Introduction
Radiotherapy is a common treatment method for a
number of types of human cancer, with approximately half of all
patients requiring radiotherapy for palliative or curative purposes
(1). However, patients undergoing
radiotherapy may develop adverse side-effects, including
hematological toxicity, cytopenia, immune suppression and mucosal
damage (2). Under ideal conditions,
tumor tissue would receive a large dose of radiation, while normal
healthy tissues would be protected from radiation injury.
Therefore, the pathogenic processes induced by ionizing radiation
and non-toxic radioprotective compounds that may protect normal
tissues against radiation injury, are currently being extensively
researched (3–5). Several compounds, including cysteine,
aminothiol and cytokines, are known radioprotectors (6–8).
Crescenti et al (9) reported
that selenium, zinc and magnesium may also have radioprotective
properties. Nishimura et al (10) reported that chitosan increased the
hematocrit and survival rate in mice exposed to sublethal X-ray
irradiation. Emami et al (11) reported that zinc exerted a protective
effect against lethality in irradiated mice.
Lactoferrin (LF) is an 80 kDa iron-binding
glycoprotein, which is a component of exocrine secretions,
including milk and saliva and is also present in neutrophil
granules (12). LF has been reported
to serve a role in host defense and has various biological
properties, including antimicrobial effects and modulation of cell
growth (13,14). In addition to serving a key role in
immune homeostasis, LF also reduces oxidative stress and may
control excessive inflammatory responses (13,15,16).
Recently, Sriramoju et al (17) reported that LF exerts various
beneficial effects on humans and animals, including inhibition of
carcinogenesis and prevention of drug-induced toxicity. Irradiated
mice on an LF diet exhibited a significantly higher survival rate
compared with mice fed a standard diet (18). The prevention of chemotherapy-induced
ovarian disorders in mice receiving oral LF has also been reported
(19). In addition, the use of a gel
containing LF in patients with oral cancer who were treated with
radiotherapy, increased salivary secretion, inhibited xerostomia
and improved oral bacterial flora (20).
However, studies on the radioprotective effects of
LF are limited. The aim of the present study was to investigate
in vivo whether LF may enhance resistance to high doses of
ionizing radiation in mice and to elucidate the possible mechanisms
of action. To determine this, the survival ratio and hematopoietic
system toxicity in mice receiving whole-body, high does (7.0 Gy)
irradiation were assessed.
Materials and methods
Animals and irradiation
Male Balb/c mice (age, 6 weeks; weight, 20–23 g)
were purchased from Unilever (Shanghai, China). All mice had free
access to water and food; they were kept in a room maintained at
60±10% relative humidity and 20±2°C with a 12 h light/dark cycle.
There were 5 mice per cage. A total of 60 mice were randomly
assigned into 3 groups (n=20 per group) as follows: i) Control
(non-irradiated mice fed a standard diet without LF); ii) IR
(whole-body irradiated mice fed a standard diet without LF); and
iii) IR+LF (whole-body irradiated mice fed a diet containing 0.1%
bovine LF; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The mice
in the IR and IR+LF groups were exposed to a sublethal radiation
dose (7.0 Gy). The control mice were sham irradiated. The mice were
irradiated using a 6-MV linear accelerator at a dose rate of 0.865
Gy/min (PRIMUS High Energy; Siemens AG, Munich, Germany). The mice
were fed for 7 days prior to irradiation and for 30 continuous days
following irradiation. The study protocol was approved by the
Ethics Committee of Qianfoshan Hospital of Shandong Province
(Jinan, China).
Peripheral blood cell counts
Blood was collected from the mice via the tail vein
in EDTA tubes (BD Biosciences, Franklin Lakes, NJ, USA) on days 0,
1, 2, 3, 9, 14, 19 and 29 following irradiation. The blood was
centrifuged at 1,000 × g for 20 min at 20±2°C and evaluated using
an automated hematology analyzer (pocH-100i; Sysmex Corporation,
Kobe, Japan) to provide the complete blood cell counts. The
measurements included leukocyte, erythrocyte and platelet (PLT)
counts, as well the hemoglobin. The normal references value of
hematological parameters were described previously (21).
