2100F transmission electron
microscope (JEOL, Ltd., Tokyo, Japan) with an acceleration of 150
kV.
SG-NCTD-LIP stability studies
SG-NCTD-LIP suspension stability was assessed over 3
months of storage at 4°C by means of particle size, PDI and EE
measurements.
In vitro drug release study
In vitro drug release was performed as
described previously (39). Dialysis
tubing (molecular weight cut off, 8,000–14,000 g/mol) was incubated
in distilled water for 12 h at room temperature. SG-NCTD-LIP (0.18
mg/ml. 8 ml) and NCTD solution (0.18 mg/ml, 8 ml) were placed in
separate dialysis tubing and each incubated with stirring in 80 ml
PBS (pH 7.4, release medium) at 37°C. Following 0.25, 0.5, 1, 1.5,
2, 4, 8 and 12 h incubation, 0.5 ml release medium was collected
and replaced with an equal volume of fresh medium. NCTD
concentration was analyzed by RP-HPLC.
Cell culture
HepG2, an immortalized cell line consisting of
hepatoblastoma cells, was obtained from the Laboratory of
Hepatobiliary and Pancreatic Surgery (Affiliated Hospital of Guilin
Medical University, Guilin, China). Cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (FBS; both Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
under 5% CO2 atmosphere at 37°C for 24 h.
Cytotoxicity study
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assays were used to study the proliferative effect of
SG-NCTD-LIP. HepG2 cells were plated in 96-well culture plates at
4,000 cells/well. After overnight incubation at 37°C, cells were
treated with varying concentrations (2.5, 5, 10, 20 and 40 µg/ml)
of SG-modified blank liposomes (SG-LIP), NCTD solution, liposomes
loaded with NCTD (NCTD-LIP) or SG-NCTD-LIP and were incubated for
48 h at 37°C. MTT (Sigma-Aldrich; Merck KGaA) solution (20 µl; 5
mg/ml) was added to each well. Following 4 h incubation at 37°C,
