Introduction
Spinal cord injury (SCI) is associated with severe
and long-lasting neurological dysfunction in humans. Currently,
there is no effective treatment to reverse the consequences
resulting from traumatic SCI. While in experimental studies,
various pharmacological agents and therapeutic approaches have
tended to improve outcomes in animal SCI models (1), unfortunately none of these therapies
has been successfully transferred into clinical practice.
After SCI the development of the lesion consists of
primary and secondary phases (2,3). The
primary phase is the direct mechanical injury to the spinal cord
tissues itself, which is followed by a secondary phase leading to a
marked enlargement of the primary lesion within several h after
SCI. This slow evolution of the lesion has been referred to as
progressive hemorrhagic necrosis or ‘autodestruction’ (4,5). It
results in secondary loss of vital spinal cord tissue and, in some
species including humans, leads to formation of a cystic cavity
surrounded by glial scar tissue.
From the clinical point of view, the secondary phase
is very important because it represents the target for therapeutic
interventions which could minimize the extent of the final lesion
(6). To this date the treatments
options for SCI are limited to surgical interventions, early
steroid administration and physical therapy. These therapeutic
interventions are effective only under specific circumstances
(7,8), and their effect on the final outcome is
modest at best.
One potential treatment which has shown promising
results over the past few decades is therapeutic hypothermia.
Although the entire range of the effects of hypothermia is not
fully understood, a few observations are notable (9–12).
Hypothermia seems to work through several mechanisms which start to
operate soon after trauma and may have an important role in the
functional outcome. Neuroprotective effects of systemic and
regional hypothermia have been described (9–11,13,14).
It has been demonstrated that hypothermia has a powerful effect on
control of the injury-induced immune response (15), apoptosis and inflammation (13,14,16–22).
Hypothermia also delays glutathione release, reduces oxidative
stress and increases spinal cord tolerance to ischemia (23–26).
There are also numerous articles illustrating the neuroprotective
effect of hypothermia on neural stem cell differentiation and
survival (15,27–35).
The published studies have defined the most
effective target temperature range for systemic hypothermia
(36–39) and they suggest that the most
effective cooling is such that i) is initiated early after injury,
ii) lasts for long periods (up to 48-h) and iii) is followed by
slow rewarming (13,38).
Relatively little experimental data are available
for local hypothermia. In general, the results indicate that longer
durations of local hypothermia (at least 48 h) yield better results
than shorter durations (14,40–43).
However, there are studies indicating that duration of hypothermia
<48 h may be sufficient. In the literature, some animal studies
found that local hypothermia improved outcomes after SCI (37,38,42,44–50),
whereas others concluded that it has no effect (51–54).
Interestingly, in a clinical study by Hansebout and Hansebout
(55), positive results were
observed with local hypothermia lasting <4 h. In this study, the
cooling was initiated 7.1 h after injury, the mean duration of the
treatment was 3.7 h and there was an uncontrolled rewarming
rate.
In the present study, we wanted to test the
protective effect of local hypothermia after precisely-controlled
SCI model in minipigs (56,57) representing a preclinical model which
mimics human patients more closely than the widely-used rodent
models. In a series of experiments, we modified i) the cooling
approach (durotomy vs. intact dura mater), ii) the kind of cooling
solutions (saline, medium or enriched medium), and iii) the degree
of hypothermia (15 or 24°C just above the SCI). The outcome
parameters involved long-term behavioral testing for the recovery
of neurological functions, and detailed histological analyses of
the spinal cord lesions involving axonal quantification using a
selective marker of mature axons (SMI-312).
Materials and methods
Animals
A total of 24 adult female
Göttingen-Minnesota-Liběchov minipigs each weighing 25–35 kg were
used in this study. The animals were maintained in standard
conditions with ad libitum access to food and water. The
experimental protocols were prepared in accordance with the EC
Council Directive (2010/63/EU) regarding the use of animals in
research and approved by the State Veterinary and Food
Administration of the Slovak Republic (decision no. 1319/13-221) as
well as by the Ethical Commission of the University of Veterinary
Medicine and Pharmacy (Kosice, Slovak Republic). All efforts were
made to minimize the size of experimental groups and animal
suffering.
Experimental groups
Two sets of experiments with modifications in
post-injury approach (durotomy vs. intact dura mater) and
temperature of perfusion solutions (15 or 24°C) were carried
out.
The animals in Experiment 1 were divided into the
following groups: i) naïve control-without anesthesia or surgical
intervention (n=3); ii) SCI at L3 level + durotomy (5 h) and
survival for 9 weeks (n=3); iii) SCI at L3 level + durotomy
followed by 4°C saline treatment (target temperature: 15°C for 5 h)
and survival for 9 weeks (n=3); iv) SCI at L3 level + durotomy
followed by 4°C medium treatment (target temperature, 15°C for 5 h)
and survival for 9 weeks (n=3); v) SCI at L3 level + durotomy
followed by enriched 4°C medium treatment (target temperature: 15°C
for 5 h) and survival for 9 weeks (n=3).
In order to relieve the edema and provide direct
contact of cooling solutions with the spinal cord, the minipigs
underwent durotomy immediately after SCI. Medium (DMEM/F12) was
composed of rh Transferrin 100 mg/l, rh Insulin 25 mg/l, Glucose
1.56 g/l, Progesterone 6.3 µg/l, Putrescine 16.1 mg/l, Selenium 5.2
µg/l. Enriched medium was composed of DMEM/F12 + rh bFGF 10 µg/l,
rh BDNF 10 µg/l, rh GDNF 10 µg/l, rh VEGF 10 µg/l, Creatine 20 mM.
The treatments were initiated 30 min after SCI and durotomy, and
maintained for 5 h (see details of hypothermic treatment
below).
The animals in Experiment 2 were divided into the
following groups: i) naïve control-without anesthesia or surgical
intervention (n=3); ii) SCI at L3 level (intact dura mater) and
survival for 9 weeks (n=3); iii) SCI at L3 level (intact dura
mater) followed by room temperature saline treatment (target
temperature: 24°C for 5 h) and survival for 9 weeks (n=3). The
treatment was initiated 30 min after SCI and maintained for 5 h
(see details of hypothermic treatment below).
Pre-contusion procedures
Three days before the surgery, the animals were
pre-treated with a combination of penicillin and streptomycin,
administered intramuscularly in a dose of 0.5 ml/30 kg (Norbrook
Laboratories, Newry, Northern Ireland). The minipigs were
premedicated 30 min before surgical procedure with an intramuscular
injection of Stresnil (2 mg/kg; Janssen Pharmaceutica, Beerse,
Belgium) and Atropin (0.5 mg/kg; Biotika, Slovenská Ľupča,
Slovakia). Anesthesia was induced by intravenous administration of
Thiopental (10 mg/kg; Czech Pharma, Czech Republic) followed by
identification of the precise location of the L3 vertebra using
plain X-rays in a lateral projection (58). Afterwards, the animals were
endotracheally intubated (diameter, 5.0–6.0 mm) and anesthesia was
maintained by inhalation of 1.5% Sevofluran (Baxter, Prague, Czech
Republic) mixed with oxygen. Analgesia was supported by
administration of Butomidor (0.4 mg/kg, i.v.; Richter Pharma,
Austria). Catheters for the administration of infusions and
medications were inserted bilaterally into the cephalic and
auricular veins. Standard monitoring of life-function indicators
(mainly heart rate, respiratory rate, blood pressure and oxygen
saturation) was performed during the whole surgical procedure and
hypothermic treatment. Hydration was maintained with lactated
Ringer's solution.
Spinal cord contusion
The animals were placed into a special
immobilization apparatus (Fig. 1A).
After disinfection of the lumbar area, a midline skin and
subcutaneous fat incision was performed from L2 to L4 segments.
