Introduction
Osteoarthritis (OA) is a disease characterized by
progressive chondrocyte degeneration, synovial hyperplasia in
joints, narrowing of the joint space and dysregulation of
extracellular matrix metabolism (1).
OA primarily affects the knee, hip and shoulder joints and the main
symptoms include joint pain, swelling, joint deformity and limited
mobility (2). The pathogenesis of OA
is associated with aging, obesity, inflammation, immunity, genetics
and many other factors (3).
Cartilage degeneration is considered to be one of the primary
pathological changes that cause OA, and this typically occurs due
to metabolic disorders of extracellular matrix synthesis and
degradation (4). Chondrocytes, as
the primary cell type found in cartilage tissue, serve important
roles in the maintenance of bone and joint structure and function
(5,6). Previous studies have demonstrated that
matrix metalloproteinases (MMPs) and collagen also serve an
important role in the development of OA (7,8). MMPs
are ion-active proteases that are ubiquitous in the cartilage and
function to degenerate chondrocytes and degrade the extracellular
matrix in the cartilage (9–11). MicroRNAs (miRNAs) are single-stranded
non-coding RNAs that are associated with the development and
progression of OA (12). It has been
reported that miRNAs serve a role in the pathogenesis of OA
regulating the expression of inflammatory mediators, vascular
endothelial growth factor and nerve growth factor (12). Further studies have revealed that
miRNAs may inhibit or promote the expression of MMPs and collagen,
resulting in the degeneration of chondrocytes and cartilage
extracellular matrix, eventually leading to OA (13,14).
Previous studies have also reported that miRNA-4784
expression is downregulated in certain types of cancer, including
breast and liver cancer, and miRNA-4784 is involved in the
development and progression of cancer by affecting the Akt signal
pathway (12,13). To the best of our knowledge, no
previous studies have assessed the expression and mechanism of
action of miRNA-4784 in OA. The aim of the present study was to
assess the expression of miRNA-4784 in OA chondrocytes and the
effect of transfection with exogenous double-stranded
(ds)-miRNA-4784 on the chondrocyte function. The results of the
present study may provide a theoretical basis for a novel treatment
of OA.
Materials and methods
Experimental reagents
Lipofectamine® 2000, Dulbecco's modified
Eagle's medium (DMEM), PBS and penicillin were purchased from Gibco
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
miR-4784 mimic was purchased from GenePharma Co., Ltd. (Shanghai,
China). Fetal bovine serum and western blotting reagents (cat no.
YJ1012465) were purchased from Yiji (Shanghai, China) and
radioimmunoprecipitation cell lysis solutions from Beyotime
Institute of Biotechnology (Haimen, China). Cell culture plates,
Col2a1 and MMP-3 rat anti-rabbit monoclonal antibodies were
purchased from Pierce (cat nos. ab185430 and ab26111; Pierce;
Abcam, Shanghai, China). The quantitative polymerase chain reaction
(qPCR) kit and the reverse transcription (RT) kit were purchased
from Fermentas (Thermo Fisher Scientific, Inc.).
Experimental animals and OA model
establishment
A total of 40 New Zealand rabbits (age, 6 months; 20
male and 20 female; weight, 2.0–3.0 kg) were purchased from the
West China Center of Medical Sciences, Sichuan University (Chengdu,
China). The rabbits were raised with controlled temperature and
light cycles (24°C and 12/12 light cycles) and free access to water
and food. The humidity was 60±10% for 2 weeks prior to surgery. The
following method was used to establish the adult rabbit OA model:
pentobarbital sodium (3%; 30 mg/kg) was injected into the ear vein
for anesthesia, and then the rabbits were fixed on an operating
table in the supine position. The right knee was shaved and
disinfected with iodophor (Abcam). The medial patella meniscus was
opened and the patella was everted. The knee joint was then buckled
and the anterior segment ligament and collateral ligament were cut
with an ophthalmic clip. The surgical site was rinsed, the joint
capsule was closed and the skin was disinfected. The right limb
remained unfixed and rabbits were kept in a separate case, and had
free access to water and food until they were sacrificed. A daily
intramuscular injection of 80×106 U of penicillin was
administered for 1 week. Right knee tissues were collected at 4 and
8 weeks following modeling to serve as groups OA at week 4 and 8,
respectively (each, n=10). The left knee of rabbit without
treatment was set as control group (n=10). The present study was
approved by the Ethics Committee of the 174th Hospital of Chinese
PLA (Chenggong Hospital Affiliated to Medical College of Xiamen
University, Xiamen, China).
