Introduction
In 2015, ~376,300 cases of colorectal cancer (CRC)
were diagnosed in China (1). CRC is
regarded as one of the most common cancers with high morbidity and
mortality (2). Despite the
improvement of diagnostic technologies, screening tools and
clinical therapy, CRC remains a global challenge for public health
due to the absence of a ‘gold standard’ for early diagnosis
(2).
The molecular carcinogenic mechanisms of CRC have
not been completely elucidated, however, CRC appears to be driven
by the accumulation of abnormal genetic and epigenetic alternations
in both oncogenes and tumor-suppressor genes (3). Cytosine modification, including DNA
methylation, is one of the basic molecular mechanisms involved in
the initiation and progression of CRC (2,4–6). Therefore, DNA methylation or epigenetic
alterations may be promising markers for the early detection of CRC
(4,7).
The endothelium PAS domain protein 1 (EPAS1) gene
product is one of the important subunits of oxygen-induced hypoxia
inducible factor (HIF) α, which regulates the primary
transcriptional response to hypoxic stress (8). Hypoxia is one of the main factors
promoting tumor angiogenesis (9,10). In
addition, angiogenesis is considered a prerequisite for a range of
biological processes, including tumorigenesis and tumor progression
(11,12). HIF-2α/EPAS1 serves a role in tumor
angiogenesis of different types of cancer, including lung cancer
(13,14), renal carcinoma (15,16),
liver cancer (17), pheochromocytoma
(18–20) and CRC (8,21).
In a previous study including 39 patients with CRC
and 43 normal controls, the expression of EPAS1 in the blood
of patients with CRC was significantly increased, and was
subsequently decreased after surgical resection of the tumor,
returning to a normal level (21).
Another study revealed significantly increased levels of
EPAS1 methylation and significantly lower levels of
EPAS1 mRNA expression in 120 primary colon adenocarcinoma
tissues compared with paracancerous tissues (8).
In the current study, quantitative
methylation-specific polymerase chain reaction (qMSP) was used to
measure EPAS1 methylation in 120 Chinese patients with CRC
and 22 healthy controls in two-stage experiments to assess whether
the methylation of EPAS1 could be used as a biomarker for
the diagnosis of CRC.
Patients and methods
Study subjects
The first phase of the association study involved 41
patients with CRC, from whom frozen tumor tissues and adjacent
tissues 5 and 10 cm away from the tumor lesions were collected.
These patients with CRC were recruited from the Third Affiliated
Hospital of Nanjing University of Traditional Chinese Medicine
(Nanjing, China) between August 2011 and March 2015 and their
average age was 64.03±11.39 years (range, 21–86 years). Of the 41
patients, 28 were male, 12 were female and 1 was missing
information. The second phase of the association study was
conducted to verify the role of EPAS1 methylation in CRC.
The second phase of the association study involved 79 CRC tumor
tissues, 79 paired adjacent tissues 5 cm away from the tumor
lesions and 22 healthy human intestinal tissues. Patients involved
in the second phase of the present study were recruited from the
Zhejiang Cancer Hospital (Hangzhou, China) and Shaoxing First
People's Hospital (Shaoxing, China) between August 2011 and January
2015. The average age of the 79 patients with CRC in the second
phase of the present association study was 60.27±11.74 years. Of
the 79 patients, 51 were male and 28 were female. All patients were
diagnosed by pathological examination. No radiotherapy or
chemotherapy was performed prior to surgery. The age and sex data
of healthy controls were not available. All clinical data were
extracted between August 2011 and March January 2015 from medical
records for subsequent analysis. The Human Research Ethics
Committee of Ningbo University (Ningbo, China) granted approval for
the present study. Each participant completed the written informed
consent form.
DNA extraction and bisulfite
conversion
DNA was extracted from frozen tissues using
E.Z.N.A.® Tissue DNA kit (Omega Bio-Tek, Inc., Norcross,
GA, USA) according to the manufacturer's protocol. DNA
concentrations measurement and bisulfite treatment were performed
as previously described (22).
SYBR-Green-based qMSP
qMSP was performed as previously described (23,24). The
following thermocycling conditions were used: Initial denaturation
at 95°C for 10 min; 45 cycles of 95°C for 20 sec, 58°C for 20 sec
and 72°C for 30 sec; melting curve analysis at 95°C for 15 sec,
58°C for 1 min and 60°C for 1 min; and a final cooling stage at
40°C for 10 min. The primer sequences for EPAS1 (95 bp) were
forward, 5′-GTTATAGATAGCGTTTGTAGAC-3′ and reverse,
5′-GATTACCACATTCCCGATA-3′; and the primer sequences for ACTB
(133 bp) were forward, 5′-TGGTGATGGAGGAGGTTTAGTAAGT-3′ and reverse,
5′-AACCAATAAAACCTACTCCTCCCTTAA-3′. The percentage of methylated
reference (PMR) of EPAS1 for each sample was calculated
using the 2−ΔΔCq quantification approach, where
ΔΔCq=sample DNA (CtEPAS1- CtACTB
control)-fully methylated DNA
(CtEPAS1-CtACTB control)
(25).