Muscles were dissected from the spinous processes and vertebral
arches and retracted, then a dorsal laminectomy at the L3 segment
was performed. Afterwards the minipigs received the muscle relaxant
sukcinylcholine and SCI was accomplished using a computer-operated
compression device (Fig. 1A)
consisting of a stepping motor (ViDiTo, Kosice, Slovakia) and a
digital force gauge connected to a 5-mm diameter circular aluminum
bar (Fig. 1B). Parameters for SCI
(velocity: 10 mm/sec; force: 18N) were preset by the computer
software (56). To control the
uniformity of the impact force, the contusion curve was recorded in
each animal (Fig. 1C). The force
impact was uniform without significant differences between animals
or groups. The real peak force in Experiment 1 was 21.08 N ± 0.79
and in Experiment 2 it was 20.72 N ± 0.97. After SCI, the
paraspinal musculature and skin were sutured with several
absorbable stitches or durotomy and subsequent hypothermic
treatments were performed depending on the experimental group. The
animals were housed in threesomes to recover. Post-operative care
included bladder expression twice a day, antibiotic administration
Penstrepten (0.5 ml/30 kg/day; Biotika) for 10 days, cleaning the
hind limbs and monitoring of skin irritation and development of
decubitus ulcers.
Implantation of perfusion chamber
In the case of animals undergoing experimental
treatment, the perfusion chamber was implanted over the exposed
spinal dural sac containing the spinal cord immediately after
performing the SCI (Fig. 1D and E).
A cone-shaped chamber with both ends opened was made from ABS
plastic using a uPrint SE 3D printer (Stratasys, Eden Prairie, MN,
USA). It had ellipse-shaped openings, the lower opening measuring
20×14 mm and the upper one 40×30 mm; the height of the chamber was
50 mm. The chamber was fitted with two tubings (inflow and
outflow). The smaller opening of the chamber was about the size of
the laminectomy, so it could be freely placed directly on the dura
mater without any fixation to the spine or paravertebral muscles.
Two superficially-placed stitches were used to partially close the
wound, making the skin edges slightly embracing the chamber.
Hypothermia
Hypothermia with cold solutions was carried out
locally with the help of the perfusion chamber. The inflow tubing
was connected to the tank with sterile cold perfusion solution. The
cooling of the injury site was initiated 30 min after SCI or SCI +
durotomy. At the beginning, the solution drip rate was manually
adapted to ensure that the temperature of fluid in the perfusion
chamber (just above the SCI) was maintained at 15°C in Experiment 1
or at 24°C in Experiment 2. Given that cooling was applied locally,
the temperature of the solution in the chamber decreased to 15°C or
24°C very rapidly, max. within 5–6 min and was measured at 30 min
intervals using a needle-tip thermometer (Omega Bio-Tek, Inc.,
Norcross, GA, USA) during the whole treatment procedure.
Hypothermic treatment of the injured spinal cord lasted for 5 h.
Continuous inflow of the perfusion solution into the chamber was
regulated by a peristaltic pump (bubble rise distance, flow 2
ml/min; Heidolph, Schwabach, Germany). The outflow tubing was used
to drain excess perfusion solution. The average temperature of
cooling solutions in the chamber (just above the site of injury)
was 15.43°C ± 3.75 in Experiment 1 and 24.06°C ± 1.95 in Experiment
2. There were no significant differences in epidural temperature
between groups during the whole treatment period. After the
therapy, the perfusion chamber was extracted, the dura mater in
Experiment 1 was closed with absorbable sutures, and the wound was
sutured in layers. These animals were housed in separated cages to
recover.
To minimize variability in body temperature and to
prevent systemic hypothermia during treatment the minipigs were
covered with an isothermal foil. The rectal temperature of each
animal was measured at 30 min intervals. When the body temperature
dropped below 36°C, the animals were heated with a stream of warm
air blown below the blanket covering the animal. The average rectal
temperature was 36.47°C ± 0.70 in Experiment 1, 36.02°C ± 0.87 in
the SCI group, and 36.10°C ± 0.59 in the saline group in Experiment
2. No significant differences were recorded in rectal temperatures
between groups during the treatment period.
Behavioral assessment
Three weeks before the surgery each minipig
underwent half an hour of training for 5 days/week to walk upon
command, up and down a rubber mat (width, 0.8 m; length, 5.2 m).
Post-operative neurological impairment was tested using a 21-point
scale, ranging from complete paraplegia (zero) to normal ambulation
(20 points) (57). The scoring
points took into consideration the hind-limb movement for each
individual joint, the capability of each animal to get up by
itself, its trunk stability and stepping coordination. The scoring
points represent the sequential recovery stages which the minipigs
attained after SCI. Porcine neurological motor scores (1–8)
represent slight or extensive movement for each individual joint of
the hind limbs. Points (9–11) represent sweeping and the ability to
get up by itself (with or without assistance; the animals were not
necessarily able to maintain balance), and points (12–20)
characterize the ability to walk from a few steps without
fore-limb/hind-limb coordination to consistent plantar-hoof
stepping and consistent fore-limb/hind-limb coordination. The
neurological impairment was tested under blinded outcome assessment
weekly for nine weeks of survival. Each minipig was allowed to walk
freely on the rubber mat for 10 min without assistance. A video
recording was made of each evaluation. In order to reduce bias,
three independent observers were involved to determine the
neurological scale. The inter-rater differences in the scores
varied by one point at most. The discrepancies between the raters
were discussed and clarified.
Spinal cord tissue preparation
At the end of the experiment, the animals were
anesthetized by intravenous administration of Thiopental (10 mg/kg;
Czech Pharma) and transcardially perfused with heparinized saline
(5l) followed by 4% paraformaldehyde in 0.1 M phosphate-buffered
saline (PBS; pH 7.4, 5l). The lumbar spinal cords encompassing the
injury site were carefully dissected out, cleaned of the dura mater
remnants and post-fixed in 4% paraformaldehyde overnight. Next day,
the spinal cords were cut into seven 1 cm segments: cranial (+3,
+2, +1), central (0) and caudal (−1, −2, −3) segments. The spinal
cord segments were cryoprotected in a solution of 30% sucrose in
PBS at 4°C for 2 days, cut into transverse serial sections (30 µm)
on a cryostat (Leica Microsystems, Wetzlar, Germany) and used for
histological and immunohistochemical analyses.
Histology
The selected sections were placed in PBS for 10 min,
in 70% alcohol for 2 h and overnight in 0.1% Luxol fast blue (LFB)
at room temperature. Next day, the sections were rinsed in
distilled water and PBS for 3 min, put into 0.05% aqueous lithium
carbonate followed by 40% alcohol, PBS and distilled water.
Afterwards, the slices were counterstained with 0.2% cresyl violet
for 4 min and rinsed in distilled water. Finally, the sections were
dehydrated through a graded series of ethanol and xylene and
covered with coverslips. Sections were visualized using an Olympus
BX51/BX52 light microscope (magnification ×5, fitted with an
Olympus DP50 digital camera coupled with a computer equipped with
Olympus DP Image software, version 3.1; Olympus Corporation, Tokyo,
Japan). Slices from each segment were evaluated. The areas of
spared white and grey matter were measured using ImageJ 1.44
(National Institutes of Health, Bethesda, MD, USA), expressed as a
percentage and compared to control (control values were expressed
as 100%). The cresyl violet counterstain enabled motor neurons to
be counted. Motor neurons located in the ventral horn (VH) with
clearly visible nuclei on both sides of the spinal cord were
counted.
Immunohistochemistry
Selected slides taken from each rostral and caudal
segment (except for the epicenter of the injury) were pre-treated
in PBS for 3×10 min and incubated in blocking solution consisting
of 5% normal goat serum in 0.1 M PBS with 0.3% Triton-X 100 (normal
goat serum; Vector Laboratories, Burlingame, CA, USA) for 2 h at
room temperature. Afterwards, the slices were incubated overnight
at 4°C with mouse monoclonal anti-neurofilament primary antibody
(Pan-Axonal Neurofilament Marker SMI-312; dilution, 1:1,000; Abcam,
Cambridge, UK) for 24 h at 4°C. In the next step, the sections were
rinsed 3 times in PBS, followed by incubation in secondary
anti-mouse antibody (RhodamineRed; dilution, 1:200; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 90 min at
room temperature. Sections were rinsed in PBS 3×10 min, mounted
with Fluoromount (Serva, Heideberg, Germany) and covered with
coverslips. SMI-312 positive axons were counted by an observer in a
blinded procedure using a ×20 lens over an area of 800×600 µm in
three specific regions of the white matter: dorsal, lateral and
ventral columns (DC, LC, VC) (Fig.
