Introduction
Acute cerebral infarction is a common disease in
clinical practice, especially in neurology, which frequently occurs
in elderly patients and has higher disability fatality rates
(1). With the gradual aggravation of
social aging, the incidence rates of cardiovascular and
cerebrovascular diseases are also increased. Acute cerebral
infarction is also known as cerebral ischemic stroke, under which
brain cells are unable to have normal blood circulation, and
varying degrees of ischemia and anoxia lead to malacia or necrosis
of brain tissue cells, resulting in disability or death of
patients, and greatly reducing the quality of life of patients
(2).
Previous data have shown that carotid artery
stenosis is the main factor among various factors that cause
ischemic encephalopathy, but with the continuous strengthening of
evidence-based basis, it has been found that rupture and erosion
caused by the instability of atherosclerotic plaque also play
important roles in promoting the occurrence of acute cerebral
infarction (3,4). On the other hand, overexpressed
inflammatory cytokines play an important role in the occurrence and
development of patients with acute cerebral infarction. The most
common inflammatory cytokines are C-reactive protein (CRP), tumor
necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which
significantly affect the pathophysiological processes of acute
cerebral infarction brain cells at the same time (5,6).
Inflammatory cytokines are closely related to the occurrence and
the severity of acute cerebral infarction. Matrix metalloproteinase
(MMP) is a substance synthesized and secreted by macrophages
(7). MMP-2 and MMP-9 are members of
various substances decomposed by it, and the main roles are to
degrade collagen fibers, elastic fibers and other extracellular
matrixes, resulting in weakened fibrous cap function and unstable
carotid plaque, thereby increasing the risk of acute cerebral
infarction (8).
Therefore, the study on the correlations of carotid
artery thickness, atherosclerotic plaque stability, serum
inflammatory factors and MMP with acute cerebral infarction has an
important reference value in clinical diagnosis and prognosis.
Patients and methods
General data
A total of 56 patients diagnosed with acute cerebral
infarction in Jingmen First People's Hospital (Jingmen, China) from
February 2016 to January 2017 were selected and divided into the
plaque stability group (n=25) and plaque instability group (n=31)
based on the stability of plaque indicated in color
ultrasonography. Among them, there were 36 males and 20 females
aged 62–91 years, with an average age of 79.34±4.82 years.
Diagnostic criteria for all enrolled patients were based on revised
standards of Chinese Fourth Conference on Cerebrovascular Disease
in combination with brain computed tomography (CT) or magnetic
resonance imaging. Inclusion criteria: patients who had complete
clinical data and signed the informed consent; patients who were
diagnosed with acute cerebral infarction. Exclusion criteria:
cerebral embolism patients with determined source of emboli, such
as atrial fibrillation and peripheral vascular disease, patients
with coma, disturbance of consciousness or disability due to other
causes, for example, drug, malignancy, trauma and arteritis.
This study was approved by the Ethics Committee of
Jingmen First People's Hospital. Signed informed consents were
obtained from the patients or guardians.
Methods
General data of patients, including age, sex,
height, weight, blood pressure, smoking history, and with or
without coronary heart disease and diabetes mellitus, were
collected. Biochemical and inflammatory indexes of selected
patients were measured. National Institutes of Health Stroke Scale
(NIHSS) score and Barthel index were calculated.
Determination of biochemical indicators: fasting
peripheral blood (10 ml) was drawn from all included patients after
fasting for solids and liquids for 10 h overnight, and the upper
serum was used to determine biochemical indicators, including
glycosylated hemoglobin A1c (HbA1c) measured through a glycosylated
hemoglobin analyzer, and total cholesterol (TC), triglyceride (TG),
low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) measured by an automatic
biochemical analyzer provided by Hitachi, Ltd. (Tokyo, Japan).
Measurement of serum inflammatory cytokines, MMP-2
and MMP-9: the enrolled patients did not eat or drink for 10 h
overnight, 10 ml peripheral blood was drawn, and the serum was
collected. Serum inflammatory cytokines were measured by
immunoturbidimetry, and serum MMP-2 and MMP-9 levels were detected
using enzyme-linked immunosorbent assay. Reagents and instruments
were provided by Shandong Biological Instrument Co. (Qingdao,
China).