the medium was replaced with 150 µl dimethyl sulfoxide to dissolve
formazan crystals. Optical densities (ODs) were measured at 490 nm
with an ELISA reader. The half-maximal inhibitory concentration
(IC50) was calculated using SPSS (version 22.0; IBM
Corp., Armonk, NY, USA). The inhibition ratio (IR) was calculated
as follows: IR=1-OD of cells treated with sample/OD of culture
medium.
Statistical analysis
Data were analyzed using SPSS software (version
22.0; IBM Corp., Armonk, NY, USA) and results are expressed as mean
± standard deviation. One-way analysis of variance followed by a
Fisher's Least Significant Difference post-hoc test was used to
assess the significance of the differences among various groups.
Correlation coefficient (R) is a variable that demonstrated the
degree of linear correlation between variables. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Effect of different preparation
variables on the EE of SG-NCTD-LIP
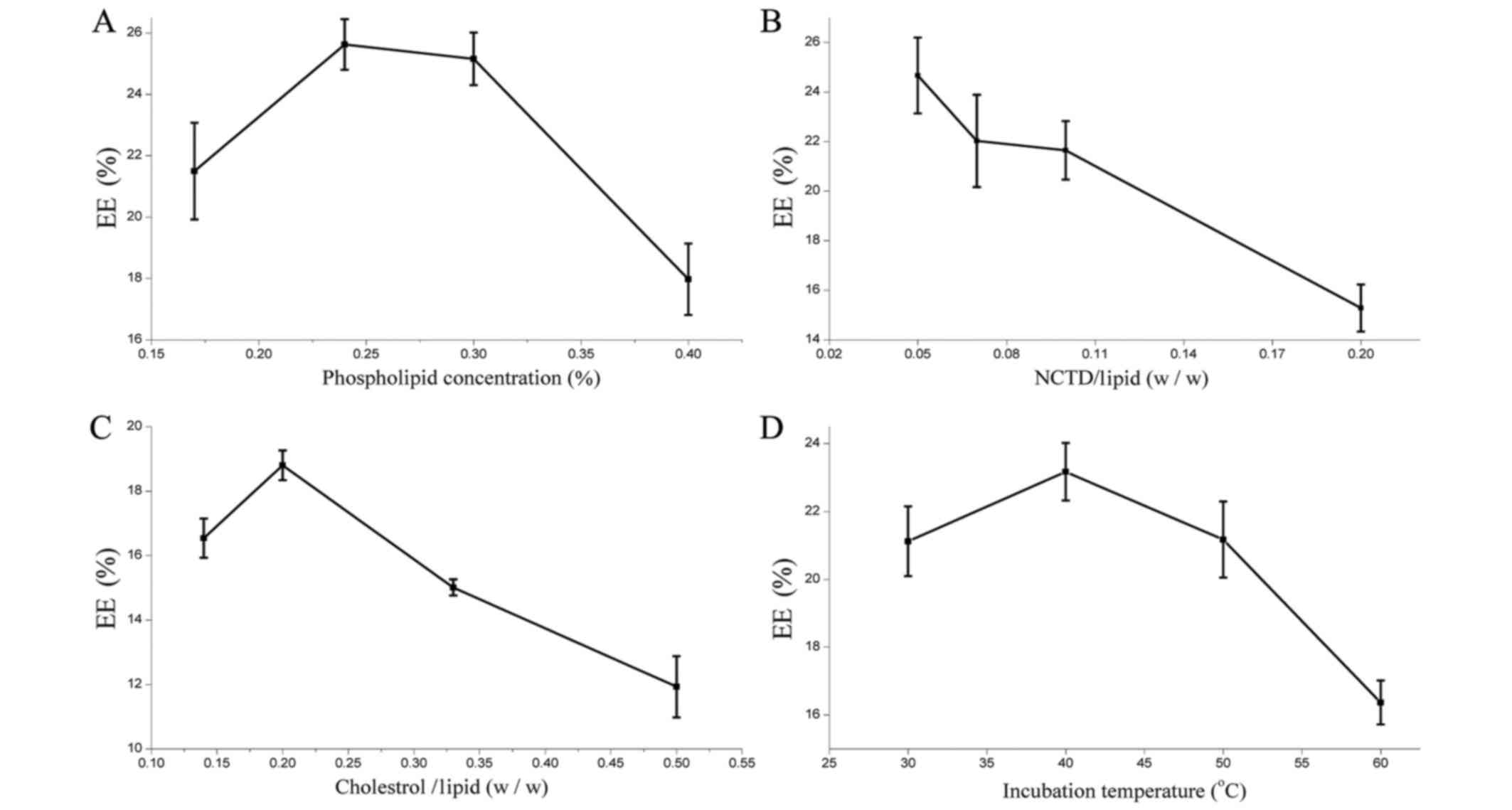
The results presented in Fig. 4A identified the highest EE at 0.24%
phospholipid concentration. However, excessively high phospholipid
resulted in the aggregation of phospholipids and decreased EE. It
was revealed that the EE of SG-NCTD-LIP was lowered from 25 to 16%
when increasing the amount of NCTD; the highest EE was observed for
a 1:20 NCTD-phospholipid ratio (Fig.
4B). Cholesterol is a lipid used to improve liposome stability
in vitro and in vivo (40). The results indicated that the highest
EE was achieved at a 1:5 cholesterol-phospholipid mass ratio
(Fig. 4C). At higher ratios,
cholesterol may induce rapid vesicle aggregation. Its rigidity
restricts the lipid bilayer flexibility and prevents NCTD
accumulation inside the vesicle. The optimum incubation temperature
was determined to be 40°C (Fig.
4D).