Cell culture
Rabbits were sacrificed at week 4 and 8 following
surgery in groups OA at week 4 and 8, respectively. Aseptic
cartilage specimens were obtained under sterile conditions.
Cartilage tissues with a thickness of 1–2 mm were scraped using a
surgical blade and digested with 0.25% trypsin for 30 min at 24°C.
Tissues were then digested with 0.2% collagen II protease for 2 h
at 37°C. This procedure was repeated 2–3 times to collect
chondrocytes, which were subsequently cultured in DMEM supplemented
with 10% fetal bovine serum and 100 U/ml penicillin at 37°C and 5%
CO2.
Transfection of ds-miRNA-4784 into OA
chondrocytes
Cells were transfected with ds-miRNA-4784 (UGAGGAGAU
GCUGGGACUGA; Thermo Fisher Scientific, Inc.) at 100 nM in
accordance with the manufacturer's instructions (Guangzhou RiboBio
Co., Ltd., Guangzhou, China). Chondrocytes were cultured overnight
to reach a cell density of 40–50% at 37°C. The miRNA-4784 mimic was
then transfected into chondrocytes using Lipofectamine®
2000 for 6 h at 37°C. Fresh DMEM was then added, and then the cells
were cultured at 37°C and 5% CO2. Cells were harvested
at 48–96 h after transfection and RT-qPCR was used to detect mRNA
expression.
Detection of miRNA and mRNA expression
using RT-qPCR
U6 snRNA was used as an endogenous control to
quantify the expression of miRNA-4784. The expression of Col2a1 and
MMP-3 was quantified using β-actin as an endogenous control before
and after transfection. Total RNA was extracted from OA
chondrocytes using TRIzol (Thermo Fisher Scientific, Inc.) and was
reverse transcribed into cDNA using the RT kit according to the
manufacturer's instructions (37°C for 15 min, 95°C for 5 min). PCR
thermocycling conditions were as follows: 94°C for 2 min, followed
by 50 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 30
sec. Cq values were processed by 2−ΔΔCq method and the
relative expression of each gene was normalized to their
corresponding endogenous controls (15). U6 forward,
5′-TGCGGGTCGTGGCTTCGGCAG-3′ and reverse, 5′-CCAGTGCAGGGTCCGAGG-3′;
β-actin forward, 5′-GCTGCGTGTGGCCCCTGAG-3′ and reverse,
5′-ACGCAGGATGGCATGAGGGA-3′; Col2a1 forward,
5′-TCCTAAGGGTGCCAATGGTGA-3′ and reverse,
5′-AGGACCAACTTTGCCTTGAGGAC-3; MMP-3 forward,
5′-ATTCCATGGAGCCAGGCTTTC-3′ and reverse,
5′-CATTTGGGTCAAACTCCAACTGTG-3′.
Detection of Col2a1 and MMP-3 protein
expression using western blotting (cat. no. YJ1012465; Yiji)
Cultured chondrocytes were rinsed with PBS and lysed
using cell lysis solution for 30 min. Cell lysate was then
transferred into a tube and centrifuged at 9,800 × g for 20 min at
4°C to collect cell supernatant. Total protein concentration was
determined using a BCA assay. Protein samples (~90 µg per lane)
were separated by 10% SDS-PAGE and transferred onto polyvinylidene
membranes. The membranes were then blocked using 5% bovine serum
albumin (Abcam) at 24°C for 2 h. Membranes were washed 3 times with
TBST and incubated with Col2a1 rat anti-rabbit monoclonal
antibodies (1:500) or MMP-3 rat anti-rabbit monoclonal antibodies
(1:500) overnight at 4°C. After further washes with TBST 3 times,
the membranes were incubated with goat anti-rat horseradish
peroxidase-labeled secondary polyclonal antibody (1:1,000; cat. no.
7077; Cell Signaling Technology, Danvers, MA, USA) for 1 h at room
temperature. Membranes were washed 3 more times with TBST and ECL
reagent (Beyotime, Shanghai, China) was added for signal
development. The relative expression level of each protein was
normalized to the endogenous control (β-actin; 1:1,000, cat. no.