Evaluation of carotid artery: carotid intima-media
thickness (IMT): carotid ultrasound examination was performed using
the Philips iE33 color Doppler ultrasound equipment. The specific
sites of the examination were bilateral common carotid artery,
bifurcation of common carotid artery and internal carotid artery
outside the brain. Each site was measured 3 times, and the average
was used as result. Determination of plaque stability: the
stability of the plaque was determined according to the nature of
echo displayed in the results of ultrasound examination: high-level
echo, stable plaque; low-level or equal echo, unstable plaque;
determination of eccentricity index (EI), total thickness of the
plaque/measured IMT.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (IBM Corp., Armonk, NY, USA) was used for data
processing. Collected data were expressed as mean ± SD. The
χ2 test was used to compare enumeration data.
Correlation analysis was carried out over two factors. Logistic
regression analysis was performed on relevant risk factors.
P<0.05 indicates that the difference was statistically
significant.
Results
Comparison of general data between plaque stability
and instability groups. There were no statistical differences in
age, sex, body mass index (BMI), HbA1c, TG, HDL-C, LDL-C, systolic
pressure, diastolic pressure, coronary heart disease, diabetes
mellitus and smoking history between the plaque stability and
instability groups (P>0.05), but the level of TC in the plaque
instability group was significantly higher than that in the plaque
stability group (P<0.05) (Table
I).
 | Table I.Comparison of general data between the
plaque stability and instability groups. |
Table I.
Comparison of general data between the
plaque stability and instability groups.
| General data | Plaque stability
group (n=25) | Plaque instability
group (n=31) | P-value |
|---|
| Age (years) | 79.53±5.08 | 81.07±4.76 | 0.312 |
| Sex
(male/female) | 16/9 | 20/11 | 0.465 |
| BMI
(kg/m2) | 24.23±1.87 | 24.96±1.93 | 0.766 |
| HbA1c (%) | 6.08±2.60 | 6.12±3.08 | 0.724 |
| TG (mmol/l) | 1.27±0.38 | 1.25±0.29 | 0.376 |
| TC (mmol/l) | 2.99±0.92 | 4.34±0.88 | 0.028 |
| HDL-C (mmol/l) | 1.09±0.41 | 1.04±0.25 | 0.065 |
| LDL-C(mmol/l) | 2.88±1.23 | 2.45±1.01 | 0.051 |
| Systolic pressure
(mmHg) | 145.37±22.12 | 150.16±20.39 | 0.072 |
| Diastolic pressure
(mmHg) | 82.53±15.13 | 85.19±17.68 | 0.142 |
| Coronary heart
disease [n (%)] | 14 (56.0) | 19 (61.3) | 0.595 |
| Diabetes mellitus [n
(%)] | 10 (40.0) | 13 (41.9) | 1.001 |
| Smoking history [n
(%)] | 5
(20.0) | 8
(25.8) | 0.119 |
Comparison of IMT, EI, NIHSS score and
Barthel index between plaque stability and instability groups
Plaque instability group had obviously increased IMT
and NIHSS scores and clearly reduced EI and Barthel index in
comparison with the plaque stability group, and the differences
were statistically significant (P<0.05) (Table II).
 | Table II.Comparison of IMT, EI, NIHSS score and
Barthel index between the plaque stability and instability
groups. |
Table II.
Comparison of IMT, EI, NIHSS score and
Barthel index between the plaque stability and instability
groups.
| Projects | Plaque stability
group (n=25) | Plaque instability
group (n=31) | P-value |
|---|
| IMT (cm) | 0.205±0.103 | 0.341±0.127 | 0.001 |
| EI | 0.57±0.14 | 0.38±0.11 | 0.001 |
| Barthel index | 69.30±19.4 | 60.62±19.3 | 0.001 |
| NIHSS score | 3.9±2.8 | 6.2±3.1 | 0.001 |
Comparison of serum inflammatory
factor levels between plaque stability and instability groups
Serum CRP, TNF-α and IL-6 levels in the plaque
instability group were overtly higher than those in the plaque
stability group, with statistically significant differences
(P<0.05) (Table III).
 | Table III.Comparison of serum inflammatory
factor levels between the plaque stability and instability
groups. |
Table III.
Comparison of serum inflammatory
factor levels between the plaque stability and instability
groups.
| Inflammatory
factors | Plaque stability
group (n=25) | Plaque instability
group (n=31) | P-value |
|---|
| CRP (mg/l) | 2.39±0.99 | 5.48±1.43 | 0.001 |
| TNF-α (ng/ml) | 6.46±1.09 | 10.23±1.19 | 0.001 |
| IL-6 (µg/l) | 7.21±1.17 | 9.24±1.66 | 0.001 |
Comparison of serum MMP-2 and MMP-9
levels between plaque stability and instability groups
Serum MMP-2 and MMP-9 levels were evidently elevated
in the plaque instability group compared with those in the plaque
stability group, and the differences were statistically significant
(P<0.05) (Table IV).
 | Table IV.Comparison of serum MMP-2 and MMP-9
levels between the plaque stability and instability groups. |
Table IV.