Lymphocyte isolation and comet
assay
A volume of 0.15 ml whole blood was layered onto the
lymphocyte separation medium (cat. no. MRGMA0; R&D Systems,
Inc., Minneapolis, MN, USA) and centrifuged for 2 min at 3,500 × g
at 20±2°C. The lymphocytes were subsequently transferred to a 1.5
ml tube containing 1.2 ml 0.1 M PBS and centrifuged for 5 min at
2,000 × g at 20±2°C. The lymphocytes were washed twice with PBS.
The cells were then suspended in PBS and the density was adjusted
to 5–6×104/ml. A comet assay was performed under neutral
conditions as described by Banath et al (22), with a slight modification.
Specifically, special comet slides were used as opposed to
conventional slides. All comet images were analyzed using CASP Lab
software (version 1.2.3b2; CASPLab, Wroclaw, Poland) (23) and the percentage of DNA in the Olive
Tail Moment (OTM) was recorded to characterize the lymphocytic DNA
damage.
Biochemical analysis
The livers were removed, fixed in 4%
paraformaldehyde solution at room temperature for 20 min and ground
30 days after radiation (5 mice per group). The obtained cells were
washed with PBS and suspended in EDTA. Superoxide dismutase (SOD)
and malondialdehyde (MDA) activities in the liver were analyzed
using SOD and MDA assay kits (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
(≥5 mice per group at each time point). Statistical analysis was
performed using one-way analysis of variance with a post hoc
Tukey's test (multiple comparison test) to determine the
significance of differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference. SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for
the analyses.
Results
LF increases the survival rate of mice
exposed to irradiation
In the present study, mice in the IR and IR+LF
groups were exposed to 7 Gy radiation. The survival rate was
monitored on days 1–30 following irradiation (Fig. 1). Kaplan-Meier analysis indicated
that survival rates were significantly higher in the IR+LF group
compared with the IR group between day 12 and 30 (P<0.05). On
day 30 the survival rate of the IR+LF group was 50% and the
survival rate of the IR group was 33%. The survival rate in the
IR+LF group was significantly higher compared with that of the IR
group (P<0.05). The differences between the IR+LF group and the
control were also statistically significant (P<0.05). These
results suggest that LF increased the survival rate of mice
following exposure to radiation.
LF reduces the radiation-induced
decrease in body weight
The body weights of the mice were measured at
various time points following irradiation and the mean weight ±
standard deviation was calculated among surviving mice (Fig. 2). The results revealed that the body
weights significantly increased in the control group, remained
mostly constant in the IR+LF group and decreased slightly in the IR
group between day 8 and 10 after irradiation. Statistical analysis
indicated that body weight was significantly higher in the IR+LF
group compared with the IR group between days 20 and 30
(P<0.05). Furthermore, the body weights of the mice in the
control group were significantly greater compared with the IR+LF
group on days 20 and 25 (P<0.05). Furthermore, on day 30, no
significant differences in body weight were identified between the
control group and the IR+LF group.
LF enhances hematological repopulation
following whole-body irradiation
Hematological parameters were recorded following
irradiation, including changes in the leukocyte count (Fig. 3). The leukocyte count in the IR+LF
group exhibited a progressive decline to 1.9×109/l on
day 3. In addition, the leukocyte count appears to stay steady
between days 9 and 14 in the IR+LF group at ~2.6×103/µl.
On day 29 the leukocyte count of the IR+LF mice had stabilized to
within the normal range (7.6×109−10.9×109/l)
(21). The significant difference
was identified between the control group and the IR+LF group except
on day 29 (P<0.05). However, the leukocyte count of the IR mice
remained low (0.35×109/l) until day 14. Between day 9
and 29, the leukocyte counts in the IR group were significantly
lower compared with the IR+LF group (P<0.05).
In the IR+LF group the erythrocyte count decreased
to 4.6×1012/l on day 9 and gradually recovered to a
value of 6.47×1012/l on day 14 (Fig. 4). No significant difference was
identified between the control group and the IR+LF group on day 29.
In the IR group the erythrocyte count decreased to
2.17×1012/l on day 9. From day 9, the erythrocyte count
in the IR+LF group was significantly greater compared with the IR
group (P<0.05). The control group was significantly greater
compared with the IR group between day 3 and 29 (P<0.05). These
results indicate that LF improved erythrocyte repopulation in the
mice.