60008-1-Ig, Proteintech, Wuhan, China) using ImageJ 1.48 (NIH,
Bethesda, MD, USA).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses and data are expressed as the
mean ± standard error of the mean. One-way analysis of variance was
used for multiple group comparisons and a post-hoc Dunnett's test
was performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
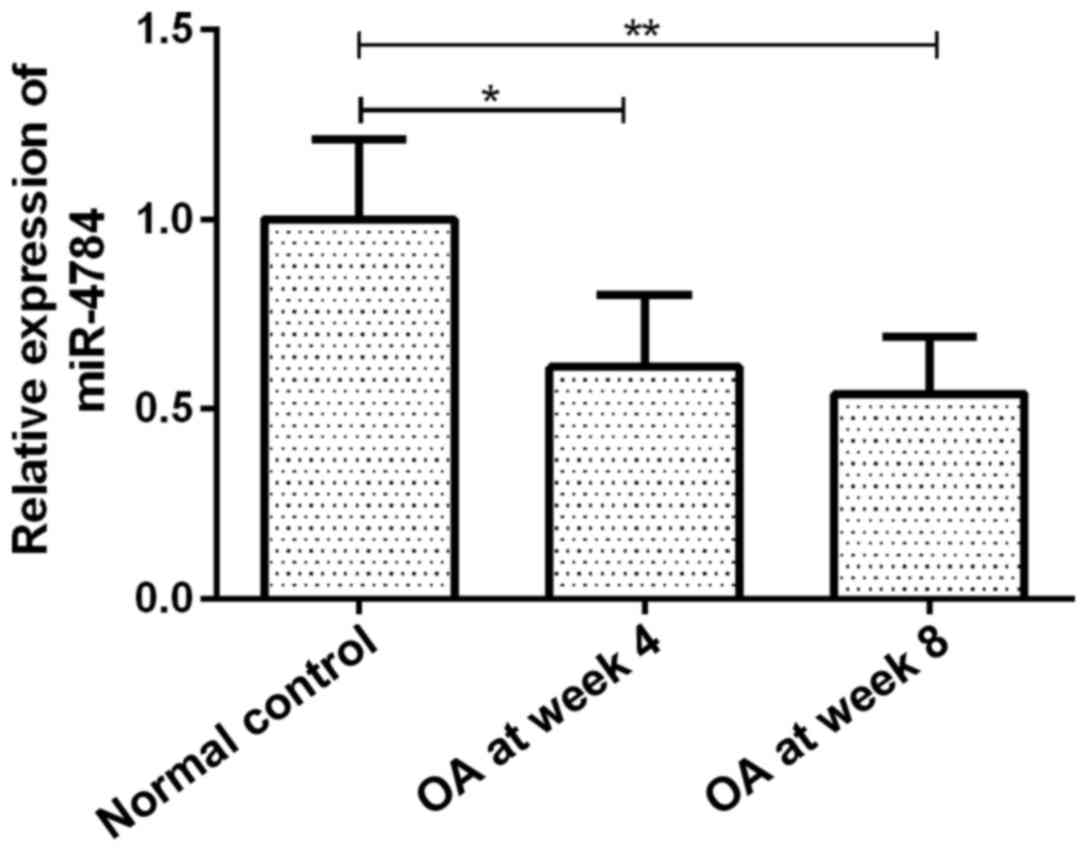
miR-4784 expression
The expression of miR-4784 in OA articular
chondrocytes gradually decreased with disease progression. miR-4784
expression in groups OA at week 4 and 8 was 61 (P<0.05) and 54%
(P<0.01) of that in the group normal control, respectively
(Fig. 1). However, no significant
difference in miR-4784 expression was observed between groups OA at
week 4 and 8 (Fig. 1).