Optimization of the SG-NCTD-LIP
formulation by orthogonal design
Four factors, NCTD-phospholipid mass ratio (factor
A), phospholipid concentration (factor B), incubation temperature
(factor C) and cholesterol-phospholipid mass ratio (factor D) were
selected as main influencing factors. Each factor was studied at
three levels and the EE was selected as the investigated indicator
of the formulation. The orthogonal factors and levels are presented
in Table I. Nine formulations were
tested, according to the L9 (34) orthogonal table.
Table II displays the orthogonal
test results and the variance analysis is presented in Table III. According to the extremum
values (R) in Table II, the
importance of the factors may be ranked as A>B>C>D. The
optimum formulation composition was identified as A1B1C2D3, with
1:5 NCTD-phospholipid mass ratio, 0.4% phospholipid and 1:7
cholesterol-phospholipid mass ratio at an incubation temperature of
50°C. The analysis of variance indicated that factors A, B and C
had a significant impact on the experimental outcome (Table III). According to the F-value, the
impact of the factors may be scored as A>B>C>D, which
supports earlier findings. Preparation and analysis of the optimal
formulation mentioned above were repeated three times and the EE
was 27.80±2.18%. Prior to optimization of the NCTD-LIP formulation,
literature was consulted (41–44) and
combined with preliminary experiments, which investigated the
effect of different masses of SG on the size, morphology, EE and
stability of liposomes. Following this, 0.04% SG was identified as
an appropriate amount of drug to be added, without affecting the
formulation parameters of the liposome.
 | Table I.Factors and levels for optimization
using orthogonal design. |
Table I.
Factors and levels for optimization
using orthogonal design.
|
| Factor |
|---|
|
|
|
|---|
| Level | A | B (%) | C (°C) | D |
|---|
| 1 | 1:5 | 0.040 | 40 | 1:3 |
| 2 | 1:15 | 0.025 | 50 | 1:5 |
| 3 | 1:30 | 0.015 | 60 | 1:7 |
 | Table II.Results of orthogonal testing. |
Table II.
Results of orthogonal testing.
| No. | A | B (%) | C (°C) | D | EE (%) |
|---|
| 1 | 1.00 | 1.00 | 1.00 | 1.00 | 25.88 |
| 2 | 1.00 | 2.00 | 2.00 | 2.00 | 23.40 |
| 3 | 1.00 | 3.00 | 3.00 | 3.00 | 22.65 |
| 4 | 2.00 | 1.00 | 2.00 | 3.00 | 27.63 |
| 5 | 2.00 | 2.00 | 3.00 | 1.00 | 20.26 |
| 6 | 2.00 | 3.00 | 1.00 | 2.00 | 23.96 |
| 7 | 3.00 | 1.00 | 3.00 | 2.00 | 17.20 |
| 8 | 3.00 | 2.00 | 1.00 | 3.00 | 15.53 |
| 9 | 3.00 | 3.00 | 2.00 | 1.00 | 17.72 |
| K1 | 71.93 | 70.71 | 65.37 | 63.86 | N/A |
| K2 | 71.85 | 59.19 | 68.75 | 64.56 | N/A |
| K3 | 50.45 | 64.33 | 60.11 | 65.81 | N/A |
| R | 21.48 | 11.52 | 8.64 | 1.95 | N/A |
 | Table III.Variance analysis. |
Table III.
Variance analysis.