Bioinformatics analysis
The genomic position of the amplified fragment was
obtained from University of California Santa Cruz genome browser
according to human (GRCh37) assembly (genome.ucsc.edu). To evaluate the association between
mRNA expression and EPAS1 methylation, data in the TCGA
colorectal adenocarcinoma cohort with 372 samples were downloaded
from cBioPortal (www.cbioportal.org).
Statistical analysis
All data were analyzed using SPSS 18.0 software
(SPSS Inc., Chicago, IL, USA). Due to the skewed distribution of
methylation levels, data were presented as the median
(interquartile range). Friedman test and Wilcoxon nonparametric
test were used to assess the difference in methylation between
samples. Mann-Whitney U nonparametric test was used to assess the
difference in methylation between groups. Contingency correlation
test was used to evaluate the association between EPAS1
methylation and clinical features. Spearman's rank correlation
coefficient was used to assess the correlation between EPAS1
methylation and gene expression. Pearson χ2 test or
Fisher's exact test were used to assess the difference in clinical
features between different sampling locations. The receiver
operating characteristic (ROC) analysis was used to evaluate the
diagnostic value of EPAS1 promoter methylation for CRC.
Two-tailed P<0.05 was considered to indicate a statistically
significant difference. All figures were plotted using GraphPad
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Sequencing results were analyzed using Chromas LITE 2.1.1 software
(Technelysium Pty, Ltd., Brisbane, Australia).
Results
DNA methylation analysis
To assess the association between methylation of
EPAS1 and CRC, a two-stage association study was conducted.
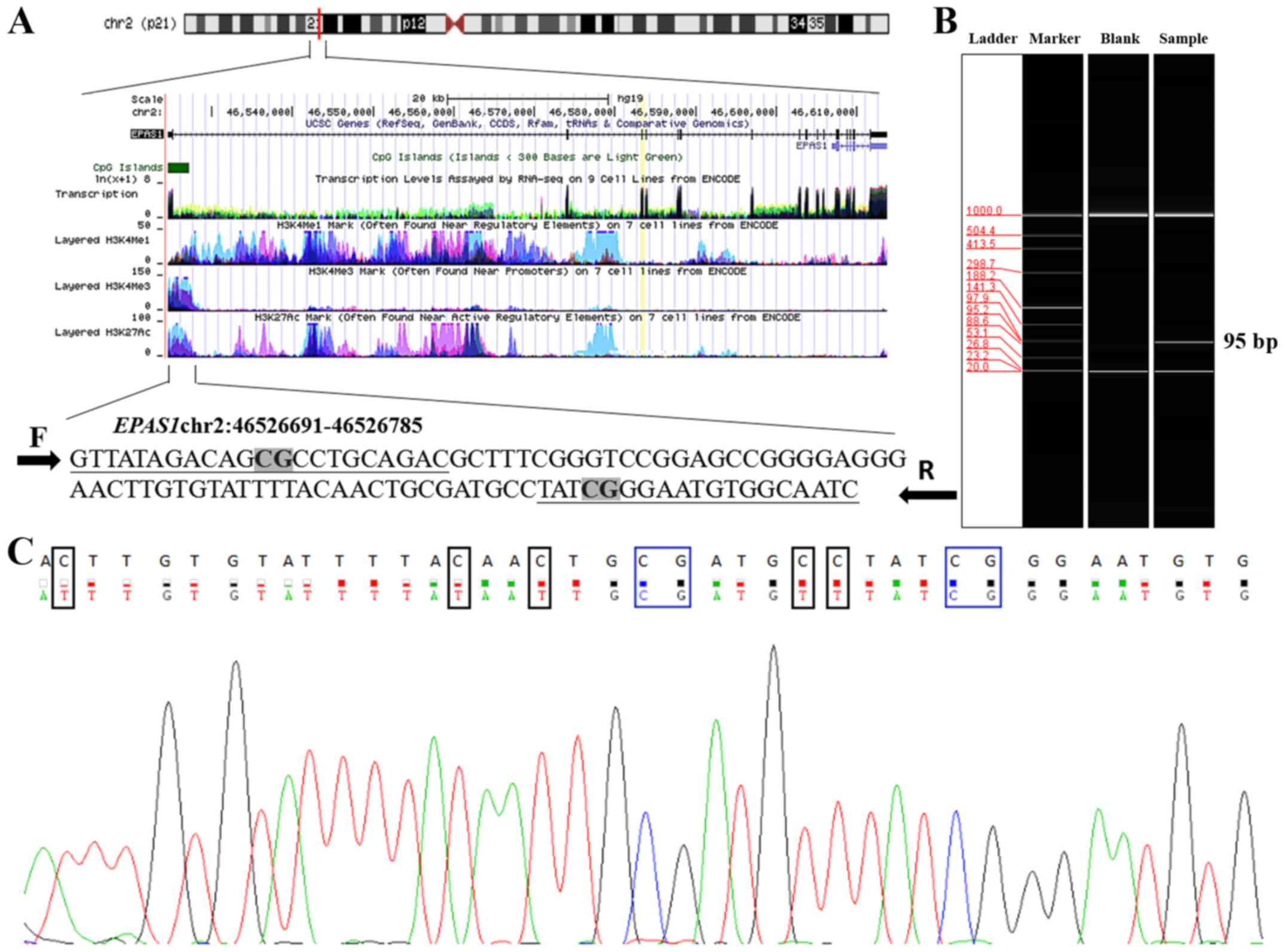
Two cytosine-phosphate-guanine (CpG) sites were identified in the
95 bp fragment of EPAS1 (hg38; chr2:46526691-46526785)
(Fig. 1A). The tested EPAS1
fragment was expected to be 95 bp (Fig.