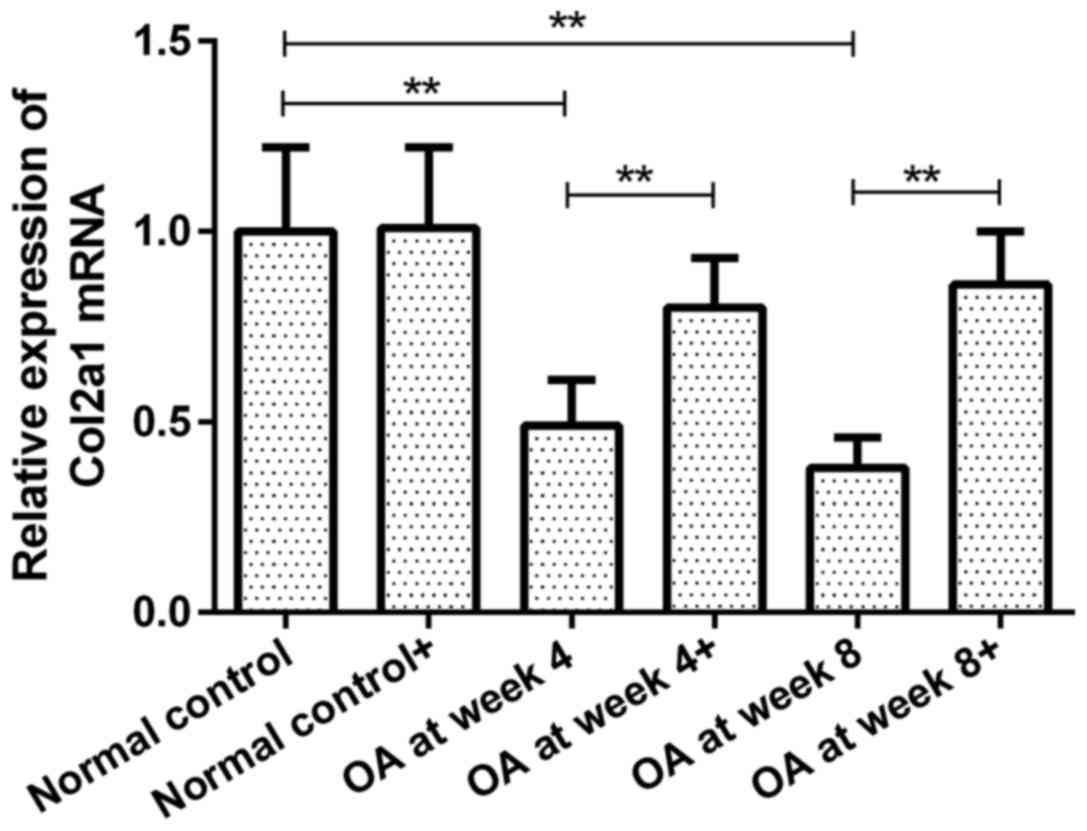
Expression of Col2a1 mRNA in
chondrocytes prior to and following ds-miRNA-4784 transfection
Col2a1 mRNA expression in groups OA at week 4 and 8
prior to transfection were 49 and 38% of that in normal control
group, respectively (P<0.01; Fig.
2). However, no significant difference was observed between
groups OA at week 4 and 8. Following ds-miRNA-4784 transfection
(ds-miRNA-4784+), no significant change in Col2a1 mRNA expression
was observed in normal control group. However, Col2a1 mRNA levels
in groups OA at week 4 and 8 were increased by 63 and 126%,
respectively, following transfection (P<0.01; Fig. 2). Furthermore, no significant
difference in Col2a1 expression was identified between groups OA at
week 4 and 8 following transfection.