| Source | S | f | MS |
F-statistic | P-value |
|---|
| A | 102.15 | 2 | 51.08 | 157.02 | <0.01 |
| B | 22.20 | 2 | 11.10 | 34.13 | <0.05 |
| C | 12.64 | 2 | 6.32 | 19.43 | <0.05 |
| D | 0.65 | 2 | 0.33 | 1.00 | >0.05 |
| Error | 0.65 | 2 | 0.33 | N/A | N/A |
SG-NCTD-LIP size and morphology
Nanoparticles, between 70–200 nm, function as drug
delivery systems and are usually in the circulation for longer than
free drugs (45,46). In addition, such nanoparticles escape
RES clearance, while larger particles (>200 nm) may be removed
by the system. A small particle size (<200 nm), below the pore
size of leaky vasculatures, along with poor lymphatic drainage may
provide a suitable condition for the accumulation and localization
in the tumor (47). The average
particle size at the optimal formulation after 3 months of storage
at 4°C was 87.5 nm with a PDI of 0.151 (Fig. 5). The morphological character of
SG-NCTD-LIPs was evaluated by TEM. Liposomes were spherical, no
aggregation or fusion occurred and the size was ~100 nm.
Stability of liposomes
SG-NCTD-LIPs
Particle size and EE were evaluated following 3
months of storage at 4°C to study stability. Initially, the average
particle size was 87.5 nm and EE was 27.80%. There were no
significant changes in the average particle size and EE following 3
months at 4°C, the average particle size was 82.9 nm and EE was
27.28%. The SG-NCTD-LIP stability was attributed to the presence of
cholesterol, which increased the rigidity of the liposomal membrane
and reduced permeability (48). The
results demonstrated that SG-NCTD-LIPs were stable at 4°C for 3
months. However, the biological efficacy of SG-NCTD-LIPs following
storage was not evaluated.
In vitro release of NCTD from
SG-NCTD-LIP
The in vitro release rate evaluation
describes an essential step for drug development and quality
control, which may reflect on the in vivo drug performance.
Q is used to denote the cumulative release percentage. The
release rate of NCTD from SG-NCTD-LIP was significantly decreased
compared with the free NCTD solution 1.5–12 h after incubation
(Fig. 6). SG-NCTD-LIP may prolong
the release of NCTD from liposomes. The release process of NCTD
from SG-NCTD-LIP may be divided into two phases: In the first 4 h,
the release rate was high, with 65% of the total dose released.
Unencapsulated NCTD and NCTD coordinating to the surface of the
lipid bilayer may be responsible for the rapid release. This phase
was defined as a rapid release phase. It was followed by a steady
release, known as the slow release phase. To investigate the
release properties of SG-NCTD-LIP, release data were fitted using
various release models, including zero-order, first-order, Higuchi
(49) and Weibull (50) kinetic models, and the optimal fit was
determined by correlation coefficient. The fitting equations of the
release curves are presented in Table
IV. The results indicated that the release from SG-NCTD-LIP
follows a first order model.
 | Table IV.Release curve-fitting equations of
norcantharidin loaded liposomes modified with stearyl
glycyrrhetinate. |
Table IV.
Release curve-fitting equations of
norcantharidin loaded liposomes modified with stearyl
glycyrrhetinate.
| Model | Fitting
equation | R |
|---|
| Zero-order |
Q=0.0972t+0.2303 | 0.9190 |
| First order |
ln(1-Q)=−0.3336t−0.2110 | 0.9902 |
| Higuchi |
Q=0.3732t1/2+0.0350 | 0.9764 |
| Weibull |
ln(1/1-Q)=0.6040
lnt+0.7481 | 0.9561 |
In vitro cytotoxicity study
The cytotoxicity of SG-LIP, free NCTD, NCTD-LIP and
SG-NCTD-LIP towards HepG2 cells was evaluated using the MTT
cytotoxicity assay. SG-LIP was assessed as a control and the
concentration of SG in SG-LIP was equal to its concentration in
SG-NCTD-LIP. Following incubation for 48 h, an IR value of ~6.35%
was determined for SG-LIP, indicating no significant cytotoxicity
against HepG2 cells (data not shown). The inhibitory effect of the
samples increased with the NCTD concentration; all treatments
revealed dose-dependent cytotoxic activity (Fig. 7). NCTD-LIP and SG-NCTD-LIP
demonstrated significantly increased toxicity compared with free
NCTD. In addition, when NCTD concentration was 5, 10 and 20 µg/ml,
SG-NCTD-LIP significantly increased cytotoxicity compared with
NCTD-LIP after 48 h incubation. The IC50 of SG-NCTD-LIP,
NCTD-LIP and free NCTD were 16.93, 24.03 and 49.79 µg/ml,
respectively. SG-NCTD-LIP was 1.42-fold more cytotoxic compared
with NCTD-LIP. SG modification increased the cytotoxicity of the
liposomes, which may be associated with the interaction between SG
exposed on the liposome surface and GA receptors on the cell
membrane.