2), using Image J graphics software (ImageJ 1.44; National
Institutes of Health). The images were converted into a binary
image using a set threshold. NF positive axons were counted
automatically using the ‘analyze particles’ tool (57,59). The
number of neurofilaments (expressed as percentage of
NF/mm2) was evaluated bilaterally from ten
randomly-selected transverse slices of each spinal cord section. In
the control group the number of neurofilaments was expressed as
100%.
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for the statistical analyses. The
differences in behavioral outcomes between individual groups were
assessed using ordinary one-way ANOVA followed by Sidak's multiple
comparisons test with statistical significance set at P<0.05 for
each week separately. The data collected from the white and grey
matter sparing, number of motoneurons and number of neurofilaments
were also subjected to ordinary one-way ANOVA followed by Sidak's
multiple comparison test; each spinal cord segment was assessed
separately. The unpaired Student t-test was used to determine the
effect of durotomy. The comparison between SCI group from
Experiment 1 and SCI group from Experiment 2 was performed and
P<0.05 was considered to indicate a statistically significant
difference. Spearman's correlation analysis was used to establish
the relationship between spinal cord tissue integrity and the
neurological outcome of the animals. The data from all 24 animals
from both experiments were utilized. All data are presented as mean
± standard deviation.
Results
Experiment 1: Spinal cord exposed by
durotomy
Behavioral assessment
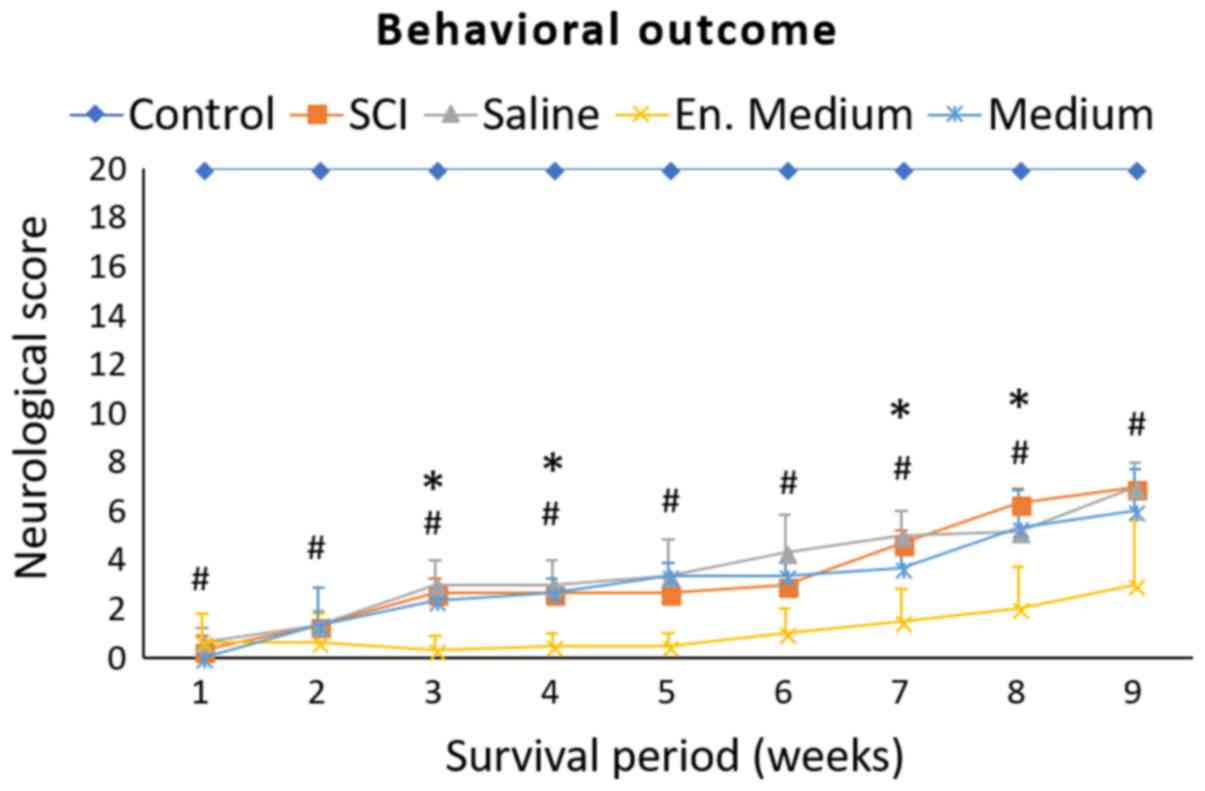
Neurological outcomes of minipigs recorded during a
9-weeks survival period are summarized in Fig. 3. All animals displayed complete
paraplegia immediately after SCI. In the SCI group, one week after
SCI, two animals suffered from complete paraplegia and one was
capable of slight movement in one hip joint (ANOVA summary for 1st
week: F (4, 10)=575.8; P<0.0001; Control vs. SCI P<0.0001).
During the whole survival period, the animals slowly recovered some
of their hind-limb functions characterized by slight movement in
all three joints. The outcome improved gradually and at the end of
the survival period their motor score reached 7±0.0 (ANOVA summary
for 9th week: F (4, 10)=59.05; P<0.0001; Control vs. SCI
P<0.0001). At the 9th week, these animals were not able to
support their body weight, and the motor activity of their
hindlimbs consisted of extensive movements in all three joints and
‘sweeping’, which was usually restricted to one pelvic
extremity.
None of the treatments improved the final motor
scores compared to the SCI group. However, there were significantly
lower scores in the 3rd, 4th, 7th and 8th weeks (SCI vs. en. medium
P<0.05) in the neurological outcome of the group where the
enriched medium was used. Final outcome was 3.0±2.65 (SCI vs. en.
medium, P=0.0545). In the group treated with 4°C saline, there was
transient improvement of motor functions between the 3rd and 7th
week of the follow up period in comparison to the SCI group but the
differences did not reach statistical significance. No differences
were observed at the end point of the survival period.
Macroscopic changes at the lesion
site
After 9 weeks, the spinal cord was markedly narrowed
at the injury site (Fig. 4Ab). When
removing the dura mater, we noted extensive fibrotic adhesions
between the dura and underlying spinal cord tissue. On
transversally-cut 1 cm segments, the central part contained a
distorted cavity spreading in both cranial and caudal directions
from the epicenter. The size of the cavity seemed to be slightly
reduced in hypothermia-treated groups. No differences could be seen
in the animals treated with medium or enriched medium.
Histological outcome
The cross-sectional areas of spared white and grey
matter tissue on histological sections, taken from seven
consecutive spinal cord segments, were measured. Nine weeks after
SCI, the lesion formed a distorted spindle-shaped cavity. The data
from morphometric analyses form a V-shaped curve which corresponds
to the amount of preserved tissue in the damaged spinal cord
(Figs. 4 and 5).
The most affected area was the epicenter of the
injury (segment 0) where the white matter sparing corresponded to
26.20%±6.99. A detailed analysis of spared white matter was carried
out by dividing it into dorsal, lateral and ventral columns
(Fig. 4). We assessed the volume of
spared white matter columns and found differences indicating the
largest loss of tissue in the dorsal columns. The volumes of
preserved white matter ranked in descending order: Ventral columns
(74.21%±6.73) > lateral columns (69.14%±6.05) > dorsal
columns (61.69%±5.45).