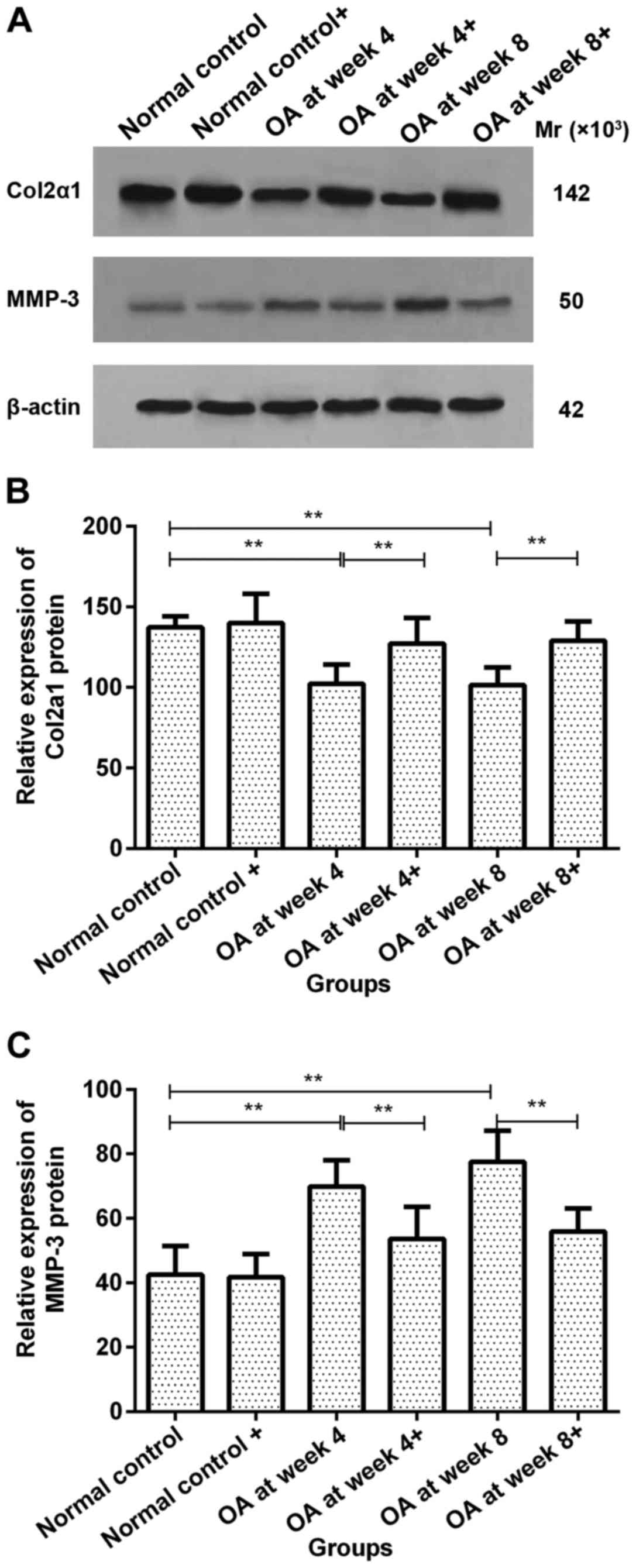
Col2a1 protein expression in
chondrocytes prior to and following ds-miRNA-4784 transfection
No significant difference in Col2a1 protein
expression was observed in normal control group prior to and
following ds-miRNA-4784 transfection. However, Col2a1 protein
expression increased significantly in groups OA at week 4 and 8
following transfection compared with pre-transfection levels
(P<0.01; Fig. 3A and B).
MMP-3 protein expression in
chondrocytes prior to and following ds-miRNA-4784 transfection
No significant difference in MMP-3 protein
expression was observed in normal control group prior to and
following ds-miRNA-4784 transfection. However, the expression of
MMP-3 protein significantly decreased following transfection
compared with pre-transfection levels in groups OA at week 4 and 8
(P<0.01; Fig. 3A and C).
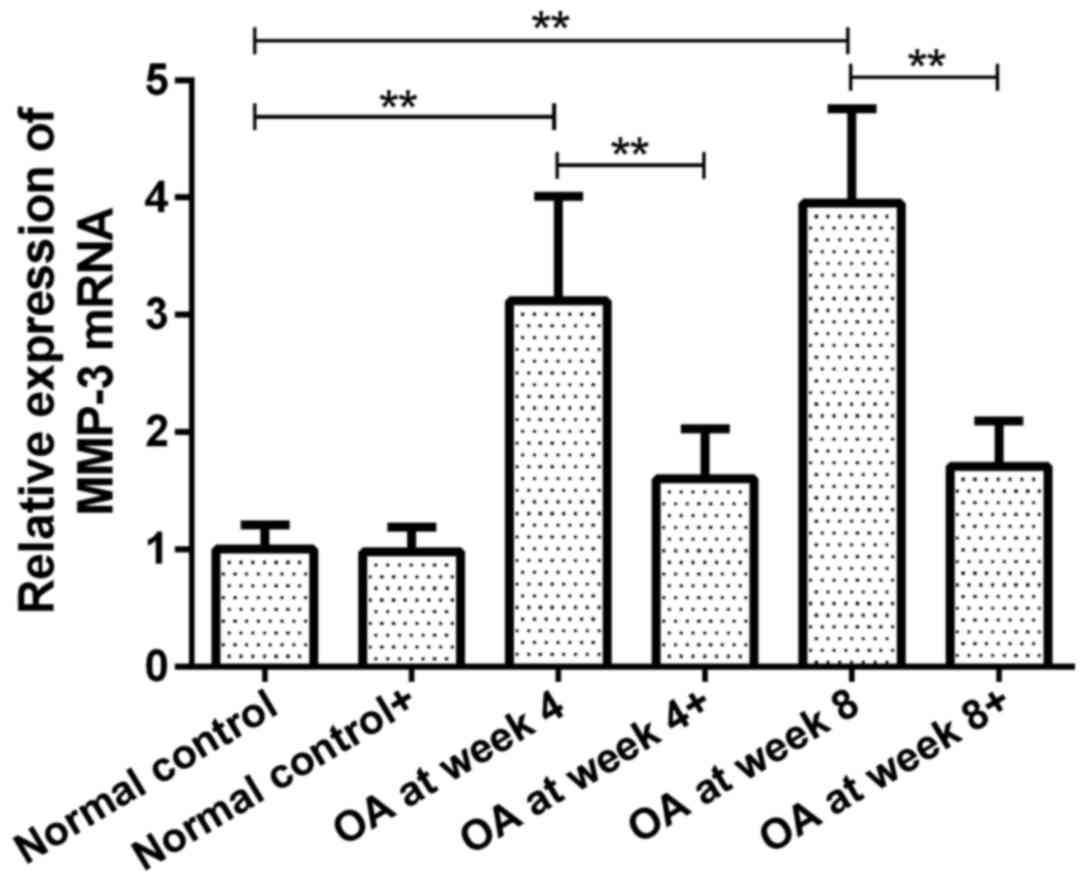
MMP-3 mRNA expression in chondrocytes
prior to and following ds-miRNA-4784 transfection
The expression of MMP-3 mRNA in groups OA at week 4
and 8 prior to transfection were 3.12 and 3.95-fold higher than
that in normal control group (P<0.01, Fig. 4). However, no significant difference
was observed between groups OA at week 4 and 8. Following
transfection with ds-miRNA-4784, no significant difference in MMP-3
mRNA expression was observed in normal control group compared with
pre-transfection level. Furthermore, the expression of MMP-3 mRNA
in groups OA at week 4 and 8 was significantly decreased following
transfection compared with pre-transfection levels (P<0.01;
Fig. 4). No significant difference
in MMP-3 mRNA expression was observed between groups OA at week 4
and 8 following transfection.
Discussion
OA is one of the most common types of chronic
arthritis and seriously affects the quality of life in the elderly