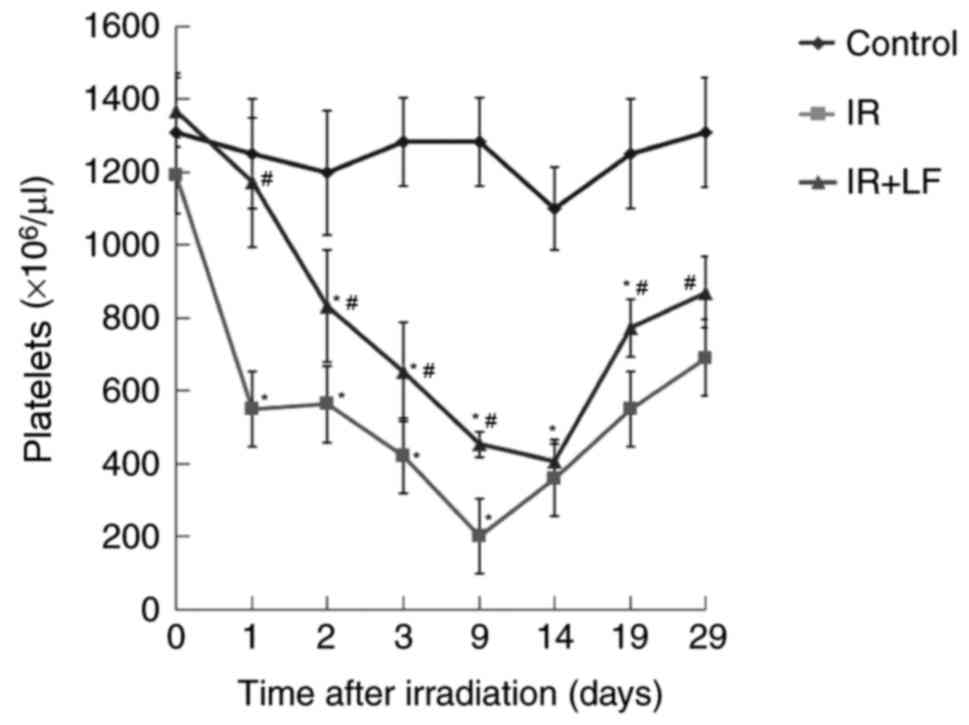
Following a decrease post irradiation, the PLT count
in the IR+LF group was restored to within a normal range on day 19
(Fig. 5) (21). However, in the IR group, the PLT
count decreased to a minimum value at day 9 and slowly increased to
a normal level (21) by day 29. The
IR+LF group was significantly greater compared with the IR group
between day 1 aand 9, and 19 and 29 (P<0.05). The control group
was significantly greater compared with the IR and IR+LF group
(P<0.05). No significant difference was identified between the
control group and the IR+LF group on day 29. These results indicate
that LF improved PLT repopulation in the mice.
The results also demonstrated that IR induced a
significant decrease in the level of hemoglobin between days 7 and
21 following irradiation. Post irradiation, the hemoglobin levels
in the IR+LF and IR groups were significantly lower compared with
the control group (P<0.05; Fig.
6). The hemoglobin level recovered faster and was consistently
increased in the IR+LF group compared with the IR group. The
hemoglobin levels in the IR+LF group were significantly higher
compared with the IR group (P<0.05). These results indicate that
LF significantly enhanced the recovery of hemoglobin during the
experimental period compared with the IR group.
LF increases antioxidant capacity
The MDA level is associated with lipid peroxidation
in the liver (24). The MDA level in
hepatic tissue was significantly lower in the IR+LF group compared
with the IR group, which suggests that the LF diet prevented
hepatic lipid peroxidation (Table
I). SOD activity indicates the generation of oxidative stress
(25). The protective response to
oxidative damage in the liver of IR mice decreased significantly
following irradiation compared with the control group. However, the
LF diet significantly prevented the change in SOD activity compared
with the IR group.
 | Table I.MDA level and SOD activity in hepatic
tissue. |
Table I.