Nine weeks after SCI, the gray matter preservation
was quite low at the lesion site (20.99±6.59%) and in segments near
the injury epicenter (48.26±7.53% at +1 and 65.00±7.56% at −1
segment) (Fig. 5A). In addition, we
counted the number of motoneurons in seven consecutive segments.
The average number of motoneurons in the VHs of control animals was
16.58±1.54. SCI + durotomy significantly reduced the cumulative
number of motoneurons to 6.17±1.73. A large necrotic focus could be
seen in the epicenter of the injury, where the number of
motoneurons dropped to 0.39±1.04. The number of motoneurons
increased gradually along with the distance from the epicenter
(Fig. 5B). Occasionally occurring
motoneurons were observed up to 1 cm in rostro-caudal direction;
there were 3.11±2.70 cells rostrally and 5.72±0.75 cells caudally.
The motoneurons were clearly detectable 2 cm from the epicenter,
although their number was still quite low (5.94±1.80 rostrally and
8.06±1.00 caudally) in comparison with controls. There was only a
slight increase in occurrence of motoneurons in segment +3
(9.5±1.79) and in segment −3 (10.44±3.05) (Fig. 5B).
Morphometric analyses indicated that individual
treatments had positive effect on tissue sparing after SCI. The
most prominent improvement was detected in the white matter, where
4°C saline positively affected tissue sparing especially in the
dorsal and lateral columns in almost all rostro-caudal segments.
Hypothermia with medium and/or enriched medium positively affected
the white matter integrity in the rostral segments of the dorsal
and lateral columns (Fig. 4B and C).
Significant sparing (P<0.05) was also found in the caudal
segment distant from the epicenter of the injury in the group
treated with enriched medium (Fig.
4C). No significant differences were noted in the ventral
columns. The hypothermia treatment was less pronounced in the grey
matter. The largest effect could be seen after 4°C saline
treatment, especially in the segments distant from the epicenter.
The most prominent improvement in number of motoneurons was noticed
after hypothermia in the caudal segments (Fig. 5B). Significant improvement in the
rostral segments was visible only in the saline or medium treated
groups (Fig. 5B).
Imunohistochemistry
SMI-312 immunolabeling was performed to visualize
individual axons in the white matter and to count their number in
individual areas of interest (Figs.
2, 6 and 7). These data characterize the density of
axons in selected areas of the dorsal, lateral and ventral columns,
and their changes after SCI (Fig.
7). Nine weeks after SCI, loss of SMI-312-positive axons was
significant in all white matter columns along the rostro-caudal
axis. The most affected were the lateral columns. The white matter
disintegrity after SCI did not allow the number of neurofilaments
to be quantified at the lesion site (segment 0).
Hypothermic treatment with 4°C saline increased the
number of NF in all columns in comparison to the SCI. However, this
improvement was significant (SCI vs. saline P<0.05) only in the
lateral (+1 segment) and ventral columns (+1 and −3 segments).
Hypothermia with medium negatively influenced the number of NF in
the caudal segments (Fig. 7A-C).
Experiment 2: Intact dura mater
Behavioral assessment
The outcomes of minipigs in this experiment were
very similar to those in Experiment 1 (Fig. 8). One week after SCI, the animals
suffered complete paraplegia with almost no movement ability in all
joints of the hind limbs (ANOVA summary for 1st week: F (2,
6)=495.5; P<0.0001; Control vs. SCI P<0.0001). At the end of
the survival period, the score was 8.7±3.35 points (ANOVA summary
for 9th week: F (2, 6)=13.41; P=0.0028; Control vs. SCI
P=0.0051).
We did not notice any significant differences
between the SCI group and the saline treated group (24°C) at any
time point during the whole survival period. The final neurological
score was 7.67±4.73 (SCI vs. Saline P=0.9683).
Histological outcome
The pattern of spinal cord tissue disintegrity after
SCI was quite similar to that described in Experiment 1 (Figs. 9 and 10). At the lesion epicenter, the
cumulative white matter sparing was 31.48%±7.45 and the spared gray
matter was 23.7%±4.35. The volumes of preserved white matter ranked
in descending order: ventral columns (77.91%±7.48) > dorsal
columns (75.54%±5.89) > lateral columns (72.23%±6.35).
After 24°C saline treatment, there was an increase
in white matter preservation (Fig.
9B-D). The area of preserved white matter was significant (SCI
vs. saline P<0.05) at several levels of the evaluated segments
in the dorsal and lateral columns. The largest differences were
noted in the lateral columns where hypothermia increased white
matter preservation by 13.71%±4.75 (Fig.
9C). This improvement was significant along the rostro-caudal
axis from +2 to −2 segment. The gray matter integrity was
influenced as well (Fig. 10).
Quantitative analysis revealed overall improvement by 16.68±5.76%
in comparison to the SCI group. The improvement was significant
(SCI vs. saline P<0.05) in all segments except for the epicenter
itself (Fig. 10A). The number of
motoneurons was also improved by saline treatment (Fig. 10B). As shown in Fig. 10B, the number of motoneurons dropped
from the initial value of 16.58±1.54 cells in the control group to
cumulative 5.7±1.78 motoneurons in the SCI group. The number of
motoneurons was significantly higher in all segments caudal to the
injury epicenter (Fig. 10B). The
cumulative number of motoneurons in the VHs in the saline-treated
group increased to 6.9±1.57 cells.
Imunohistochemistry
Nine weeks after SCI, the density of neurofilaments
strongly decreased in all white matter columns (Figs. 11 and 12). Again, the lowest density of SMI-312
positive axons (59.16±5.57% of spared neurofilaments) was
identified in the lateral columns.
Although saline hypothermia had beneficial influence
on neurofilament's preservation in all white matter columns
(Figs. 11 and 12), the effect of treatment was more
prominent in the dorsal and the lateral columns than in the ventral
columns (Fig. 12).
Durotomy vs. intact dura mater
To determine the effect of durotomy, the data from
both SCI groups from Experiment 1 (durotomy) and Experiment 2
(intact dura mater) are summarized in Fig. 13. We detected significantly better
neurological outcome in animals with intact dura mater at the
4th-6th weeks (P<0.05), but the differences were non-significant
at the end of the survival period (Fig.
13A).
The animals with intact dura mater also showed
improvement in white matter preservation (segments +3, +2, −2, −3)
and in density of SMI-312 positive axons (from +3 to +1 segments)
(Fig. 13B and D). In contrast, both
the grey matter sparing and preservation of motoneurons
deteriorated in segments distant from the lesion site (Fig. 13C and D).
Correlation
To determine the sensitivity of the individual
outcome parameters, we utilized the data from all groups from
experiments 1 and 2 (n=24) and performed a Spearman's correlation
analysis between individual outcome parameters and final behavioral
scores (Fig. 14).
We found moderately positive correlation between the
neurological score and the number of neurofilaments at 9 weeks
(r=0.6746; P=0.0003) followed by weakly positive correlation
between neurological score and number of motoneurons in the VHs
(r=0.4660; P=0.0217), cumulative white matter sparing (r=0.4708;
P=0.0202) and cumulative grey matter sparing (r=0.4760;
P=0.0187).
Discussion
In the present study, we tested the efficacy of
local hypothermia on the outcome parameters in a minipig SCI model
utilizing a computer-controlled impact device. Two different
approaches to local hypothermia treatment were compared: i)
Post-SCI durotomy allowing direct access of perfusion solutions to
the spinal cord tissue, and ii) post-SCI treatment with intact dura
mater.
Comparison of the outcomes from both SCI groups (in
Experiments 1 and 2) demonstrates that the durotomy itself had no
impact on the final neurological outcome. However, the recovery of
the animals treated with durotomy was noticeably slower and the
neurological score of these minipigs was significantly worse from
the 4th to 6th week. This finding was supported by our histological
observations where the white matter outcome parameters showed
significantly worsened tissue and axonal preservation in the group
treated with durotomy. Based on data from the literature, durotomy
itself performed soon after SCI could be beneficial. The positive
effects are generally attributed to alleviated intraspinal
pressure, subsequent improvement in blood perfusion of the injured
tissues, and also to reduction of lesion volume due to attenuation
of post-traumatic cystic cavitation (60–63).