population (1–3). Major pathological features of OA
include a series of biological and/or morphological changes, such
as the degeneration of articular cartilage, hyperosteogeny and/or
sclerosis (16). Currently, OA is
typically treated conservatively, with the main aim being the pain
relief; however, effective radical treatment remains insufficient
(17). Furthermore, certain patients
may experience disease recurrence due to ineffective treatment
(18).
The occurrence of OA is associated with many
parameters, including genetic and immune factors; however, the
exact pathogenesis remains unclear (3). The roles of various miRNAs in OA
chondrocytes have been widely studied and have provided a novel
research direction, which has helped to elucidate the pathogenesis
of OA (13). miRNAs are a group of
ubiquitous endogenous small single-stranded non-coding RNAs that
are associated with the regulation of 30% of genes (19). The abnormal expression of various
miRNAs, including miRNA-140, −126 and −146, has been observed in
patients with OA (13,14). These miRNAs advance the development
of OA by promoting the expression of MMPs and reducing the
mechanism of collagen (20).
In the present study, a rabbit OA model was
established to assess the changes in miRNA-4784 expression in
chondrocytes at 4 and 8 weeks following the model construction. The
results revealed that the expression of miRNA-4784 in chondrocytes
gradually decreased with prolonged duration of the disease,
indicating that miRNA-4784 is downregulated in OA chondrocytes.
However, the mechanism by which this occurs remains unclear.
Previous studies have revealed that the expression of a series of
miRNAs, including miRNA-140, decrease in chondrocytes during early
OA; this change may be associated with miRNA inhibition by
cytokines such as metalloproteinases or collagen (13,20).