Conclusions
SG-NCTD-LIP was successfully prepared using the
ethanol injection method and the determined properties, including
particle size, EE, release profile and stability, may meet the
potential requirements of liver cancer therapy based on the in
vitro experiments, which overcome the limitations of
conventional chemotherapy by improving the bioavailability and
stability of the drug molecules, and minimizing side effects by
site-specific and targeted delivery of the drugs (51–53). The
in vitro cytotoxicity study confirmed that SG modification
of NCTD-LIP enhanced the inhibitory effects on hepatoblastoma
cells. Therefore, SG-NCTD-LIP may be a potential drug delivery
system for NCTD-targeted liver cancer therapy. Future experiments
may include cytotoxicity studies against HepG2 cells compared with
L02 normal liver cells, to confirm the safety and efficacy of
SG-NCTD-LIP, further uptake studies and in vivo
experiments.
Acknowledgements
Not applicable.
Funding
The present study was funded by grants from the
National Natural Science Foundation of China (grant no. 81560653)
and the Natural Science Foundation of Guangxi Province of China
(grant no. 2016GXNSFAA380081).
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available due to the patent
application but are available from the corresponding author on
reasonable request.
Authors' contributions
WW was involved in the design of the study. JZ and
WZ performed all experiments. DW performed the in vitro
cytotoxicity studies. SL performed statistical analyses. All
authors participated in manuscript preparation and involved in the
discussion of the results. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bei YY, Chen XY, Liu Y, Xu JY, Wang WJ, Gu
ZL, Xing KL, Zhu AJ, Chen WL, Shi LS, et al: Novel
norcantharidin-loaded liver targeting chitosan nanoparticles to
enhance intestinal absorption. Int J Nanomedicine. 7:1819–1827.
2012.PubMed/NCBI
|
|
2
|
Chen YC, Chang SC, Wu MH, Chuang KA, Wu
JY, Tsai WJ and Kuo YC: Norcantharidin reducedcyclins and cytokines
production in human peripheral blood mononuclearcells. Life Sci.
84:218–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei CM, Wang BJ, Ma Y, Sun ZP, Li XL and
Guo RC: Pharmacokinetics and biodistributionof 3H-norcantharidin in
mice. Yao Xue Xue Bao. 42:516–519. 2007.(In Chinese). PubMed/NCBI
|
|
4
|
Chen YN, Chen JC, Yin SC, Wang GS, Tsauer
W, Hsu SF and Hsu SL: Effect ormechanisms of norcantharidin-induced
mitotic arrest and apoptosis inhuman hepatoma cells. Int J Cancer.
100:158–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCluskey A and Sakoff JA: Small molecule
inhibitors of serine/threonine protein phosphatases. Mini Rev Med
Chem. 1:43–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng LC, Yang MD, Kuo CL, Lin CH, Fan MJ,
Chou YC, Lu HF, Huang WW, Peng SF and Chung JG:
Norcantharidin-induced apoptosis of ags human gastric cancer cells
through reactive oxygen species production, and caspase- and
mitochondria-dependent signaling pathways. Anticancer Res.