MDA level and SOD activity in hepatic
tissue.
| Group | SOD (U/ml) | MDA (pmol/l) |
|---|
| Control | 41.25±0.41 | 4.31±0.02 |
| IR |
21.52±0.24a |
7.31±0.12a |
| IR+LF |
42.56±0.71b |
4.98±0.42b |
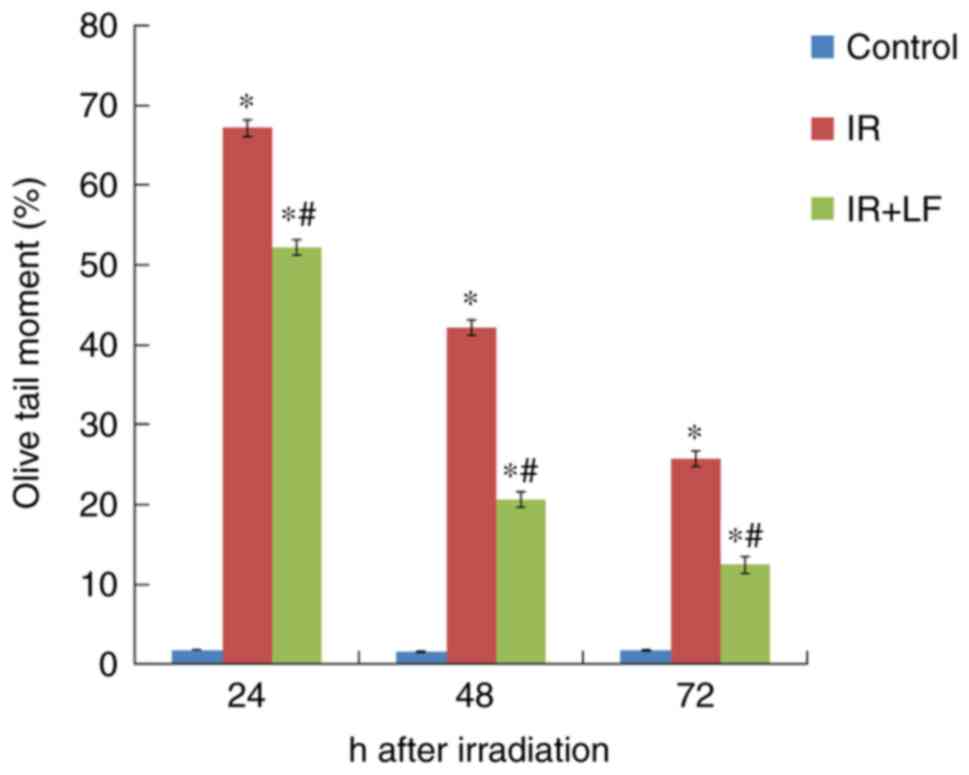
LF decreases the OTM of lymphocytes
following irradiation
Irradiation led to the breakage of DNA chains. The
OTM percentage 24, 48 and 72 h post irradiation in the IR+LF group
was significantly greater and lesser compared with the control and
IR groups, respectively (P<0.05; Fig.
7). Following unwinding, DNA was affected by the electric field
in the electrophoresis liquid, forming the distinctive comet tail
formation (Fig. 8).
Discussion
A number of substances with radioprotective
properties have been previously reported (26). Intraperitoneal injection of purified
ginseng extract following 6.5 Gy X-ray irradiation significantly
increased the 30 day survival rate in mice (27). In addition, Shigoka extract prepared
from Acathopanax senticosus was also reported to increase
the post-irradiation survival rate in mice (28). The aim of the present study was to
demonstrate the protective effects exerted by LF against
radiation-induced injury in mice. The results demonstrated that at
day 30 following irradiation the survival rate of the mice was 17%
higher in IR+LF group compared with the mice in the IR group,
demonstrating that an LF diet significantly improves survival
rates.
It has been previously established that the survival
rate of mice following exposure to a sublethal dose of radiation
depends, on the recovery of the hematopoietic system (29,30). To
determine whether LF protects mice from IR-induced hematopoietic
system injury, the mice were exposed to X-ray irradiation at a dose
of 7.0 Gy.
It is known that the number of leukocytes is
correlated with the radiation dose (31). The IR+LF group exhibited a rapid
increase in the leukocyte count from day 14 onwards and on day 29
the count was restored to normal levels. However, in the IR group,
the leukocyte count began to increase at day 14 in the IR group,
but the count remained at a lower level. These results indicate
that LF stimulated the recovery of leukocytes and exerted a
radioprotective effect.