Changes in the expression of Col2a1 and MMP-3 in OA
chondrocytes were also assessed in the present study. Col2a1
expression serves an important role in maintaining the normal
function of chondrocytes, the downregulation of which indicates the
degeneration of articular cartilage (21–23).
MMP-3 is an important chondroitin-degrading enzyme whose
upregulation promotes the degeneration of articular cartilage
(24). In the present study,
compared with normal control group, the expression of Col2a1 in OA
chondrocytes was significantly reduced at the miRNA and protein
levels, which is consistent with the results of previous studies
(25–27). Furthermore, an increase in MMP-3
expression was observed in OA chondrocytes, indicating that there
was a degenerative change in the cartilage during early stage OA.
Additionally, miRNA-4784 levels increased following transfection
with exogenous ds-miRNA-4784, while Col2a1 expression also
increased in OA chondrocytes. The expression of MMP-3 mRNA and
protein were decreased, suggesting that ds-miRNA-4784 transfection
improves the function of OA chondrocytes.
The results of the present study also demonstrated
that miRNA-4784 expression gradually decreases with prolonged
disease duration. Transfection-induced miRNA-4784 upregulation
maintained the stability of chondrocytes, promoted the expression
of Col2a1 and inhibited the expression of MMP-3. These results
confirm that miRNA-4784 serves a role in the development and
progression of OA. In this investigation, we only studied the
mechanism of miRNA-4784 in the pathogenesis of OA from experimental
animals, but we did not detect the cytokines and RNA in clinical
patients. Therefore, more research is needed to further confirm the
role of miRNA-4784 in the pathogenesis of OA. The present study may
provide novel insights into the pathogenesis of OA and a
theoretical basis for the application of gene therapy in future
treatments for OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and QY were major contributors in writing the
manuscript and participated in the analysis and discussion of the
data. JL and YYa were responsible for the cell culture. XC and YYe
performed RT-qPCR and western blotting. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the 174th Hospital of Chinese PLA (Chenggong Hospital
Affiliated to Medical College of Xiamen University, Xiamen,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taheri P, Vahdatpour B, Asl MM and
Ramezanian H: Effects of taping on pain and functional outcome of
patients with knee osteoarthritis: A pilot randomized single-blind
clinical trial. Adv Biomed Res. 6:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brazilian Medical Association, . Silvinato
A and Bernardo WM: Inflammatory arthritis or osteoarthritis of the
knee - efficacy of intra-joint infiltration of methylprednisolone
acetate versus triamcinolone acetonide or triamcinolone
hexacetonide. Rev Assoc Med Bras (1992). 63:827–836. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang M, Jiang L, Wang Q, Chen H and Xu G:
Traditional Chinese medicine for knee osteoarthritis: An overview
of systematic review. PLoS One. 12:e01898842017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitney KE, Liebowitz A, Bolia IK, Chahla
J, Ravuri S, Evans TA, Philippon MJ and Huard J: Current
perspectives on biological approaches for osteoarthritis. Ann NY
Acad Sci. 1410:26–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nielsen FK, Egund N, Jørgensen A and Jurik
AG: Risk factors for joint replacement in knee osteoarthritis; a
15-year follow-up study. BMC Musculoskelet Disord. 18:5102017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duarte N, Rodrigues AM, Branco JDC, Canhão
H, Hughes SL and Paúl C: Health and lifestyles factors associated
with osteoarthritis among older adults in Portugal. Front Med
(Lausanne). 4:1922017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo S, Shi Q, Chen J, Wang H, Wu W and Zha
Z: Expression and significance of MMPs in synovial fluid, serum and
PBMC culture supernatant stimulated by LPS in osteoarthritis
patients with or without diabetes. Exp Clin Endocrinol Diabetes.
Dec 21–2017.(Epub ahead of print).
|
|
8
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barlas IO, Sezgin M, Erdal ME, Sahin G,
Ankarali HC, Altintas ZM and Türkmen E: Association of (−1,607)
1G/2G polymorphism of matrix metalloproteinase-1 gene with knee
osteoarthritis in the Turkish population (knee osteoarthritis and
MMPs gene polymorphisms). Rheumatol Int. 29:383–388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhtar N, Khan NM, Ashruf OS and Haqqi TM:
Inhibition of cartilage degradation and suppression of PGE2 and
MMPs expression by pomegranate fruit extract in a model of
posttraumatic osteoarthritis. Nutrition. 33:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu P, Yao J and Hou W: Relationships
between COL2A1 gene polymorphisms and knee osteoarthritis in Han
Chinese women. Mol Biol Rep. 38:2377–2381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao L, Zeng Y, Wang J, Liu Z, Shen B, Ge
J, Liu Y, Guo Y and Qiu J: Differential microRNA expression in
aristolochic acid-induced upper urothelial tract cancers ex vivo.