1B). Sequencing results indicated that the amplified fragment
matched the target sequence (Fig.
1C).
Association between EPAS1
hypomethylation in patients with CRC and clinical features
The results of the present study indicated that the
EPAS1 methylation was not associated with sex, age, TNM
stage, differentiation, tumor size or lymph node metastasis (all
P>0.05) (Table I). Methylation
levels in the tissues from 41 patients with CRC (Jiangsu, China)
and 79 patients with CRC (Zhejiang, China) were examined. The
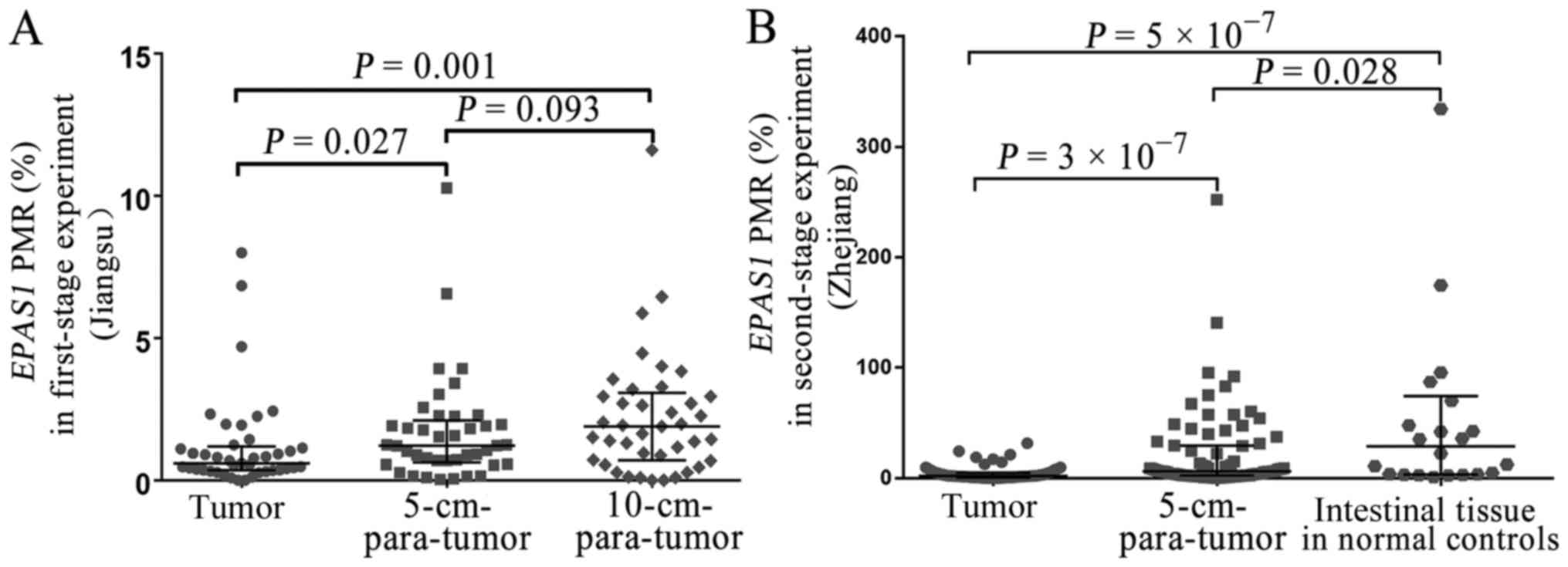
results indicated that EPAS1 promoter methylation levels in
CRC tissues (Jiangsu, China) were significantly lower compared with
those of the 5-cm-para-tumor tissues (median PMR, 0.59 vs. 1.22%;
P=0.027) (Fig. 2A) and
10-cm-para-tumor tissues (median PMR, 0.59 vs. 1.89%; P=0.001)
(Fig. 2A). Second-stage experiment
was used to further validate the role of EPAS1 methylation
in CRC. The results indicated that EPAS1 promoter
methylation was significantly lower in CRC tissues (Zhejiang,
China) compared with 5-cm-para-tumor tissues (median PMR, 1.91 vs.