In the IR group the PLT count exhibited an initial
decline following X-ray irradiation and on day 9 the count was at
its lowest level, however it returned to normal by day 29. The
IR+LF group exhibited a faster increase in PLTs compared with the
IR group and they recovered to near normal levels at day 19. It has
been previously reported that when infants were fed an
LF-supplemented infant formula, their hemoglobin value was
increased compared with the group fed a conventional infant formula
(32); similar results were also
observed in female marathon runners (33). In the present study, the red blood
cell count and hemoglobin levels were increased in the IR+LF group
compared with the IR group following irradiation, which indicates
that LF exerted hematopoietic or radioprotective effects.
Radiation may increase the oxidative capacity and
induce damage to cellular molecules; previous biochemical studies
have been performed to define normal MDA and SOD levels in liver
tissue (34–36). The results of the present study
revealed that the MDA level in the hepatic tissue was significantly
lower in the IR+LF group compared with the IR group, while SOD
activity was significantly increased. These results reveal that LF
exerted a protective effect on cellular molecules against
radiation-induced oxidative damage.
The comet assay, which detects DNA damage, has been
widely used in radiation biology (37–40). The
comet assay is a rapid and sensitive microdosimetric technique,
particularly useful in radiation accidents (41). In the IR+LF and IR groups, the comet
assay was used to observe the degree of DNA damage by irradiation.
The IR group exhibited a substantial increase in DNA damage, even
at 30 days post irradiation, while the IR+LF mice exhibited
significantly reduced DNA damage. The present study demonstrated
that significant differences were identified between the IR group
and IR+LF group following irradiation. Therefore, the comet assay
demonstrated that LF effectively reduced radiation-induced DNA
injury.
In conclusion, the results of the present study
suggest that LF increases PLT and leukocyte counts and reduces DNA
damage in mice following high-dose irradiation. In the future LF
may have potential as a radioprotector to reduce the adverse
effects of radiotherapy. However, the exact mechanism of action of
LF has not yet been fully elucidated. Therefore, further studies
are required to determine whether radioscavenging or trapping is
involved in this effect and to clarify the value of LF within the
field of radiation protection.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong (grant no. 2010GSF10251), the
Natural Science Foundation of Shandong (grant no. ZR2014YL027), the
Natural Science Foundation of Inner Mongolia Autonomous Region of
China (grant no. 2016MS0814) and the National Natural Science
Foundation of China (grant no. 81760567).
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
LF fed the animals, collected blood from the mice
and was a major contributor in the writing of the manuscript. DG
performed the irradiation. DPD performed the histological
examination. HYD performed the superoxide dismutase and
malondialdehyde ELISAs. LQ analyzed the peripheral blood cells. JGL
performed the lymphocyte isolation and comet assays. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qianfoshan Hospital Affiliated to Shandong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jung J, Kim H, Yoon SM, Cho B, Kim YJ,
Kwak J and Kim JH: Targeting accuracy of image-guided stereotactic
body radiation therapy for hepatocellular carcinoma in real-life
clinical practice: In vivo assessment using hepatic parenchymal
changes on Gd-EOB-DTPA-enhanced magnetic resonance images. Int J
Radiat Oncol Biol Phys. S0360-3016:30811–30813. 2018.
|
|
2
|
Kavitha M, Mubeen K and Vijayalakshmi KR:
A study on Evaluation of efficacy of bethanechol in the management
of chemoradiation-induced xerostomia in oral cancer patients. J
Oral Maxillofac Pathol. 21:459–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szybalski W: Molecular events resulting in
radiation injury, repair and sensitization of DNA, Radiation
research. Supplement. 7:147–159. 1967.
|
|
4
|
Yazdi Keramati F, Monfared Shabestani A,
Tashakkorian H, Mahmoudzadeh A and Borzoueisileh S: Radioprotective
effect of Zamzam (alkaline) water: A cytogenetic study. J Environ
Radioact. 167:166–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kirakosyan G, Torgomyan H, Malakyan M,
Bajinyan S and Trchounian A: Protective effect of some amino acids
synthesized derivatives and their chelates on Escherichia coli
under X-ray irradiation. Indian J Biochem Biophys. 50:289–295.