36:6031–6042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Li G, Ma X, Wang Y, Liu G, Feng
L, Zhao Y, Zhang G, Wu Y, Ye X, et al: Norcantharidin enhances
ABT-737-induced apoptosis in hepatocellular carcinoma cells by
transcriptional repression of Mcl-1. Cell Signal. 24:1803–1809.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan XY, Zhai XF, Jiang YP, Han T, Zhang QY
and Xin HL: Antimetastatic effects of norcantharidin on
hepatocellular carcinoma cells by up-regulating FAM46C expression.
Am J Transl Res. 9:155–166. 2017.PubMed/NCBI
|
|
9
|
Xiong X, Wu M, Zhang H, Li J, Lu B, Guo Y,
Zhou T, Guo H, Peng R, Li X, et al: Atg5 siRNA inhibits autophagy
and enhances norcantharidin-induced apoptosis in hepatocellular
carcinoma. Int J Oncol. 47:1321–1328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin X, Zhang B, Zhang K, Zhang Y, Wang J,
Qi N, Yang S, He H and Tang X: Preclinical evaluations of
norcantharidin-loaded intravenous lipid microspheres with low
toxicity. Expert Opin Drug Deliv. 9:1449–1462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie J, Zhang Y, Hu X, Lv R, Xiao D, Jiang
L and Bao Q: Norcantharidin inhibits Wnt signal pathway via
promoter demethylation of WIF-1 in human non-small cell lung
cancer. Med Oncol. 32:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu MC, Liu L, Wang XR, Shuai WP, Hu Y,
Han M and Gao JQ: Folate receptor-targeted liposomes loaded with a
diacid metabolite of norcantharidin enhance antitumor potency for
H22 hepatocellular carcinoma both in vitro and in vivo. Int J
Nanomedicine. 11:1395–1412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Q and Sun M:
Poly(lactide-co-glycolide) nanoparticles as carriers for
norcantharidin. Materials Science and Engineering: C. 29:708–713.
2009. View Article : Google Scholar
|
|
14
|
Ding XY, Hong CJ, Liu Y, Gu ZL, Xing KL,
Zhu AJ, Chen WL, Shi LS, Zhang XN and Zhang Q: Pharmacokinetics,
tissue distribution, and metabolites of a
polyvinylpyrrolidone-coated norcantharidin chitosan nanoparticle
formulation in rats and mice, using LC-MS/MS. Int J Nanomedicine.
7:1723–1735. 2012.PubMed/NCBI
|
|
15
|
Yan D, Ni LK, Chen HL, Chen LC, Chen YH
and Cheng CC: Amphiphilic nanoparticles of
resveratrol-norcantharidin to enhance the toxicity in zebrafish
embryo. Bioorg Med Chem Lett. 26:774–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding XY, Hong CJ and Zhou X: Mechanism of
Polyvinylpyrrolidone-coated norcantharidin chitosan nanoparticle.
Current Nanoscience. 9:401–406. 2013. View Article : Google Scholar
|
|
17
|
Torchilin VP: Recent advances with
liposomes as pharmaceutical carriers. Nat Rev Drug Discov.
4:145–160. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bangham AD, Standish MM and Watkins JC:
Diffusion of univalent ions across the lamellae of swollen
phospholipids. J Mol Biol. 13:238–252. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irie T, Watarai S and Kodama H: Humoral
immune response of carp (Cyprinus carpio) induced by oral
immunization with liposome-entrapped antigen. Dev Comp Immunol.