6.25%; P=3×10−7) (Fig.
2B) and normal intestinal tissues from healthy controls (median
PMR, 1.91 vs. 28.84%; P=5×10−7) (Fig. 2B). Additionally, a significantly
lower EPAS1 promoter methylation was found in the paired
5-cm-para-tumor tissues (Zhejiang, China) compared with normal
intestinal tissues of healthy controls (median PMR, 6.25 vs.
28.84%; P=0.028) (Fig. 2B). A
negative correlation between mRNA expression and EPAS1
methylation was identified in 372 TCGA colorectal adenocarcinoma
samples (r=−0.329, P=8×10−11) (Fig. 3).
 | Table I.Association between EPAS1
hypermethylation and clinicopathological characteristics of
patients with CRC in the two-stage experiment. |
Table I.
Association between EPAS1
hypermethylation and clinicopathological characteristics of
patients with CRC in the two-stage experiment.
|
| First-stage
experiment |
| Second-stage
experiment |
|
|---|
|
|
|
|
|
|
|---|
| Clinical
characteristic | N | EPAS1
hypomethylation | EPAS1
hypermethylation | P-value | N | EPAS1
hypomethylation | EPAS1
hypermethylation | P-value |
|---|
| Total cases | 41 | 31 | 10 |
| 79 | 57 | 22 |
|
| Sexa |
|
|
| 1.000 |
|
|
| 0.534 |
|
Male | 28 | 21 | 7 |
| 51 | 38 | 13 |
|
|
Female | 12 | 9 | 3 |
| 28 | 19 | 9 |
|
| Age,
yearsa |
|
|
| 0.859 |
|
|
| 0.534 |
|
≤65 | 21 | 16 | 5 |
| 57 | 40 | 17 |
|
|
>65 | 19 | 14 | 5 |
| 22 | 17 | 5 |
|
|
Differentiationa |
|
|
| NA |
|
|
| 0.440 |
|
Poor | 0 | NA | NA |
| 14 | 9 | 5 |
|
|
Moderate + well | 41 | 31 | 10 |
| 63 | 47 | 16 |
|
| TNM
stageb |
|
|
| NA |
|
|
| 0.945 |
|
I+II | NA | NA | NA |
| 40 | 29 | 11 |
|
|
III+IV | NA | NA | NA |
| 39 | 28 | 11 |
|
| Tumor size,
cma |
|
|
| 0.859 |
|
|
| 0.289 |
| ≤5 | 21 | 16 | 5 |
| 40 | 31 | 9 |
|
| 5 | 19 | 14 | 5 |
| 39 | 26 | 13 |
|
| Lymphatic
metastasisa |
|
|
| 1.000 |
|
|
| 0.314 |
|
Yes | 20 | 15 | 5 |
| 36 | 28 | 8 |
|
| No | 20 | 15 | 5 |
| 43 | 29 | 14 |
|
The present study demonstrated that EPAS1
methylation levels of tumor tissues from patients from Zhejiang
province were significantly higher compared with tumor tissues from
Jiangsu province (median PMR, 1.91 vs. 0.59%; P=4×10−7)
(Table II). Significantly higher
EPAS1 methylation levels were observed in the adjacent
non-tumor tissues from patients from Zhejiang province compared
with patients from Jiangsu province (median PMR, 6.25 vs. 1.22%;
P=4×10−7) (Table II).
However, the difference in EPAS1 methylation levels between
tumor and non-tumor tissues
(D=PMRtumor-PMRnon-tumor) was not significant
between Jiangsu province and Zhejiang province (P=0.066) (Table III). To clarify the differences in
methylation status between the two provinces, the present study
further investigated the association between sample locations and
the clinical phenotypes of patients with CRC. The results indicated
that there was a significant difference in the age at diagnosis
between Zhejiang (57/79; 72.2%) compared with Jiangsu (21/41,
51.2%) (χ2=4.541; P=0.033) (Table III). In addition, there was
statistically significant difference in differentiation between
Jiangsu and Zhejiang province (P=0.002) (Table III). However, there were no
significant differences in EPAS1 methylation levels between
age subgroups and between differentiation subgroups in both
provinces (P>0.05) (Table
II).
 | Table II.Subgroup analysis by age and
differentiation. |
Table II.