Comparison of serum MMP-2 and MMP-9
levels between the plaque stability and instability groups.
| MMP | Plaque stability
group (n=25) | Plaque instability
group (n=31) | P-value |
|---|
| MMP-2 (ng/ml) | 63.21±0.56 | 71.38±0.45 | 0.001 |
| MMP-9 (ng/ml) | 301.74±31.19 | 356.92±30.46 | 0.001 |
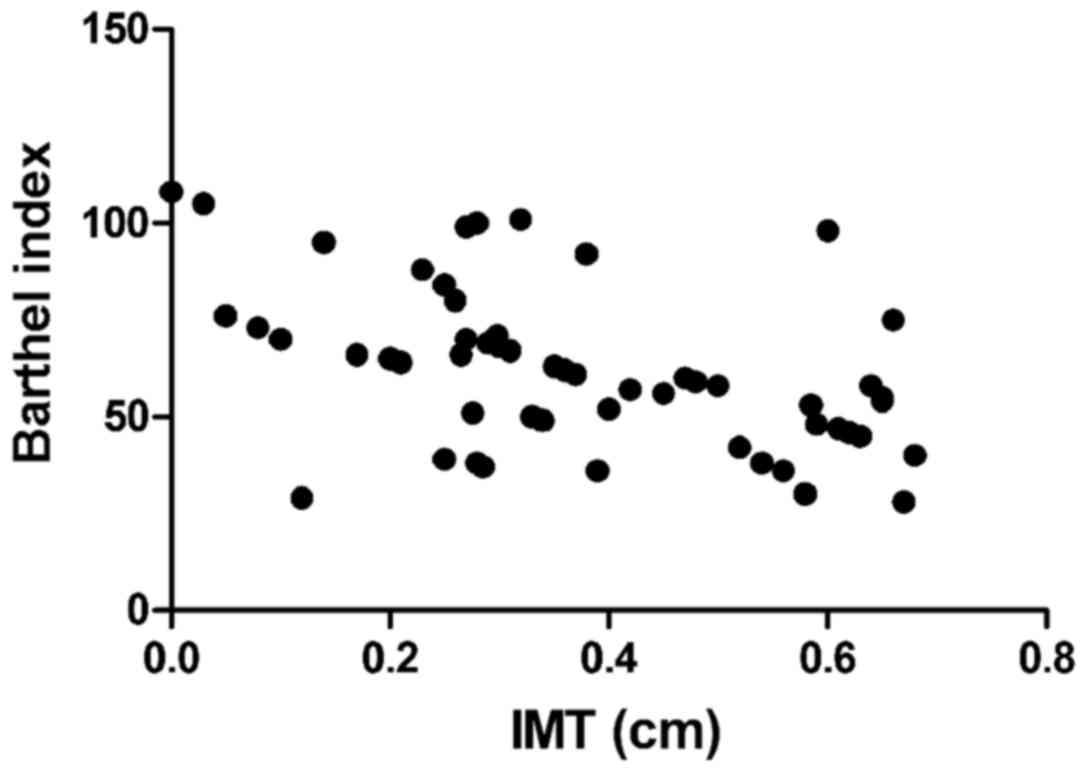
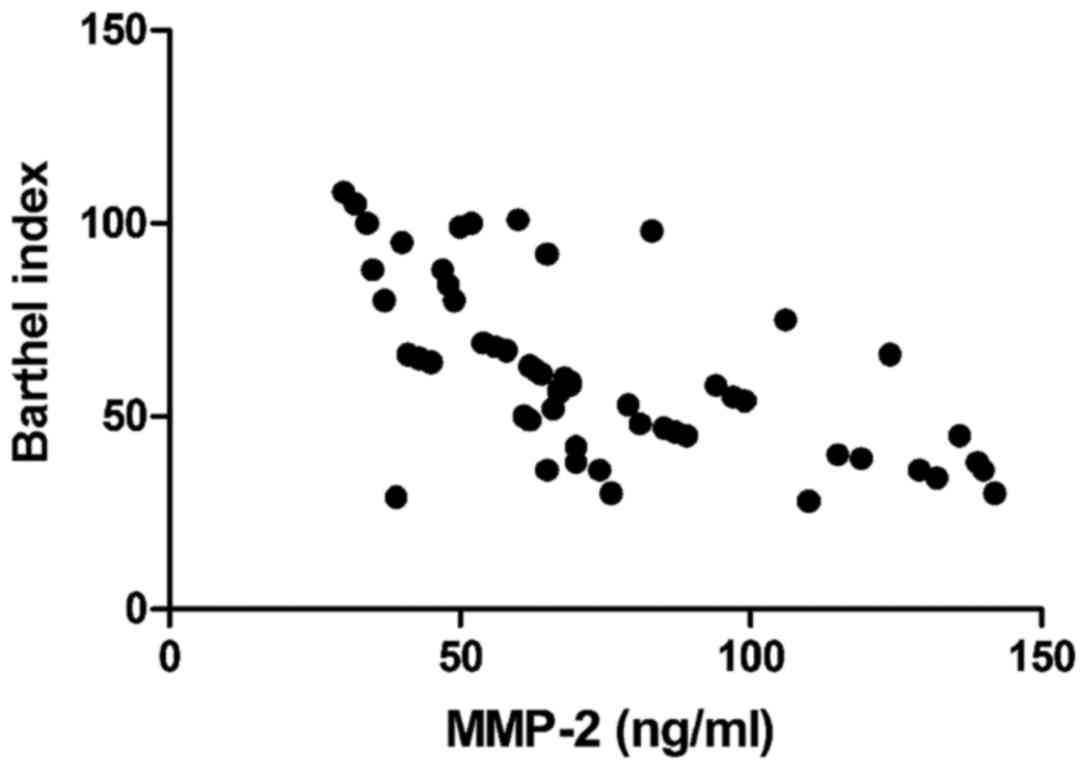
Correlation analyses of IMT, EI, serum
inflammatory factors and MMP-2 with Barthel index
Barthel index was negatively correlated with IMT
(r=−0.693, P<0.01), CRP (r=−0.765, P<0.01), and MMP-2
(r=−0.605, P<0.01), but positively associated with EI (r=0.811,
P<0.01) (Figs. 1–4).
Logistic regression analyses on the
prediction of risk factors for acute cerebral infarction
HbA1c, TC, systolic pressure, coronary heart
disease, diabetes mellitus, IMT, EI, serum inflammatory cytokines
(CRP, TNF-α and IL-6), MMP-2 and MMP-9 had independent prognostic
values for acute cerebral infarction, with statistical significance
(P<0.05) (Table V).
 | Table V.Logistic regression analyses on the
prediction of risk factors for acute cerebral infarction. |
Table V.
Logistic regression analyses on the
prediction of risk factors for acute cerebral infarction.
| Factors | P-value | OR | 95% CI |
|---|
| Age | 0.108 | 1.094 | 0.957–1.149 |
| Sex | 0.779 | 0.852 | 0.993–5.979 |
| BMI | 0.851 | 0.069 | 0.443–18.305 |
| HbA1c | 0.032 | 1.006 | 0.929–1.113 |
| TG | 0.527 | 1.447 | 0.465–4.491 |
| TC | 0.024 | 1.396 | 0.576–3.572 |
| HDL-C | 0.322 | 0.989 | 0.991–5.035 |
| LDL-C | 0.961 | 0.082 | 0.445–20.307 |
| Systolic
pressure | 0.013 | 7.543 | 1.918–8.147 |