Mol Med Rep. 12:6533–6546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhen Y, Xinghui Z, Chao W, Yi Z, Jinwen C,
Ruifang G, Chao Z, Min Z, Chunlei G, Yan F, et al: Several
microRNAs could predict survival in patients with hepatitis
B-related liver cancer. Sci Rep. 7:451952017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soyocak A, Kurt H, Ozgen M, Cosan Turgut
D, Colak E and Gunes HV: miRNA-146a, miRNA-155 and JNK expression
levels in peripheral blood mononuclear cells according to grade of
knee osteoarthritis. Gene. 627:207–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brisson NM, Stratford PW and Maly MR:
Relative and absolute test-retest reliabilities of biomechanical
risk factors for knee osteoarthritis progression: Benchmarks for
meaningful change. Osteoarthritis Cartilage. 26:220–226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abbate LM, Jeffreys AS, Coffman CJ,
Schwartz TA, Arbeeva L, Callahan LF, Negbenebor NA, Kohrt WM,
Schwartz RS, Vina E, et al: Demographic and clinical factors
associated with nonsurgical osteoarthritis treatment use among
patients in outpatient clinics. Arthritis Care Res (Hoboken).
70:1141–1149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Snochowska A, Szmigielska P,
Brzeziańska-Lasota E and Tomaszewski W: Genetic and epigenetic
interactions in the etiopathogenesis of osteoarthritis. Selected
molecular factors in OA etiopathogenesis. Ortop Traumatol Rehabil.
19:227–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopańska M, Szala D, Czech J, Gabło N,
Gargasz K, Trzeciak M, Zawlik I and Snela S: MiRNA expression in
the cartilage of patients with osteoarthritis. J Orthop Surg Res.
12:512017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nugent M: MicroRNAs: Exploring new
horizons in osteoarthritis. Osteoarthritis Cartilage. 24:573–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai Y, Kong X, Liu N, Ren S, Guo H and
Zhao K: Mutational analysis and prenatal diagnosis of COL1A1 and
COL1A2 genes in four Chinese families affected with osteogenesis
imperfecta. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 34:705–708.
2017.(In Chinese). PubMed/NCBI
|
|
22
|
Loughlin J, Sinsheimer JS, Mustafa Z, Carr
AJ, Clipsham K, Bloomfield VA, Chitnavis J, Bailey A, Sykes B and
Chapman K: Association analysis of the vitamin D receptor gene, the
type I collagen gene COL1A1, and the estrogen receptor gene in
idiopathic osteoarthritis. J Rheumatol. 27:779–784. 2000.PubMed/NCBI
|
|
23
|
Aerssens J, Dequeker J, Peeters J,
Breemans S and Boonen S: Lack of association between osteoarthritis
of the hip and gene polymorphisms of VDR, COL1A1, and COL2A1 in
postmenopausal women. Arthritis Rheum. 41:1946–1950. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong Z, Liu Y, Chen B, Yan L and Hao D:
Association between MMP3 and TIMP3 polymorphisms and risk of
osteoarthritis. Oncotarget. 8:83563–83569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi J, Zhang C, Yi Z and Lan C: Explore
the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early
stage of osteoarthritis. IUBMB Life. 68:293–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen JJ, Huang JF, Du WX and Tong PJ:
Expression and signi- ficance of MMP3 in synovium of knee joint at
different stage in osteoarthritis patients. Asian Pac J Trop Med.
7:297–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kubota E, Imamura H, Kubota T, Shibata T
and Murakami K: Interleukin 1 beta and stromelysin (MMP3) activity
of synovial fluid as possible markers of osteoarthritis in the
temporomandibular joint. J Oral Maxillofac Surg. 55:20–28. 1997.
View Article : Google Scholar : PubMed/NCBI
|