Subgroup analysis by age and
differentiation.
|
| Tumor PMR (%) |
| Non-tumor PMR
(%) |
|
|---|
|
|
|
|
|
|
|---|
| Variable | Jiangsu | Zhejiang | P-value | Jiangsu | Zhejiang | P-value |
|---|
| Total | 0.59 (0.35,
1.19) | 1.91 (0.79,
4.90) |
4×10−7 | 1.22 (0.63,
2.11) | 6.25 (2.35,
29.63) |
4×10−7 |
| Age, years |
|
|
|
|
|
|
|
≤65 | 0.83 (0.38,
1.54) | 1.91 (0.83,
5.22) | 0.008 | 1.06 (0.56,
1.92) | 7.35 (7.24,
30.43) |
3×10−6 |
|
>65 | 0.48 (0.28,
1.24) | 1.83 (0.65,
4.02) | 0.009 | 1.53 (0.69,
2.27) | 3.90 (2.33,
24.86) |
2×10−4 |
|
P-value | 0.236 | 0.577 |
| 0.630 | 0.418 |
|
|
Differentiation |
|
|
|
|
|
|
|
Poor | NA | 2.39 (1.28,
6.03) | NA | NA | 9.16 (2.32,
39.14) | NA |
|
Moderate + well | 0.59(0.35,
1.19) | 1.89 (0.79,
4.72) |
2×10−4 | 1.22 (0.60,
2.18) | 6.19 (2.35,
29.63) |
8×10−9 |
|
P-value | NA | 0.235 |
| NA | 0.530 |
|
 | Table III.Association between sampling location
and clinical characteristics. |
Table III.
Association between sampling location
and clinical characteristics.
| Clinical
characteristic | Number | Jiangsu | Zhejiang | P-value |
|---|
| Total cases | 120 | 41 | 79 |
|
| Sexa |
|
|
| 0.553b |
|
Male | 79 | 28 | 51 |
|
|
Female | 40 | 12 | 28 |
|
| Age,
yearsa |
|
|
| 0.033b |
|
≤65 | 78 | 21 | 57 |
|
|
>65 | 41 | 19 | 22 |
|
| Tumor
sizea |
|
|
| 0.847b |
| <5
cm | 61 | 21 | 40 |
|
| ≥5
cm | 58 | 19 | 39 |
|
|
Differentiationa |
|
|
| 0.002c |
| Low and
none | 14 | 0 | 14 |
|
| High
and medium | 103 | 40 | 63 |
|
| Lymph node
metastasisa |
|
|
| 0.842b |
|
Negative | 55 | 19 | 36 |
|
|
Positive | 64 | 21 | 43 |
|
| EPAS1
methylation |
|
|
| 0.066b |
|
Hypomethylation | 91 | 27 | 64 |
|
|
Hypermethylation | 29 | 14 | 15 |
|
ROC curve analysis
ROC curve analysis was used to measure the
diagnostic value of EPAS1 hypomethylation for CRC. The
second-stage association results indicated that EPAS1
hypomethylation yielded a significant AUC of 0.731 (95% CI,
0.653–0.808) with a sensitivity of 58.2% and a specificity of 78.5%
between cancer tissues and 5-cm-para-tumor tissues (Fig. 4A); a significant AUC of 0.851 (95%
CI, 0.760–0.942) with a sensitivity of 95.5% and a specificity of
60.8% between CRC tissues and normal intestinal tissues of healthy
controls (Fig. 4B); and a
significant AUC of 0.654 (95% CI, 0.522–0.786) with a sensitivity
of 63.6% and a specificity of 65.8% between 5-cm-para-tumor tissues
and normal intestinal tissues of healthy controls (Fig. 4C). All of the above data supported
the hypothesis that hypomethylation of EPAS1 may be a
potential diagnostic biomarker for CRC.
Discussion
CRC is regarded as a threat to human health
(2), and the study of methylation in
the context of CRC is a field of growing interest. In the present
study, EPAS1 promoter methylation significantly decreased
according to the results of both the first-stage and the
second-stage association tests. These results led to a hypothesis
that EPAS1 hypomethylation may be associated with the
development of CRC. In the subsequent experiment, EPAS1
hypomethylation yielded an AUC of 0.851 (sensitivity, 95.5%;
specificity, 60.8%) to distinguish the CRC tumor tissues from
normal intestinal tissues, suggesting that EPAS1
hypomethylation could serve as a promising diagnostic biomarker for
CRC.
Numerous studies demonstrated that EPAS1
served a role in angiogenesis of human cancer (26–28) at
the post-transcriptional level (27). Yoshimura et al (11) demonstrated that EPAS1 was
overexpressed in aggressive colorectal carcinoma and exhibited a
significant direct correlation with tumor microvessel count. Cho
et al (16) identified that
EPAS1 was bound by TP2399 in the PAS B domain, which diminished its
ability to bind to ARNT, causing tumor regression in preclinical
kidney cancer models. This means that EPAS1 might be an important
factor in the processes of tumorigenesis and cancer progression. In
human breast cancer cells, methyl-CpG-binding domain protein 3 can
remove the methylation of CpG sites near the promoter of
EPAS1, and, therefore, significantly increase the expression
of EPAS1 (27). In epithelial
cells, DNA (cytosine-5)-methyltransferase 3A (DNMT3A) can silence
the expression of EPAS1 (29,30).