2013.PubMed/NCBI
|
|
6
|
Greenberger JS, Clump D, Kagan V, Bayir H,
Lazo JS, Wipf P, Li S, Gao X and Epperly MW: Strategies for
discovery of small molecule radiation protectors and radiation
mitigators. Front Oncol. 1:592012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunwar A, Adhikary B, Jayakumar S, Barik
A, Chattopadhyay S, Raghukumar S and Priyadarsini KI: Melanin, a
promising radioprotector: Mechanisms of actions in a mice model.
Toxicol Appl Pharmacol. 264:202–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rostami A, Moosavi SA, Changizi V and
Ardakani Abbasian A: Radioprotective effects of selenium and
vitamin-E against 6MV X-rays in human blood lymphocytes by
micronucleus assay. Med J Islam Repub Iran. 30:3672016.PubMed/NCBI
|
|
9
|
Crescenti E, Croci M, Medina V, Sambuco L,
Bergoc R and Rivera E: Radioprotective potential of a novel
therapeutic formulation of oligoelements Se, Zn, Mn plus Lachesis
muta venom. J Radiat Res. 50:537–544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura Y, Kim HS, Ikota N, Arima H, Bom
HS, Kim YH, Watanabe Y, Yukawa M and Ozawa T: Radioprotective
effect of chitosan in sub-lethally X-ray irradiated mice. J Radiat
Res. 44:53–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emami S, Hosseinimehr SJ, Taghdisi SM and
Akhlaghpoor S: Kojic acid and its manganese and zinc complexes as
potential radioprotective agents. Bioorg Med Chem Lett. 17:45–48.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruni N, Capucchio MT, Biasibetti E,
Pessione E, Cirrincione S, Giraudo L, Corona A and Dosio F:
Antimicrobial activity of lactoferrin-related peptides and
applications in human and veterinary medicine. Molecules. 21:pii:
E752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baveye S, Elass E, Mazurier J, Spik G and
Legrand D: Lactoferrin: A multifunctional glycoprotein involved in
the modulation of the inflammatory process. Clin Chem Lab Med.
37:281–286. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berlutti F, Pantanella F, Natalizi T,
Frioni A, Paesano R, Polimeni A and Valenti P: Antiviral properties
of lactoferrin-a natural immunity molecule. Molecules.
16:6992–7018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inamori M, Togawa J, Matsumoto S, Harad K,
Matsuura M, Iida H, Akimoto K, Endo H, Nonaka T, Takahashi H, et
al: Protective effect of lactoferrin on acute acid reflux-induced
esophageal mucosal damage. Hepatogastroenterology. 61:1595–1600.
2014.PubMed/NCBI
|
|
16
|
Kruzel ML and Zimecki M: Lactoferrin and
immunologic dissonance: Clinical implications. Arch Immunol Ther
Exp (Warsz). 50:399–410. 2002.PubMed/NCBI
|
|
17
|
Sriramoju B, Kanwar RK and Kanwar JR:
Lactoferrin induced neuronal differentiation: A boon for brain
tumours. Int J Dev Neurosci. 41:28–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakai M, Matsushita T, Hoshino R, Ono H,
Ikai K and Sakai T: Identification of the protective mechanisms of
Lactoferrin in the irradiated salivary gland. Sci Rep. 7:97532017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horiuchi Y, Higuchi T, Tatsumi K, Takakura
K, Fujii S and Konishi I: Lactoferrin is associated with a decrease
in oocyte depletion in mice receiving cyclophosphamide. Fertil
Steril. 91 5 Suppl:S2069–S2078. 2009. View Article : Google Scholar
|
|
20
|
Nagy K, Urban E, Fazekas O, Thurzo L and
Nagy E: Controlled study of lactoperoxidase gel on oral flora and
saliva in irradiated patients with oral cancer. J Craniofac Surg.
18:1157–1164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bella LM, Fieri I, Tessaro FHG, Nolasco
EL, Nunes FPB, Ferreira SS, Azevedo CB and Martins JO: Vitamin D
modulates hematological parameters and cell migration into
peritoneal and pulmonary cavities in alloxan-diabetic mice. Biomed
Res Int. 2017:76518152017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banath JP, Fushiki M and Olive PL:
Rejoining of DNA single- and double-strand breaks in human white
blood cells exposed to ionizing radiation. Int J Radiat Biol.
73:649–660. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Końca K, Lankoff A, Banasik A, Lisowska H,
Kuszewski T, Góźdź S, Koza Z and Wojcik A: A cross-platform public
domain PC image-analysis program for the comet assay. Mutat Res.