Beneficial influence achieved through durotomy applied after acute
SCI was described as early as in 1988 by Perkins and Deane
(64). Based on later studies,
spinal cord decompression with durotomy has substantial effect on
neurological outcome (60,62,63). It
has been described, in terms of its application, not worsening the
neurological state of the patients in clinical studies (65,66).
Iannotti et al (67) reported
beneficial effects of durotomy itself and combined with an
allograft patch performed 4 h after injury. These researchers found
that cases with durotomy showed improved recovery and significant
reduction in lesion volume.
On the other hand, durotomy disrupts the natural
circulation of cerebrospinal fluid (CSF), which can be associated
with adverse effects. Smith et al (63) showed that durotomy itself had
detrimental effect and increased scar and cavity formation. There
are evidences that even more severe complications (e.g.,
low-CSF-pressure headache with meningitis or transient
quadriplegia) could be associated with durotomy (68). Jones et al (69,70)
performed two separate studies utilizing the porcine model of
weight drop and compression SCI followed by decompression surgery
four h after primary injury. Both studies describe in detail the
eventual consequences of the performed durotomy. The first study
showed that decompression following SCI resulted in varying degrees
of spinal cord swelling, occlusion of the subarachnoid space and
blockage of CSF flow. According to them, an important factor in
this process is the initial severity of the injury. These
observations may partly explain the lack of benefit of
decompression in some patients. They suggest a need to reduce
spinal cord swelling in order to optimize the clinical outcome
after acute SCI (69). The second
study posits that intradural swelling may induce secondary
pathology due to interruption of normal CSF flow and constraints on
swelling by the surrounding meninges, leading to inconclusive
results of decompression and neurological outcome following SCI
(70). In our study we observed
spinal cord swelling in all groups which underwent durotomy. In
spite of the treatment procedures, the swelling persisted after
five h, when the dura mater was sutured in order to stop CSF
leakage and development of meningitis. We assume that delayed
closure of the dura encircling the swollen segments of the spinal
cord could be one of the reasons for the worsened outcomes noted in
the animals that underwent durotomy.
Schumacher et al (71) reported an association between
neurofilament loss and impaired hind-limb motor function after SCI.
Significantly lower white matter integrity and reduction of
neurofilaments in durotomy-treated animals could reflect multiple
factors causing variable outcomes following the interruption of
long-projection pyramidal (lateral corticospinal) and
extrapyramidal (rubrospinal and reticulospinal) tracts.
Neurofilaments are particularly abundant in large myelinated axons
and are essential for axon radial growth and axon caliber
maintenance during development (72,73).
Wang et al (74) reported
that NF gene transcriptional regulation is crucial for NF
expression, predominantly in axonal regeneration and degenerative
diseases.
The opposite effect was seen in gray matter.
Quantitative analysis confirmed the loss of gray matter and
motoneurons at the lesion site and in rostro-caudal segments up to
2 cm in both SCI models. These changes were less dramatic in the
SCI-durotomy group but only in segments (+3 and −3), i.e., distant
from the lesion site. The response of motoneurons to SCI depends on
their ability to handle calcium. Previous results have indicated an
unusually high influx of Ca2+ into motoneurons upon
their stimulation (75–77). We have reported increased expression
of parvalbumin, a Ca2+-binding protein, in motoneurons
ten days after Th9 transection (78). The results suggest that this
Ca2+-binding protein may rescue trauma-affected neuronal
circuits involved in motor control.
Effects of hypothermia in Experiment 1
When planning to carry out therapeutic local
hypothermia in the acute stage of spinal cord compression in
minipigs, we took into consideration two critical aspects, i.e.,
the cooling conditions and the penetration of perfusion solutions,
both essential for the success of treatment. Under certain
circumstances, radical hypothermia appears more promising than
modest hypothermia (79–81). Vanický et al (82) reported that deep spinal cord
hypothermia (t<15°C) via epidural cooling with 5°C saline
provided effective protection against long-lasting spinal cord
ischemia in rabbits. We have previously shown the benefit of five
hours of local hypothermia (t=19°C) in an 8N and 15N
computer-controlled compression model of SCI in minipig (57). The results indicated that modulation
of the microcircuits leading to better functional outcome may
depend on the preservation of axons in the lateral columns of
cranial and caudal segments immediately adjacent to the lesion, and
the protection of neurofilaments in the same segments of lateral
funiculi (57).
In the present study, durotomy allowed direct
penetration of solutions (saline, medium or enriched medium)
through the outer surface of the uncovered spinal cord within 3 cm
in cranio-caudal direction. However, none of these treatments
improved the final neurological outcome, and quite surprisingly,
the application of the cold enriched medium caused significant
worsening of the scores which became apparent at the 4th week and
persisted almost until the end of the survival period. Morphometric
analyses of the lesion size indicated that individual treatment
procedures did improve some tissue preservation in both the white
and grey matter. Saline hypothermia activated the processes that
led to better regeneration of the white matter within the dorsal
and lateral columns in almost all cranio-caudal segments. We also
found improvements in neurofilament expression and the sparing of
grey matter/motoneurons. Earlier in vitro study showed that
a particular neuronal population in monolayer cultures of
dissociated murine spinal cord was especially sensitive to
hypothermic stress (83). When the
spinal cord cultures were exposed to the temperatures below 17°C,
the neuronal perikarya and dendrites swelled, with the majority of
the swollen neurons dying during the phase of rewarming to 37°C,
while glial and other nonneuronal cell types were unaffected. The
authors claimed that prolonged exposure (2–6 h) of monolayer
cultures to the temperatures below 17°C caused significant neuronal
death mediated primarily by the NMDA receptor-ion channel complexes
in the dendro-somatic membranes. In the present study we report
that the temperature of the saline (at 15 or 24°C) significantly
reduced motoneuron death. Surface cooling of the spinal cord
creates a substantial temperature gradient within the cord tissue
(84) which may differently
influence the viability of spinal motoneurons in in vivo
experiments.
These protective effects can be attributed to
transient hypothermia. Previously, strong protective effects of
local spinal cord hypothermia had been demonstrated in spinal cord
ischemia experiments (24,82,85,86).
Local hypothermia was utilized in experimental studies after
traumatic SCI as well. Cooling was generally initiated no later
than 30 min after acute SCI, its duration varied between 20 min and
48 h and the temperature varied between 19°C up to 35°C. In
general, these studies showed that hypothermia is more effective
when initiated early after injury and lasts for a longer time.
Shorter duration may require more profound cooling to achieve
similar effect (35). In general,
these data suggest that long periods of local hypothermia are
tolerated well and may improve long-term tissue sparing and
functional outcome (14,35,40–43,47,57,87).
Treatment with cold medium and/or enriched medium
significantly preserved the white matter in dorsal (+3 segment) and
lateral (+3 and +1 segment) columns located cranially to the lesion
site. The medium negatively affected neurofilaments predominantly
in the caudal segments and led to non-significantly lower
behavioral outcome than in the SCI + durotomy group from the 7th to
9th week. In the case of the enriched medium-treated minipigs, the
neurological outcome was very low during the whole survival period.
It appears that low temperature of medium and/or enriched medium
influenced the blood flow and slowed down the cell metabolism
resulting in cell inability to profit from the ingredients
contained in the cooling solutions. The mechanisms underlying
protection by hypothermia are believed to be slowing down the
enzymatic activity of reactions that require adenosine triphosphate
(88). The results of our study
confirm that exposure to low temperatures may be stressful for the
cells treated with medium and/or enriched medium. It is still
questionable whether increasing the target temperature during
enriched medium/medium hypothermia could be able to improve
histological/neurological outcomes.