DNMT3A deficiencies were reported in primary tumors and malignant
cells, leading to demethylation of EPAS1 promoter (31) and resulting in the growth of cancer
cells under hypoxia (31–34). Re-introducing DNMT3A can restore the
silencing of EPAS1, and prevent cell proliferation and
viability under hypoxia, inhibiting tumor occurrence (8,31). In
addition, our data mining of The Cancer Genome Atlas database
discovered that there was an inverse EPAS1
methylation-expression correlation in different cancers. Therefore,
the present study further hypothesized that angiogenesis induced by
increased expression of EPAS1 could be a reason underlying
the occurrence of CRC. Future studies should verify the function of
EPAS1 in CRC.
Although several types of biomarkers have been
studied in CRC (35,36), a biomarker for early diagnosis
remains to be identified. Carcinoembryonic antigen (CEA), a
biomarker used globally, did not exhibit the desired diagnostic
value according to one study (37).
However, Peng et al (38)
reported an AUC of 0.690 for CEA in the detection of CRC. In
addition, there are two main methods widely used in clinical
diagnosis of CRC, including fecal occult blood test (FOBT) and
colonoscopy (37). However, FOBT is
susceptible to bias resulting from external factors including drugs
and diet, and colonoscopy is associated with significant costs and
can often cause pain (37). The
present qMSP-based study indicated an AUC of 0.851 (sensitivity,
95.5%; specificity, 60.8%) between CRC tissues and normal
intestinal tissues of healthy controls.
A previous study indicated that patients with CRC
exhibited significantly elevated EPAS1 expression in blood,
which decreased significantly to normal levels following surgical
resection of the tumor (21).
Further analyses of EPAS1 methylation in blood samples of
patients with CRC before and after surgery should be conducted to
further verify the diagnostic value of EPAS1
hypomethylation.
Benign colorectal disease can gradually progress to
advanced adenoma and, subsequently, to invasive adenocarcinoma
(39–41). In addition, aberrant methylation
patterns exhibit a malignant potential in hyperplastic polyps
(39–42). Numerous studies have indicated that
there were significant differences in DNA methylation levels
between CRC tissues, benign colorectal disease tissues and healthy
intestinal tissues (43–45). However, methylation data for benign
colorectal disease were not available in the present study. Further
investigation is necessary to determine whether EPAS1
methylation serves a role in benign colorectal disease.
Plasma circulating DNA (cell-free DNA) of patients
with cancer may originate from circulating tumor cells, which can
indicate the occurrence of micrometastasis and invasion of cancer
cells (46,47). The present study indicated that the
levels of EPAS1 methylation in the tumor tissues were lower
compared with the adjacent non-tumor tissues. The detection of
EPAS1 methylation in plasma cf-DNA could be performed in the
future to avoid variation resulting from differences in tissue
sampling sites, surgical techniques and so on.
In conclusion, the present study suggested that
EPAS1 hypomethylation may be a diagnostic biomarker for CRC.
Further study is necessary to clarify the molecular mechanisms by
which EPAS1 hypomethylation may exert its role in
carcinogenesis.
Acknowledgements
Not applicable.
Funding
The research was supported in part by K.C. Wong
Magna Fund in Ningbo University (to SD) and grant no. U01CA217078
in Northwestern University (to WZ).
Availability of data and materials
Not applicable.
Authors' contributions
SD and RP conceived and designed the experiments.
JY, DW, CZ, XY, JZ, HY, JD, BW, YM and YZ performed the
experiments. RP, CZ and HY analyzed the data. RP, WZ and SD
contributed to the completion of figures and tables, and writing of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EPAS1
|
endothelial PAS domain-containing
protein 1
|
|
CRC
|
colorectal cancer
|
|
qMSP
|
quantitative methylation-specific
polymerase chain reaction
|
|
PMR
|
percent of methylated reference
|
|
CpG
|
cytosine-phosphate-guanine
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carethers JM and Jung BH: Genetics and
genetic biomarkers in sporadic colorectal cancer. Gastroenterology.
149:1177–1190.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging biomarkers.
Gastroenterology. 149:1204–1225.e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou D, Tang W, Su G, Cai M, An HX and
Zhang Y: PCDH18 is frequently inactivated by promoter methylation
in colorectal cancer. Sci Rep. 7:28192017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rezvani N, Alibakhshi R, Vaisi-Raygani A,
Bashiri H and Saidijam M: Detection of SPG20 gene
promoter-methylated DNA, as a novel epigenetic biomarker, in plasma
for colorectal cancer diagnosis using the MethyLight method. Oncol
Lett. 13:3277–3284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Zhang X, Lu X, You L, Song Y, Luo Z,
Zhang J, Nie J, Zheng W, Xu D, et al: 5-Hydroxymethylcytosine
signatures in circulating cell-free DNA as diagnostic biomarkers
for human cancers. Cell Res. 27:1243–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rawłuszko-Wieczorek AA, Horbacka K,
Krokowicz P, Misztal M and Jagodziński PP: Prognostic potential of
DNA methylation and transcript levels of HIF1A and EPAS1 in
colorectal cancer. Mol Cancer Res. 12:1112–1127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian H, McKnight SL and Russell DW:
Endothelial PAS domain protein 1 (EPAS1), a transcription factor
selectively expressed in endothelial cells. Genes Dev. 11:72–82.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robins JC, Akeno N, Mukherjee A, Dalal RR,
Aronow BJ, Koopman P and Clemens TL: Hypoxia induces
chondrocyte-specific gene expression in mesenchymal cells in
association with transcriptional activation of Sox9. Bone.