534:15–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Mihi KA, Kenawy HI, El-Karef A,
Elsherbiny NM and Eissa LA: Naringin attenuates
thioacetamide-induced liver fibrosis in rats through modulation of
the PI3K/Akt pathway. Life Sci. 187:50–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jindal A, Mahesh R, Bhatt S and Pandey D:
Molecular modifications by regulating cAMP signaling and
oxidant-antioxidant defence mechanisms, produce antidepressant-like
effect: A possible mechanism of etazolate aftermaths of impact
accelerated traumatic brain injury in rat model. Neurochem Int.
111:3–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith TA, Kirkpatrick DR, Smith S, Smith
TK, Pearson T, Kailasam A, Herrmann KZ, Schubert J and Agrawal DK:
Radioprotective agents to prevent cellular damage due to ionizing
radiation. J Transl Med. 15:2322017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verma P, Jahan S, Kim TH and Goyal PK:
Management of radiation injuries by panax ginseng extract. J
Ginseng Res. 35:261–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jagetia GC and Baliga MS: Polyherbal
extract of septilin protects mice against whole body lethal dose of
gamma radiation. Phytother Res. 18:619–623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu C, Liu J, Hao Y, Gu Y, Yang Z, Li H
and Li R: 6,7,3′,4′-Tetrahydroxyisoflavone improves the survival of
whole-body-irradiated mice via restoration of hematopoietic
function. Int J Radiat Biol. 93:793–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li ZT, Wang LM, Yi LR, Jia C, Bai F, Peng
RJ, Yu ZY, Xiong GL, Xing S, Shan YJ, et al: Succinate ester
derivative of δ-tocopherol enhances the protective effects against
60Co γ-ray-induced hematopoietic injury through
granulocyte colony-stimulating factor induction in mice. Sci Rep.
7:403802017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Erexson GL, Kligerman AD, Bryant MF,
Sontag MR and Halperin EC: Induction of micronuclei by X-radiation
in human, mouse and rat peripheral blood lymphocytes. Mutat Res.
253:193–198. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
King JC Jr, Cummings GE, Guo N, Trivedi L,
Readmond BX, Keane V, Feigelman S and de Waard R: A double-blind,
placebo-controlled, pilot study of bovine lactoferrin
supplementation in bottle-fed infants. J Pediatr Gastroenterol
Nutr. 44:245–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koikawa N, Nagaoka I, Yamaguchi M, Hamano
H, Yamauchi K and Sawaki K: Preventive effect of lactoferrin intake
on anemia in female long distance runners. Biosci Biotechnol
Biochem. 72:931–935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yaribeygi H, Mohammadi MT and Sahebkar A:
Crocin potentiates antioxidant defense system and improves
oxidative damage in liver tissue in diabetic rats. Biomed
Pharmacother. 98:333–337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koc M, Taysi S, Buyukokuroglu Emin M and
Bakan N: The effect of melatonin against oxidative damage during
total-body irradiation in rats. Radiat Res. 160:251–255. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang B, Su Y, Ai G, Wang Y, Wang T and
Wang F: Involvement of peroxiredoxin I in protecting cells from
radiation-induced death. J Radiat Res. 46:305–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoffmann H and Speit G: Assessment of DNA
damage in peripheral blood of heavy smokers with the comet assay
and the micronucleus test. Mutat Res. 581:105–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Wang Y, DU L, Xu C, Cao J, Wang Q,
Liu Q and Fan F: Nested PCR for mtDNA-4977-bp deletion and comet
assay for DNA damage-a combined method for radiosensitivity
evaluation of tumor cells. Oncol Lett. 7:1083–1087. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Olive PL, Banáth JP and Durand RE:
Heterogeneity in radiation-induced DNA damage and repair in tumor
and normal cells measured using the ‘comet’ assay. 1990. Radiat
Res. 178:AV35–AV42. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seidel C, Lautenschlager C, Dunst J and
Muller AC: Factors influencing heterogeneity of radiation-induced
DNA-damage measured by the alkaline comet assay. Radiat Oncol.
7:612012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sirota NP and Kuznetsova EA: The comet
assay application in radiobiological investigations. Radiats Biol
Radioecol. 50:329–339. 2010.(In Russian). PubMed/NCBI
|