Effect of hypothermia in Experiment 2
In Experiment 2 we tested a less invasive saline
(24°C) treatment through the intact dura mater, minimizing the risk
of spinal parenchymal infection. This moderate hypothermia
treatment had significant effect on tissue preservation, but this
effect was not associated with improved motor recovery. Several
studies described similar results, where spinal cord hypothermia
protected tissue from excessive loss without significant
improvement in neurological function (41,57).
Similarly, Teh et al (87)
observed that hypothermia improved tissue preservation and
neurological outcome during survival, but not the final
neurological outcome. On the other hand, Casas et al
(40) showed that regional cooling
applied 30 min after moderate contusive SCI was not beneficial or
detrimental in terms of tissue sparing, neuronal preservation, or
locomotor outcome. They suggested, that this method of cooling may
reduce blood flow in the injured spinal cord and exacerbate
secondary injury.
The results of this pilot study extend the use of
our reproducible minipig model for preclinical SCI experiments. The
data indicate that further therapeutic interventions can modify the
outcomes we have followed. The degree of injury we have used (18N
SCI) is perhaps too severe for experimental therapy, and for
observing any changes in neurological outcome, and less severe
degrees of injury would be preferable. Our observations indicate
that posttraumatic edema is a serious pathophysiological mechanism
contributing to injury. When planning interventions with durotomy,
prolonged duration of post-traumatic swelling should be taken into
consideration. The optimal degree of hypothermia and its timing
should be studied systematically. Further experimental/clinical
studies are required to determine the optimal cooling parameters
(therapeutic window, optimal temperature and duration) and to test
the effectiveness of hypothermic therapy in various trauma-induced
SCI models using large experimental animals.
Acknowledgements
The authors would like to thank Dr S. Gancarcikova
from the University of Veterinary Medicine and Pharmacy (Kosice,
Slovakia) for preparation of protocols approved by the State
Veterinary and Food Administration and Mrs. A. Kosova for her
technical assistance.
Funding
The present study was supported by the project:
Formation and Development of a Diagnostic Procedure in the
Rreatment of Trauma-Injured Spinal Cord (grant no. ITMS
26220220202), supported by the Research and Development Operational
Program funded by the ERDF.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
MMars, NL and JG were involved in creating the
study concept and designing the experiments. VL, AT, IC and PR were
involved in anesthetizing animals and post-surgical animal care.
MMars, IS, MG, IL, JK, IS Jr., JG and JP were involved in spinal
cord compression and implantation of the perfusion chamber. JG, SP
and MZ prepared the perfusion chamber. JG, MZ, SP and JP assisted
in hypothermic treatment administration. JP, MZ, SP, JG, KB and MB
were invovled in transcardial perfusion and spinal cord tissue
preparation. MZ, SP, ER, MMart, AS and AK performed the
immunohistochemical staining and histological procedures. PR, MZ,
SP and JG performed the behavioral assessments. MZ, NL and DM
performed data analysis. MZ and NL wrote the paper.
Ethics approval and consent to
participate
The experimental protocols were prepared in
accordance with the EC Council Directive (2010/63/EU) regarding the
use of animals in research and approved by the State Veterinary and
Food Administration of the Slovak Republic (decision no.
1319/13-221) as well as by the Ethical Commission of the University
of Veterinary Medicine and Pharmacy in Kosice, Slovak Republic. All
efforts were made to minimize the size of experimental groups and
animal suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
References
|
1
|
Tator CH: Review of treatment trials in
human spinal cord injury: Issues, difficulties, and
recommendations. Neurosurgery. 59:957–987. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tator CH: Update on the pathophysiology
and pathology of acute spinal cord injury. Brain Pathol. 5:407–413.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McDonald JW and Sadowsky C: Spinal-cord
injury. Lancet. 359:417–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balentine JD: Pathology of experimental
spinal cord trauma. I. The necrotic lesion as a function of
vascular injury. Lab Invest. 39:236–253. 1978.PubMed/NCBI
|
|
5
|
Simard JM, Woo SK, Aarabi B and Gerzanich
V: The Sur1-Trpm4 channel in spinal cord injury. J Spine Suppl.
4:0022013.
|
|
6
|
Rowland JW, Hawryluk GW, Kwon B and
Fehlings MG: Current status of acute spinal cord injury
pathophysiology and emerging therapies: Promise on the horizon.
Neurosurg Focus. 25:E22008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bracken MB, Shepard MJ, Collins WF,
Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers
L, Maroon J, et al: A randomized, controlled trial of
methylprednisolone or naloxone in the treatment of acute
spinal-cord injury. Results of the Second National Acute Spinal
Cord Injury Study. N Engl J Med. 322:1405–1411. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J and Pearse DD: Therapeutic
hypothermia in spinal cord injury: The status of its use and open
questions. Int J Mol Sci. 16:16848–16879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon BK, Okon E, Hillyer J, Mann C,
Baptiste D, Weaver LC, Fehlings M and Tetzlaff W: A systematic
review of non-invasive pharmacologic neuroprotective treatments for
acute spinal cord injury. J Neurotrauma. 28:1545–1588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tetzlaff W, Okon EB, Karimi-Abdolrezaee S,
Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D,
Smithson LJ, et al: A systematic review of cellular transplantation
therapies for spinal cord injury. J Neurotrauma. 28:1611–1682.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang B, Armstrong JS, Reyes M, Kulikowicz
E, Lee JH, Spicer D, Bhalala U, Yang ZJ, Koehler RC, Martin LJ and
Lee JK: White matter apoptosis is increased by delayed hypothermia
and rewarming in a neonatal piglet model of hypoxic ischemic
encephalopathy. Neuroscience. 316:296–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martirosyan NL, Patel AA, Carotenuto A,
Kalani MY, Bohl MA, Preul MC and Theodore N: The role of
therapeutic hypothermia in the management of acute spinal cord
injury. Clin Neurol Neurosurg. 154:79–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ok JH, Kim YH and Ha KY: Neuroprotective
effects of hypothermia after spinal cord injury in rats:
Comparative study between epidural hypothermia and systemic
hypothermia. Spine (Phila Pa 1976). 37:E1551–E1559. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ha KY and Kim YH: Neuroprotective effect
of moderate epidural hypothermia after spinal cord injury in rats.
Spine (Phila Pa 1976). 33:2059–2065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lo TP Jr, Cho KS, Garg MS, Lynch MS,
Marcillo AE, Koivisto DL, Stagg M, Abril RM, Patel S, Dietrich WD
and Pearse DD: Systemic hypothermia improves histological and
functional outcome after cervical spinal cord contusion in rats. J
Comp Neurol. 514:433–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crowe MJ, Bresnahan JC, Shuman SL, Masters
JN and Beattie MS: Apoptosis and delayed degeneration after spinal
cord injury in rats and monkeys. Nat Med. 3:73–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shuman SL, Bresnahan JC and Beattie MS:
Apoptosis of microglia and oligodendrocytes after spinal cord
contusion in rats. J Neurosci Res. 50:798–808. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumont RJ, Okonkwo DO, Verma S, Hurlbert
RJ, Boulos PT, Ellegala DB and Dumont AS: Acute spinal cord injury,
part I: Pathophysiologic mechanisms. Clin Neuropharmacol.
24:254–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibuya S, Miyamoto O, Janjua NA, Itano T,
Mori S and Norimatsu H: Posttraumatic moderate systemic hypothermia
reduces TUNEL positive cells following spinal cord injury in rat.
Spinal Cord. 42:29–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dietrich WD, Atkins CM and Bramlett HM:
Protection in animal models of brain and spinal cord injury with
mild to moderate hypothermia. J Neurotrauma. 26:301–312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grulova I, Slovinska L, Nagyova M, Cizek M
and Cizkova D: The effect of hypothermia on sensory-motor function
and tissue sparing after spinal cord injury. Spine J. 13:1881–1891.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seo JY, Kim YH, Kim JW, Kim SI and Ha KY:
Effects of therapeutic hypothermia on apoptosis and autophagy after
spinal cord injury in rats. Spine (Phila Pa 1976). 40:883–890.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strauch JT, Lauten A, Spielvogel D, Rinke
S, Zhang N, Weisz D, Bodian CA and Griepp RB: Mild hypothermia
protects the spinal cord from ischemic injury in a chronic porcine
model. Eur J Cardiothorac Surg. 25:708–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshitake A, Mori A, Shimizu H, Ueda T,
Kabei N, Hachiya T, Okano H and Yozu R: Use of an epidural cooling
catheter with a closed countercurrent lumen to protectagainst
ischemic spinal cord injury in pigs. J Thorac Cardiovasc Surg.