27:413–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirby C, Clarke J and Gregoriadis G:
Effect of the cholesterol content of small unilamellar liposomes on
their Stability in vivo and in vitro. Biochem J. 186:591–598. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Gu FX, Chan JM, Wang AZ, Langer
RS and Farokhzad OC: Nanoparticles in medicine: Therapeutic
applications and developments. Clin Pharmacol Ther. 83:761–769.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ara MN, Matsuda T, Hyodo M, Sakurai Y,
Hatakeyama H, Ohga N, Hida K and Harashima H: An aptamer ligand
based liposomal nanocarrier system that targets tumor endothelial
cells. Biomaterials. 35:7110–7120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang H, O'Donoghue MB, Liu H and Tan W: A
liposome-based nanostructure for aptamer directed delivery. Chem
Commun (Camb). 46:249–251. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moghimi SM, Hunter AC and Murray JC:
Long-circulating andtarget-specific nanoparticles: Theory to
practice. Pharmacol Rev. 53:283–318. 2001.PubMed/NCBI
|
|
25
|
Maeda H: Tumor-selective delivery of
macromolecular drugs via the EPReffect: Background and future
prospects. Bioconjug Chem. 21:797–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kibria G, Hatakeyama H, Ohga N, Hida K and
Harashima H: Dual-ligand modification of PEGylated liposomes shows
better cell selectivity and efficient gene delivery. J Control
Release. 153:141–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darvishi B, Manoochehri S,
Esfandyari-Manesh M, Samadi N, Amini M, Atyabi F and Dinarvand R:
Enhanced cellular cytotoxicity and antibacterial activity of
18-β-Glycyrrhetinic Acidby Albumin-conjugated PLGA Nanoparticles.
Drug Res. 65:617–623. 2015. View Article : Google Scholar
|
|
28
|
Kuang P, Zhao W, Su W, Zhang Z, Zhang L,
Liu J, Ren G and Wang X: 18β-glycyrrhetinic acid inhibits
hepatocellular carcinoma development by reversing hepatic stellate
cell-mediated immunosuppression in mice. IntJ Cancer.
132:1831–1841. 2013. View Article : Google Scholar
|
|
29
|
Manns MP, Wedemeyer H, Singer A,
Khomutjanskaja N, Dienes HP, Roskams T, Goldin R, Hehnke U and
Inoue H: European SNMC Study Group: Glycyrrhizin in patients who
failed previous interferon alpha-basedtherapies: Biochemical and
histological effects after 52 weeks. J Viral Hepat. 19:537–546.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang ZH, Li T, Chang LL, Zhu H, Tong YG,
Chen XP, Wang YT and Lu JJ: Glycyrrhetinic Acid triggers a
protectiveautophagy by activation of extracellular regulated
protein kinases in hepatocellular carcinoma cells. J Agric Food
Chem. 62:11910–11916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Irie A, Fukui T, Negishi M, Nagata N and
Ichikawa A: Glycyrrhetinic acid bound to 11 beta-hydroxysteroid
dehydrogenase in rat liver microsomes. Biochim Biophys Acta.
1160:229–234. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Negishi M, Irie A, Nagata N and Ichikawa
A: Specific binding of glycyrrhetinic acid to the rat liver
membrane. Biochim Biophys Acta. 1066:77–82. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian Q, Zhang CN, Wang XH, Wang W, Huang
W, Cha RT, Wang CH, Yuan Z, Liu M, Wan HY and Tang H:
Glycyrrhetinic acid-modified chitosan/poly (ethylene glycol)
nanoparticles for liver-targeted delivery. Biomaterials.
31:4748–4756. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian Q, Wang XH, Wang W, Zhang CN, Wang P
and Yuan Z: Self-assembly and liver targeting of sulfated chitosan
nanoparticles functionalized with glycyrrhetinic acid.
Nanomedicine. 8:870–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Zhang M, Ji J, Fang X, Pan X,
Wang Y, Wu C and Chen M: Glycyrrhetinic acid-mediated polymeric
drug delivery targeting the acidic microenvironment of
hepatocellular carcinoma. Pharm Res. 32:3376–3390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chintharlapalli S, Papineni S, Jutooru I,
McAlees A and Safe S: Structure-dependent activity of
glycyrrhetinic acid derivatives asperoxisome proliferator-activated
receptor {gamma} agonists in colon cancer cells. Mol Cancer Ther.