37:313–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshimura H, Dhar DK, Kohno H, Kubota H,
Fujii T, Ueda S, Kinugasa S, Tachibana M and Nagasue N: Prognostic
impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal
cancer patients: Correlation with tumor angiogenesis and
cyclooxygenase-2 expression. Clin Cancer Res. 10:8554–8560. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanigawa N, Amaya H, Matsumura M, Lu C,
Kitaoka A, Matsuyama K and Muraoka R: Tumor angiogenesis and mode
of metastasis in patients with colorectal cancer. Cancer Res.
57:1043–1046. 1997.PubMed/NCBI
|
|
13
|
Putra AC, Eguchi H, Lee KL, Yamane Y,
Gustine E, Isobe T, Nishiyama M, Hiyama K, Poellinger L and
Tanimoto K: The A Allele at rs13419896 of EPAS1 is associated with
enhanced expression and poor prognosis for non-small cell lung
cancer. PLoS One. 10:e01344962015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwamoto S, Tanimoto K, Nishio Y, Putra AC,
Fuchita H, Ohe M, Sutani A, Kuraki T, Hiyama K, Murakami I, et al:
Association of EPAS1 gene rs4953354 polymorphism with
susceptibility to lung adenocarcinoma in female Japanese
non-smokers. J Thorac Oncol. 9:1709–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia G, Kageyama Y, Hayashi T, Kawakami S,
Yoshida M and Kihara K: Regulation of vascular endothelial growth
factor transcription by endothelial PAS domain protein 1 (EPAS1)
and possible involvement of EPAS1 in the angiogenesis of renal cell
carcinoma. Cancer. 91:1429–1436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho H, Du X, Rizzi JP, Liberzon E,
Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA,
et al: On-target efficacy of a HIF-2α antagonist in preclinical
kidney cancer models. Nature. 539:107–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sena JA, Wang L, Heasley LE and Hu CJ:
Hypoxia regulates alternative splicing of HIF and non-HIF target
genes. Mol Cancer Res. 12:1233–1243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Welander J, Andreasson A, Brauckhoff M,
Bäckdahl M, Larsson C, Gimm O and Söderkvist P: Frequent
EPAS1/HIF2α exons 9 and 12 mutations in non-familial
pheochromocytoma. Endocr Relat Cancer. 21:495–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baba Y, Nosho K, Shima K, Irahara N, Chan
AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS and Ogino S:
HIF1A overexpression is associated with poor prognosis in a cohort
of 731 colorectal cancers. Am J Pathol. 176:2292–2301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Favier J, Plouin PF, Corvol P and Gasc JM:
Angiogenesis and vascular architecture in pheochromocytomas:
Distinctive traits in malignant tumors. Am J Pathol. 161:1235–1246.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collado M, Garcia V, Garcia JM, Alonso I,
Lombardia L, Diaz-Uriarte R, Fernández LA, Zaballos A, Bonilla F
and Serrano M: Genomic profiling of circulating plasma RNA for the
analysis of cancer. Clin Chem. 53:1860–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Yang Y, Liu J, Li B, Xu Y, Li C,
Xu Q, Liu G, Chen Y, Ying J and Duan S: NDRG4 hypermethylation is a
potential biomarker for diagnosis and prognosis of gastric cancer
in Chinese population. Oncotarget. 8:8105–8119. 2017.PubMed/NCBI
|
|
23
|
Li B, Chen X, Jiang Y, Yang Y, Zhong J,
Zhou C, Hu H and Duan S: CCL2 promoter hypomethylation is
associated with gout risk in Chinese Han male population. Immunol
Lett. 190:15–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Chen X, Hu H, Jiang Y, Yu H, Dai
J, Mao Y and Duan S: Elevated UMOD methylation level in peripheral
blood is associated with gout risk. Sci Rep. 7:111962017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kristensen LS, Mikeska T, Krypuy M and
Dobrovic A: Sensitive melting analysis after real time-methylation
specific PCR (SMART-MSP): High-throughput and probe-free
quantitative DNA methylation detection. Nucleic Acids Res.
36:e422008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Lou Y, Zhang J, Fu Q, Wei T, Sun
X, Chen Q, Yang J, Bai X and Liang T: Hypoxia-inducible factor-2α
promotes tumor progression and has crosstalk with Wnt/β-catenin
signaling in pancreatic cancer. Mol Cancer. 16:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui J, Duan B, Zhao X, Chen Y, Sun S, Deng
W, Zhang Y, Du J, Chen Y and Gu L: MBD3 mediates epigenetic
regulation on EPAS1 promoter in cancer. Tumour Biol.