134:1220–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue S, Mori A, Shimizu H, Yoshitake A,
Tashiro R, Kabei N and Yozu R: Combined use of an epidural cooling
catheter and systemic moderate hypothermia enhances spinal cord
protection against ischemic injury in rabbits. J Thorac Cardiovasc
Surg. 146:696–701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saito T, Saito S, Yamamoto H and Tsuchida
M: Neuroprotection following mild hypothermia after spinal cord
ischemia in rats. J Vasc Surg. 57:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fay T: Early experiences with local and
generalized refrigeration of the human brain. J Neurosurg.
16:239–260. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tuzgen S, Kaynar MY, Güner A, Gümüştaş K,
Belce A, Etuş V and Ozyurt E: The effect of epidural cooling on
lipid peroxidation after experimental spinal cord injury. Spinal
Cord. 36:654–657. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalayci M, Coskun O, Cagavi F, Kanter M,
Armutcu F, Gul S and Acikgoz B: Neuroprotective effects of ebselen
on experimental spinal cord injury in rats. Neurochem Res.
30:403–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji X, Luo Y, Ling F, Stetler RA, Lan J,
Cao G and Chen J: Mild hypothermia diminishes oxidative DNA damage
and pro-death signaling events after cerebral ischemia: A mechanism
for neuroprotection. Front Biosci. 12:1737–1747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duz B, Kaplan M, Bilgic S, Korkmaz A and
Kahraman S: Does hypothermic treatment provide an advantage after
spinal cord injury until surgery? An experimental study. Neurochem
Res. 34:407–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Topuz K, Colak A, Cemil B, Kutlay M,
Demircan MN, Simsek H, Ipcioglu O, Kucukodaci Z and Uzun G:
Combined hyperbaric oxygen and hypothermia treatment on oxidative
stress parameters after spinal cord injury: An experimental study.
Arch Med Res. 41:506–512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karamouzian S, Akhtarshomar S, Saied A and
Gholamhoseinian A: Effects of methylprednisolone on neuroprotective
effects of delay hypothermia on spinal cord injury in rat. Asian
Spine J. 9:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang D and Zhang J: Effects of hypothermia
combined with neural stem cell transplantation on recovery of
neurological function in rats with spinal cord injury. Mol Med Rep.
11:1759–1767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu X, Li N, Zhu L, Zhou Y and Cheng H:
Beneficial effects of local profound hypothermia and the possible
mechanism after experimental spinal cord injury in rats. J Spinal
Cord Med. 39:220–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morino T, Ogata T, Takeba J and Yamamoto
H: Microglia inhibition is a target of mild hypothermic treatment
after the spinal cord injury. Spinal Cord. 46:425–431. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Horiuchi T, Kawaguchi M, Kurita N, Inoue
S, Nakamura M, Konishi N and Furuya H: The long-term effects of
mild to moderate hypothermia on gray and white matter injury after
spinal cord ischemia in rats. Anesth Analg. 109:559–566. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Levi AD, Green BA, Wang MY, Dietrich WD,
Brindle T, Vanni S, Casella G, Elhammady G and Jagid J: Clinical
application of modest hypothermia after spinal cord injury. J
Neurotrauma. 26:407–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmad FU, Wang MY and Levi AD: Hypothermia
for acute spinal cord injury-a review. World Neurosurg. 82:207–214.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Casas CE, Herrera LP, Prusmack C, Ruenes
G, Marcillo A and Guest JD: Effects of epidural hypothermic saline
infusion on locomotor outcome and tissue preservation after
moderate thoracic spinal cord contusion in rats. J Neurosurg Spine.
2:308–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morochovic R, Chudá M, Talánová J, Cibur
P, Kitka M and Vanický I: Local transcutaneous cooling of the
spinal cord in the rat: Effects on long-term outcomes after
compression spinal cord injury. Int J Neurosci. 118:555–568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morizane K, Ogata T, Morino T, Horiuchi H,
Yamaoka G, Hino M and Miura H: A novel thermoelectric cooling
device using Peltier modules for inducing local hypothermia of the
spinal cord: The effect of local electrically controlled cooling
for the treatment of spinal cord injuries in conscious rats.
Neurosci Res. 72:279–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barbosa MO, Cristante AF, Santos GB,
Ferreira R, Marcon RM and Barros Filho TE: Neuroprotective effect
of epidural hypothermia after spinal cord lesion in rats. Clinics
(Sao Paulo). 69:559–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Albin MS, White RJ, Locke GS, Massopust LC
Jr and Kretchmer HE: Localized spinal cord hypthermia-anesthetic
effects and application to spinal cord injury. Anesth Analg.
46:8–16. 1967.PubMed/NCBI
|
|
45
|
Negrin J Jr: Spinal cord hypothermia in
the neurosurgical management of the acute and chronic
post-traumatic paraplegic patient. Paraplegia. 10:336–343. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yashon D, Vise WM, Dewey RC and Hunt WE:
Temperature of the spinal cord during local hypothermia in dogs. J
Neurosurg. 39:742–745. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dimar JR, Shields CB, Zhang YP, Burke DA,
Raque GH and Glassman SD: The role of directly applied hypothermia
in spinal cord injury. Spine (Phila Pa 1976). 25:2294–2302. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chatzipanteli K, Yanagawa Y, Marcillo AE,
Kraydieh S, Yezierski RP and Dietrich WD: Posstraumatic hypothermia
reduced polymorphonuclear leukocyte accumulation following spinal
cord injury in rats. J Neurotrauma. 17:321–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cappuccino A, Bisson LJ, Carpenter B,
Marzo J, Dietrich WD and Cappuccino H: The use of systemic
hypothermia for the treatment of an acute cervical spinal cord
injury in a professional football player. Spine (Phila Pa 1976).
35:E57–E62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Levi AD, Casella G, Green BA, Dietrich WD,
Vanni S, Jagid J and Wang MY: Clinical outcomes using modest
intravascular hypothermia after acute cervical spinal cord injury.
Neurosurgery. 66:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Albin MS, White RJ, Yashon D and Harris
LS: Effects of localized cooling on spinal cord trauma. J Trauma.
9:1000–1008. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Black P and Markowitz RS: Experimental
spinal cord injury in monkeys: Comparison of steroids and local
hypothermia. Surg Forum. 22:409–411. 1971.PubMed/NCBI
|
|
53
|
Dididze M, Green BA, Dietrich WD, Vanni S,
Wang MY and Levi AD: Systemic hypothermia in acute cervical spinal
cord injury: A case-controlled study. Spinal Cord. 51:395–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
MacLaren R, Gallagher J, Shin J, Varnado S
and Nguyen L: Assessment of adverse events and predictors of
neurological recovery after therapeutic hypothermia. Ann
Pharmacother. 48:17–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hansebout RR and Hansebout CR: Local
cooling for traumatic spinal cord injury: Outcomes in 20 patients
and review of the literature. J Neurosurg Spine. 20:550–561. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Navarro R, Juhas S, Keshavarzi S, Juhasova
J, Motlik J, Johe K, Marsala S, Scadeng M, Lazar P, Tomori Z, et
al: Chronic spinal compression model in minipigs: A systematic
behavioral, qualitative, and quantitative neuropathological study.