| Diastolic
pressure | 0.051 | 1.408 | 0.569–4.018 |
| Coronary heart
disease | 0.042 | 1.279 | 0.472–3.961 |
| Diabetes
mellitus | 0.037 | 1.162 | 0.620–4.083 |
| Smoking
history | 0.059 | 1.477 | 0.625–4.199 |
| IMT | 0.017 | 8.053 | 1.814–9.525 |
| EI | 0.035 | 1.274 | 0.631–3.929 |
| CRP | 0.028 | 1.053 | 0.985–1.064 |
| IL-6 | 0.026 | 1.029 | 0.974–1.081 |
| TNF-α | 0.005 | 7.349 | 1.918–20.143 |
| MMP-2 | 0.019 | 1.045 | 0.997–1.323 |
| MMP-9 | 0.047 | 1.118 | 0.493–5.017 |
Discussion
Clinically, diseases with the highest morbidity and
mortality rates are cardiovascular and cerebrovascular diseases, of
which acute cerebral infarction is the most common disease among
cerebrovascular diseases (9). The
pathogenesis of acute cerebral infarction is based on arterial
diseases in and outside the brain. The common arterial diseases
include carotid artery stenosis or obstruction caused by the
formation of carotid atherosclerotic plaques, plaque rupture and
erosion (10). Among many risk
factors of acute cerebral infarction, carotid lesions, especially
plaque formation, plaque thickness, stability and other properties
are the most common and important risk factors (11). Studies have shown that plaque
instability increases the onset risk of acute cerebral infarction,
and plaque instability is caused by factors such as fibrous cap
thickness and fixation degree. Among various conditions determining
the stability of the plaque, EI is also an important indicator in
addition to echo and degree of surface smoothness. The lower the
EI, the more unstable the plaque (12,13).