37:13455–13467. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu DM, Li DC, Zhang ZX and Zhang XY:
Effect of endothelial PAS domain protein 1 and hypoxia inducible
factor 1alpha on vascular endothelial growth factor expression in
human pancreatic carcinoma. Chin Med J (Engl). 121:2258–2264.
2008.PubMed/NCBI
|
|
29
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers, and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim CM, Vocke C, Torres-Cabala C, Yang Y,
Schmidt L, Walther M and Linehan WM: Expression of hypoxia
inducible factor-1alpha and 2alpha in genetically distinct early
renal cortical tumors. J Urol. 175:1908–1914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lachance G, Uniacke J, Audas TE, Holterman
CE, Franovic A, Payette J and Lee S: DNMT3a epigenetic program
regulates the HIF-2α oxygen-sensing pathway and the cellular
response to hypoxia. Proc Natl Acad Sci USA. 111:7783–7788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gordan JD, Bertout JA, Hu CJ, Diehl JA and
Simon MC: HIF-2alpha promotes hypoxic cell proliferation by
enhancing c-myc transcriptional activity. Cancer Cell. 11:335–347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim WY, Perera S, Zhou B, Carretero J, Yeh
JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, et
al: HIF2alpha cooperates with RAS to promote lung tumorigenesis in
mice. J Clin Invest. 119:2160–2170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Franovic A, Holterman CE, Payette J and
Lee S: Human cancers converge at the HIF-2alpha oncogenic axis.
Proc Natl Acad Sci USA. 106:21306–21311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ravegnini G, Zolezzi Moraga JM, Maffei F,
Musti M, Zenesini C, Simeon V, Sammarini G, Festi D, Hrelia P and
Angelini S: Simultaneous analysis of SEPT9 promoter methylation
status, micronuclei frequency, and folate-related gene
polymorphisms: The potential for a novel blood-based colorectal
cancer biomarker. Int J Mol Sci. 16:28486–28497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang J, Tan ZR, Yu J, Li H, Lv QL, Shao
YY and Zhou HH: DNA hypermethylated status and gene expression of
PAX1/SOX1 in patients with colorectal carcinoma. OncoTargets Ther.
10:4739–4751. 2017. View Article : Google Scholar
|
|
37
|
Li Y, Song L, Gong Y and He B: Detection
of colorectal cancer by DNA methylation biomarker SEPT9: Past,
present and future. Biomark Med. 8:755–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng HX, Yang L, He BS, Pan YQ, Ying HQ,
Sun HL, Lin K, Hu XX, Xu T and Wang SK: Combination of preoperative
NLR PLR and CEA could increase the diagnostic efficacy for I-III
stage CRC. J Clin Lab Anal. 31:2017. View Article : Google Scholar
|
|
39
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stella S, Bruzzese A and Chiarini S:
Biological bases of the colorectal adenoma-carcinoma sequence. G
Chir. 17:473–476. 1996.(In Italian). PubMed/NCBI
|
|
41
|
Takami K, Yana I, Kurahashi H and Nishisho
I: Multistep carcinogenesis in colorectal cancers. Southeast Asian
J Trop Med Public Health. 26 Suppl 1:S190–S196. 1995.
|
|
42
|
Wynter CV, Walsh MD, Higuchi T, Leggett
BA, Young J and Jass JR: Methylation patterns define two types of
hyperplastic polyp associated with colorectal cancer. Gut.
53:573–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang HL, Liu P, Zhou PY and Zhang Y:
Promoter methylation of the RASSF1A gene may contribute to
colorectal cancer susceptibility: A meta-analysis of cohort
studies. Ann Hum Genet. 78:208–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YC, Yu ZH, Liu C, Xu LZ, Yu W, Lu J,
Zhu RM, Li GL, Xia XY, Wei XW, et al: Detection of RASSF1A promoter
hypermethylation in serum from gastric and colorectal
adenocarcinoma patients. World J Gastroenterol. 14:3074–3080. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li YW, Kong FM, Zhou JP and Dong M:
Aberrant promoter methylation of the vimentin gene may contribute
to colorectal carcinogenesis: A meta-analysis. Tumour Biol.
35:6783–6790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Couraud S, Vaca-Paniagua F, Villar S,
Oliver J, Schuster T, Blanché H, Girard N, Trédaniel J,
Guilleminault L, Gervais R, et al: Noninvasive diagnosis of
actionable mutations by deep sequencing of circulating free DNA in
lung cancer from never-smokers: A proof-of-concept study from
BioCAST/IFCT-1002. Clin Cancer Res. 20:4613–4624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jung K, Fleischhacker M and Rabien A:
Cell-free DNA in the blood as a solid tumor biomarker-a critical
appraisal of the literature. Clin Chim Acta. 411:1611–1624. 2010.
View Article : Google Scholar : PubMed/NCBI
|