J Neurotrauma. 29:499–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gedrova S, Galik J, Marsala M, Zavodska M,
Pavel J, Sulla I, Gajdos M, Lukac I, Kafka J, Ledecky V, et al:
Neuroprotective effect of local hypothermia in a
computer-controlled compression model in minipig: Correlation of
tissue sparing along the rostro-caudal axis with neurological
outcome. Exp Ther Med. 15:254–270. 2018.PubMed/NCBI
|
|
58
|
Sulla I, Boldizar M, Racekova E and Balik
V: Experience with a thoracic laminectomy in minipigs. Folia
Veterinaria. 56:35–39. 2012.
|
|
59
|
Lim SN, Gladman SJ, Dyall SC, Patel U,
Virani N, Kang JX, Priestley JV and Michael-Titus AT: Transgenic
mice with high endogenous omega-3 fatty acids are protected from
spinal cord injury. Neurobiol Dis. 51:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tator CH and Fehlings MG: Review of the
secondary injury theory of acute spinal cord trauma with emphasis
on vascular mechanisms. J Neurosurg. 75:15–26. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rosenfeld JF, Vaccaro AR, Albert TJ, Klein
GR and Cotler JM: The benefits of early decompression in cervical
spinal cord injury. Am J Orthop (Belle Mead NJ). 27:23–28.
1998.PubMed/NCBI
|
|
62
|
Fehlings MG and Perrin RG: The timing of
surgical intervention in the treatment of spinal cord injury: A
systematic review of recent clinical evidence. Spine (Phila Pa
1976). 31 (11 Suppl):S28–S36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Smith JS, Anderson R, Pham T, Bhatia N,
Steward O and Gupta R: Role of early surgical decompression of the
intradural space after cervical spinal cord injury in an animal
model. J Bone Joint Surg Am. 92:1206–1214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Perkins PG and Deane RH: Long-term
follow-up of six patients with acute spinal injury following dural
decompression. Injury. 19:397–401. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Phang I, Werndle MC, Saadoun S, Varsos G,
Czosnyka M, Zoumprouli A and Papadopoulos MC: Expansion duroplasty
improves intraspinal pressure, spinal cord perfusion pressure, and
vascular pressure reactivity index in patients with traumatic
spinal cord injury: Injured spinal cord pressure evaluation study.
J Neurotrauma. 32:865–874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kong CY, Hosseini AM, Belanger LM, Ronco
JJ, Paquette SJ, Boyd MC, Dea N, Street J, Fisher CG, Dvorak MF and
Kwon BK: A prospective evaluation of hemodynamic management in
acute spinal cord injury patients. Spinal Cord. 51:466–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Iannotti C, Zhang YP, Shields LB, Han Y,
Burke DA, Xu XM and Shields CB: Dural repair reduces connective
tissue scar invasion and cystic cavity formation after acute spinal
cord laceration injury in adult rats. J Neurotrauma. 23:853–865.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Desai A, Ball PA, Bekelis K, Lurie J,
Mirza SK, Tosteson TD and Weinstein JN: SPORT: Does incidental
durotomy affect long-term outcomes in cases of spinal stenosis?
Neurosurgery. 69:38–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jones CF, Cripton PA and Kwon BK: Gross
morphological changes of the spinal cord immediately after surgical
decompression in a large animal model of traumatic spinal cord
injury. Spine (Phila Pa 1976). 37:E890–E899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jones CF, Newell RS, Lee JH, Cripton PA
and Kwon BK: The pressure distribution of cerebrospinal fluid
responds to residual compression and decompression in an animal
model of acute spinal cord injury. Spine (Phila Pa 1976).
37:E1422–E1431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schumacher PA, Siman RG and Fehlings MG:
Pretreatment with calpain inhibitor CEP-4143 inhibits calpain I
activation and cytoskeletal degradation, improves neurological
function, and enhances axonal survival after traumatic spinal cord
injury. J Neurochem. 74:E1646–E1655. 2000. View Article : Google Scholar
|
|
72
|
Nishida F, Sisti MS, Zanuzzi CN, Barbeito
CG and Portiansky EL: Neurons of the rat cervical spinal cord
express vimentin and neurofilament after intraparenchymal injection
of kainic acid. Neurosci Lett. 643:103–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lépinoux-Chambaud C and Eyer J: Review on
intermediate filaments of the nervous system and their pathological
alterations. Histochem Cell Biol. 140:13–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang H, Wu M, Zhan C, Ma E, Yang M, Yang X
and Li Y: Neurofilament proteins in axonal regeneration and
neurodegenerative diseases. Neural Regen Res. 7:620–626.
2012.PubMed/NCBI
|
|
75
|
Greig A, Donevan SD, Mujtaba TJ, Parks TN
and Rao MS: Characterization of the AMPA-activated receptors
present on motoneurons. J Neurochem. 74:179–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vandenberghe W, Ihle EC, Patneau DK,
Robberecht W and Brorson JR: AMPA receptor current density, not
desensitization, predicts selective motoneuron vulnerability. J
Neurosci. 20:7158–7166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Van Den Bosch L and Robberecht W:
Different receptors mediate motor neuron death induced by short and
long exposures to excitotoxicity. Brain Res Bull. 53:383–388. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lukáčová N, Kisucká A, Pavel J, Hricová L,
Kucharíková A, Gálik J, Maršala M, Langfort J and Chalimoniuk M:
Spinal cord transection modifies neuronal nitric oxide synthase
expression in medullar reticular nuclei and in the spinal cord and
increases parvalbumin immunopositivity in motoneurons below the
site of injury in experimental rabbits. Acta Histochem.
114:518–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gillinov AM, Redmond JM, Zehr KJ, Troncoso
JC, Arroyo S, Lesser RP, Lee AW, Stuart RS, Reitz BA and
Baumgartner WA: Superior cerebral protection with profound
hypothermia during circulatory arrest. Ann Thorac Surg.
55:1432–1439. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mezrow CK, Midulla PS, Sadeghi AM, Gandsas
A, Wang W, Dapunt OE, Zappulla R and Griepp RB: Evaluation of
cerebral metabolism and quantitative electroencephalography after
hypothermic circulatory arrest and low-flow cardiopulmonary bypass
at different temperatures. J Thorac Cardiovasc Surg. 107:1006–1019.
1994.PubMed/NCBI
|
|
81
|
Lima B, Williams JB, Bhattacharya SD, Shah
AA, Andersen N, Gaca JG and Hughes GC: Results of proximal arch
replacement using deep hypothermia for circulatory arrest: Is
moderate hypothermia really justifiable? Am Surg. 77:1438–1444.
2011.PubMed/NCBI
|
|
82
|
Vanický I, Marsala M, Gálik J and Marsala
J: Epidural perfusion cooling protection against protracted spinal
cord ischemia in rabbits. J Neurosurg. 79:736–741. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lucas JH, Wang GF and Gross GW: NMDA
antagonists prevent hypothermic injury and death of mammalian
spinal neurons. J Neurotrauma. 7:229–236. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Romero-Sierra C, Hansebout R, Sierhuis A
and Lewin M: A new method for localized spinal cord cooling. Med
Biol Enf. 12:188–193. 1974. View Article : Google Scholar
|
|
85
|
Marsala M, Vanicky I, Galik J, Radonak J,
Kundrat I and Marsala J: Panmyelic epidural cooling protects
against ischemic spinal cord damage. J Surg Res. 55:21–31. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Marsala M, Galik J, Ishikawa T and Yaksh
TL: Technique of selective spinal cord cooling in rat: Methodology
and application. J Neurosci Methods. 74:97–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Teh DBL, Chua SM, Prasad A, Kakkos I,
Jiang W, Yue M, Liu X and All AH: Neuroprotective assessment of
prolonged local hypothermia post contusive spinal cord injury in
rodent model. Spine J. 18:507–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Camara AK, Riess ML, Kevin LG, Novalija E
and Stowe DF: Hypothermia augments reactive oxygen species detected
in the guinea pig isolated perfused heart. Am J Physiol Heart Circ
Physiol. 286:H1289–H1299. 2004. View Article : Google Scholar : PubMed/NCBI
|