6:1588–1598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marzban E, Alavizadeh SH, Ghiadi M,
Khoshangosht M, Khashayarmanesh Z, Abbasi A and Jaafari MR:
Optimizing the therapeutic efficacy of cisplatin PEGylated
liposomes via incorporation of different DPPG ratios: In vitro and
in vivo studies. Colloids Surf B Biointerfaces. 136:885–891. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chi L, Na MH, Jung HK, Vadevoo SM, Kim CW,
Padmanaban G, Park TI, Park JY, Hwang I, Park KU, et al: Enhanced
delivery of liposomes to lung tumor through targeting interleukin-4
receptor on both tumor cells and tumor endothelial cells. J Control
Release. 209:327–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu D, Xing J, Xiong F, Yang F and Gu N:
Preparation and in vivo safety evaluations of
antileukemichomoharringtonine-loaded PEGylated liposomes. Drug Dev
Ind Pharm. 43:652–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kirby C, Clarke J and Gregoriadis G:
Effect of the cholesterol content of small unilamellar liposomeson
their stability in vivo and in vitro. Biochem J. 186:591–598. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Xu H, Ke X and Tian J: The
anti-tumor performance of docetaxel liposomes surface-modified with
glycyrrhetinic acid. J Drug Targeting. 20:467–473. 2012. View Article : Google Scholar
|
|
42
|
Chen J, Jiang H, Wu Y, Li Y and Gao Y: A
novel glycyrrhetinic acid-modified oxaliplatin liposome for
liver-targeting and in vitro/vivo evaluation. Drug Des Dev Ther.
9:2265–2275. 2015.
|
|
43
|
Cheng M, Gao X, Wang Y, Chen H, He B, Xu
H, Li Y, Han J and Zhang Z: Synthesis of glycyrrhetinic
acid-modified chitosan5-fluorouracil nanoparticles and its
inhibition of liver cancercharacteristics in vitro and in vivo. Mar
Drugs. 11:3517–3536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tian Q, Wang XH, Wang W, Zhang CN, Wang P
and Yuan Z: Self-assembly and liver targeting of sulfated
chitosannanoparticles functionalized with glycyrrhetinic acid.
Nanomedicine. 8:870–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ossipov DA: Nanostructured hyaluronic
acid-based materials for active delivery tocancer. Expert Opin Drug
Deliv. 7:681–703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang T, Choi MK, Cui FD, Kim JS, Chung SJ,
Shim CK and Kim DD: Preparationand evaluation of paclitaxel-loaded
PEGylated immunoliposome. J Control Release. 120:169–177. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ravar F, Saadat E, Gholami M,
Dehghankelishadi P, Mahdavi M, Azami S and Dorkoosh FA: Hyaluronic
acid-coated liposomes for targeted delivery of paclitaxel, in-vitro
characterization and in-vivo evaluation. J Control Release.
229:10–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan MH, Xu SY, Xia SQ and Zhang XM:
Preparation of salidroside nano-liposomes by ethanol injection
method and in vitro release study. Eur Food Res Technol.
227:167–174. 2008. View Article : Google Scholar
|
|
49
|
Higuchi T: Mechanism of sustained-action
medication. Theoretical analysis of rate of release of solid drugs
dispersed in solid matrices. J Pharm Sci. 52:1145–1149. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weibull W: A statistical distribution of
wide applicability. J Appl Mech. 18:293–297. 1951.
|
|
51
|
Dutta R and Mahato RI: Recent advances in
hepatocellular carcinoma therapy. Pharmacol Ther. 173:106–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sarfraz M, Afzal A, Raza SM, Bashir S,
Madni A, Khan MW, Ma X and Xiang G: Liposomal co-delivered
oleanolic acid attenuates doxorubicin-induced multi-organ toxicity
in hepatocellular carcinoma. Oncotarget. 8:47136–47153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Himanshu P, Radha R and Vishnu A: Liposome
and their applications in cancer therapy. Braz Arch Boil Technol.
59:e161504772016.
|