NIHSS score and Barthel index are generally used to judge the
neurologic status in patients with acute cerebral infarction. A
higher NIHSS score and a lower Barthel index indicate more severe
neurologic impairment (14). In this
study, it was found that IMT and NIHSS scores in the plaque
instability group were significantly higher than those in the
plaque stability group, while EI and Barthel index in the plaque
instability group were significantly lower than those in the plaque
stability group, and the differences were statistically significant
(P<0.05). In addition, correlation analyses revealed that IMT
and Barthel index were negatively correlated, while EI and Barthel
index were positively related, suggesting that the thicker the
carotid artery thickness is, and the more unstable the plaque is,
the more serious the condition of acute cerebral infarction will
be.
With continuous and in-depth studies on the
pathogenesis of acute cerebral infarction, it has been found that
in the early stage of acute cerebral infarction, over-reaction of
the inflammatory system in ischemic and infarct regions is also an
important factor promoting its development (15). In the process leading to
over-reaction of the inflammatory system, neutrophils, macrophages
and lymphocytes play a key role, and the excessive synthesis and
release of inflammatory cytokines released by the above cells such
as interleukin and CRP further lead to inflammatory cascade,
increasing the trauma of brain histiocyte (16,17). In
addition, blood-brain barrier is damaged in the over-reaction
process of the inflammation system, resulting in increased levels
of inflammatory cytokines and MMP expression (18). MMP-2 and MMP-9 are important
components of MMP. In patients with acute cerebral infarction,
increased activity of MMP causes edema in brain histiocytes, and
measurement of MMP content can be used to determine the severity of
acute cerebral infarction (19,20). The
relationship between inflammatory cytokines and acute cerebral
infarction was investigated in this study, and it was found that
the levels of serum inflammatory cytokines in the plaque
instability group were distinctly higher than those in the plaque
stability group, and the differences were statistically significant
(P<0.05). Both CRP and MMP-2 had a negative correlation with
Barthel index. Meanwhile, IMT, EI, serum inflammatory cytokines
(CRP, TNF-α and IL-6), MMP-2 and MMP-9 had independent predictive
values for acute cerebral infarction.
In conclusion, examining carotid artery thickness,
atherosclerotic plaque stability, and levels of serum inflammatory
factors, MMP-2 and MMP-9 has important significance in judging the
severity of acute cerebral infarction and guiding its treatment and
prognosis. Risk factors of acute cerebral infarction should be
deeply understood, so as to prevent or timely and correctly treat
the disease.
Acknowledgements
This study was supported by the Key Science and
Technology Project of Jingmen, Hubei (no. YFZD2016045).
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LC and QY designed the study and performed the
experiments. LC, RD and DL collected the data. QY and ZC analyzed
the data. LC and QY prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Jingmen First People's Hospital (Jingmen, China). Signed informed
consents were obtained from the patients or guardians.
Patients consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Kim HM, Shin HY, Jeong HJ, An HJ, Kim NS,
Chae HJ, Kim HR, Song HJ, Kim KY, Baek SH, et al: Reduced IL-2 but
elevated IL-4, IL-6, and IgE serum levels in patients with cerebral
infarction during the acute stage. J Mol Neurosci. 14:191–196.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dziedzic T: Clinical significance of acute
phase reaction in stroke patients. Front Biosci. 13:2922–2927.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liapis CD, Kakisis JD and Kostakis AG:
Carotid stenosis: Factors affecting symptomatology. Stroke.
32:2782–2786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma LL, Song L, Yu XD, Yu TX, Liang H and
Qiu JX: The clinical study on the treatment for acute cerebral
infarction by intra-arterial thrombolysis combined with mild
hypothermia. Eur Rev Med Pharmacol Sci. 21:1999–2006.
2017.PubMed/NCBI
|
|
5
|
Segers D, Helderman F, Cheng C, van Damme
LC, Tempel D, Boersma E, Serruys PW, de Crom R, van der Steen AF,
Holvoet P, et al: Gelatinolytic activity in atherosclerotic plaques
is highly localized and is associated with both macrophages and
smooth muscle cells in vivo. Circulation. 115:609–616. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moreno PR, Purushothaman KR, Fuster V,
Echeverri D, Truszczynska H, Sharma SK, Badimon JJ and O'Connor WN:
Plaque neovascularization is increased in ruptured atherosclerotic
lesions of human aorta: Implications for plaque vulnerability.
Circulation. 110:2032–2038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Casas JP, Shah T, Hingorani AD, Danesh J
and Pepys MB: C-reactive protein and coronary heart disease: A
critical review. J Intern Med. 264:295–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doyle B and Caplice N: Plaque
neovascularization and antiangiogenic therapy for atherosclerosis.
J Am Coll Cardiol. 49:2073–2080. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang N, Lin M, Wang BG, Zeng WY, He YF,
Peng HY, Zeng J, Wu ZY and Zhong Y: Low level of low-density
lipoprotein cholesterol is related with increased hemorrhagic
transformation after acute ischemic cerebral infarction. Eur Rev
Med Pharmacol Sci. 20:673–678. 2016.PubMed/NCBI
|
|
10
|
Bazan HA, Smith TA, Donovan MJ and
Sternbergh WC III: Future management of carotid stenosis: Role of
urgent carotid interventions in the acutely symptomatic carotid
patient and best medical therapy for asymptomatic carotid disease.
Ochsner J. 14:608–615. 2014.PubMed/NCBI
|
|
11
|
Bauzá A, Mooibroek TJ and Frontera A:
Corrigendum: The bright future of unconventional σ/π-hole
interactions. Chemphyschem. 16:31302015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hermus L, Tielliu IF, Wallis de Vries BM,
van den Dungen JJ and Zeebregts CJ: Imaging the vulnerable carotid
artery plaque. Acta Chir Belg. 110:159–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuttolomondo A, Di Raimondo D, Pecoraro R,
Arnao V, Pinto A and Licata G: Atherosclerosis as an inflammatory
disease. Curr Pharm Des. 18:4266–4288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azzurri A, Sow OY, Amedei A, Bah B, Diallo
S, Peri G, Benagiano M, D'Elios MM, Mantovani A and Del Prete G:
IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are
tools for monitoring inflammation and disease activity in
Mycobacteriumtuberculosis infection. Microbes Infect.
7:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mairuhu AT, Peri G, Setiati TE, Hack CE,
Koraka P, Soemantri A, Osterhaus AD, Brandjes DP, van der Meer JW,
Mantovani A, et al: Elevated plasma levels of the long pentraxin,
pentraxin 3, in severe dengue virus infections. J Med Virol.
76:547–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muller B, Peri G, Doni A, Torri V,
Landmann R, Bottazzi B and Mantovani A: Circulating levels of the
long pentraxin PTX3 correlate with severity of infection in
critically ill patients. Crit Care Med. 29:1404–1407. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sprong T, Peri G, Neeleman C, Mantovani A,
Signorini S, van der Meer JW and van Deuren M: Pentraxin 3 and
C-reactive protein in severe meningococcal disease. Shock.
31:28–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bevelacqua V, Libra M, Mazzarino MC,
Gangemi P, Nicotra G, Curatolo S, Massimino D, Plumari A, Merito P,
Valente G, et al: Long pentraxin 3: A marker of inflammation in
untreated psoriatic patients. Int J Mol Med. 18:415–423.
2006.PubMed/NCBI
|
|
19
|
Wang J, Yang Z, Liu C, Zhao Y and Chen Y:
Activated microglia provide a neuroprotective role by balancing
glial cell-line derived neurotrophic factor and tumor necrosis
factor-α secretion after subacute cerebral ischemia. Int J Mol Med.
31:172–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yilmaz G, Arumugam TV, Stokes KY and
Granger DN: Role of T lymphocytes and interferon-gamma in ischemic
stroke. Circulation. 113:2105–2112. 2006. View Article : Google Scholar : PubMed/NCBI
|