Introduction
Ventilator-associated pneumonia (VAP) is a common
type of nosocomial infection in children following cardiac surgery
(1,2). Transient systemic immune suppression
(3) and the prolonged use of
mechanical ventilation increase the risk of VAP following cardiac
surgery (2). It has been reported
that VAP increases the duration of mechanical ventilation,
prolonging the hospital stay (4) and
even contributing to a 13% mortality rate (5). However, it is a major challenge to
accurately diagnose VAP in patients immediately following cardiac
surgery as systemic inflammatory response syndrome is induced by
surgical trauma as well as the interaction of the blood with the
cardiopulmonary bypass (6), and
imaging studies may reveal pulmonary opacities caused by the
surgical manipulation, atelectasis or alveolar haemorrhage
(4). As a result, accurate pathogen
detection to discern advisable treatment and early diagnosis are
key to improving the prognosis of patients with VAP following heart
surgery.
The gold standard for the diagnostic confirmation of
VAP is lung tissue examination and culturing (7,8),
however, as an interventional procedure is required to get lung
biopsy samples, clinical applicability is limited (8). The triggering receptor expressed on
myeloid cells-1 (TREM-1) is a member of the immunoglobulin
superfamily, is secreted by neutrophils, macrophages and monocytes,
and amplifies the inflammatory response following the exposure of
cells to bacteria and fungi (9). A
soluble form of TREM-1 (sTREM-1) has been proposed as a novel
biomarker, and has been tested for in patients with acute
infections with different diagnostic and prognostic results
(9–11). Elevated levels of sTREM-1 were
identified in the serum, bronchoalveolar lavage fluid (BALF) and
the exhaled ventilator condensate (EVC) in patients with VAP
(12,13). sTREM-1 in exhaled breath condensate
(EBC) and BALF has been demonstrated to be a good diagnostic factor
in differentiating patients who have suffered an ischaemic stroke
with VAP from those without (14),
however the measurement of sTREM-1 in EVC has been demonstrated to
be useful for the diagnosis of VAP after cardiac surgery (13). Whether the detection of sTREM-1 in
EVC and BALF improves the accuracy of a VAP diagnosis following
cardiac surgery remains to be explored. Additionally, polymerase
chain reaction (PCR) assays allow for rapid molecular testing, as
traditional culturing methods may not be sufficiently sensitive to
detect even the more common bacterial pathogens and viruses
(15–17). Studies using PCR techniques for
pathogen detection have involved severe sepsis and bloodstream
infections (17,18). A previous study demonstrated that PCR
assay rapidly detected pathogens, and was more sensitive and rapid
than traditional cultures (19). In
the present study, a PCR assay was used to define the pathogens in
BALF for patients with suspected VAP, and to evaluate whether the
use of sTREM-1 improved the accuracy of VAP diagnosis in children
undergoing cardiac surgery.
Materials and methods
Study design
The current study was a prospective cohort study
conducted in the Cardiac Intensive Care Unit (CICU) of Shanghai
Children's Medical Center (Shanghai, China) on children with
congenital heart disease undergoing cardiac surgery between August
2016 and October 2017. The present study was approved by the Ethics
Committee of Shanghai Jiaotong University School of Medicine
(Shanghai, China; approval no. SCMCIRB-K2015040) and written
informed consent was obtained from the patients' parents.
Diagnosis, treatment and prevention of
VAP
Patients with suspected VAP who remained intubated
and mechanically ventilated for ≥48 h after surgery were included.
VAP was suspected if the patient had a radiographic infiltrate that
was novel or progressive, together with clinical findings that were
suggestive of infection, including the onset of fever (temperature
≥38.3°C) or hypothermia (temperature ≤36.5°C), leucocytosis
(≥10×109/l or ≤4×109/l), purulent sputum and
a decline in oxygenation (oxygen saturation <90%). The exclusion
criteria were a preoperative diagnosis of pneumonia and/or sepsis.
The BALF samples were collected and analysed as described below.
Patients with a positive detection of bacteria were determined as
the VAP group, whilst the control group was determined as the
non-VAP group, patients with a negative detection of bacteria.
The protocol for VAP treatment and prevention
followed standard protocols based on the British Thoracic Society
guideline for advanced diagnostic and therapeutic flexible
bronchoscopy in adults (20). In
addition, VAP care bundles for the prevention of VAP were also
available (20).
Clinical assessment
The baseline assessment included the evaluation of
demographic data (age, sex and weight), medical history, Paediatric
Risk of Mortality score (21), Risk
Adjustment for Congenital Heart Surgery score (22), modified clinical pulmonary infection
score (23), the ratio of partial
oxygen to the fraction of inspired oxygen
(PaO2/FiO2) (23), cardiopulmonary bypass time, aortic
cross clamp time, the level of inflammatory biomarkers
procalcitonin and C-reactive protein, the time of intubation, CICU
length of stay and hospital length of stay. The levels of
procalcitonin in blood plasma were determined using a procalcitonin
detecting kit and measured by Getein 1600 Immunofluorescence
Quantitative Analyzer (both Getein Biotech, Inc., Nanjing, China).
The C-reactive protein levels in blood were determined using a
C-reactive protein detecting kit (Goldsite, Inc., Shenzhen, China)
and detected by Astep C Reactive Analyzer (GOLDSITE, Inc.).
Sample processing and measurement
A bronchoscopy was performed on the day when VAP was
suspected and BALF and EVC samples were collected for measurement
on the same day. The diagnostic flexible bronchoscopy guideline of
the British Thoracic Society was also followed (8). To obtain the BALF sample, a total of 9
ml sterile saline was instilled into the middle lobes of the right
and left lungs, and was then gently suctioned out. One-third of the
BALF sample was centrifuged at 200 × g for 15 min at room
temperature, and the cell-free supernatants were aliquoted. In
addition, EVC samples, the liquid of exhaled gases and vapours
collected in a portable condenser, were collected from the trap
located in the expiratory limb of the ventilator circuit and 3 ml
was required for the measurement on the same day. Part of the BALF
and EVC samples were sent to the laboratory immediately following
the collection to measure the sTREM-1 protein concentration using
the Human TREM-1 Quantikine ELISA kit (cat. no. DTRM10B; R&D
Systems Inc., Minneapolis, MN, USA). The remainder of the BALF
samples were analysed by quantitative PCR (qPCR) and
microbiological culture using PMseq™ infection high-throughput gene
detection analysis performed by Beijing Genomics Institute
(Beijing, China).
Statistical analysis
The statistical analysis was performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). Data are expressed as
the median (range). The VAP positive and VAP negative data were
compared using a Mann-Whitney U test for equal proportion. The
statistical tests performed were two-sided. All the analyses were
performed on an intention-to-treat basis and a two-sided P<0.05
indicated that the difference between groups was statistically
significant. The association of characteristics with VAP was
assessed by Spearman's correlation. A Receiver Operating
Characteristic (ROC) curve was constructed to determine the cut-off
value of sTREM-1 expression in the EVC for the diagnosis of VAP.
The figures were drawn using GraphPad prism version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) and Medcalc11.4.2 (MedCalc
Software bvba, Ostend, Belgium).
Results
Patient characteristics following
clinical assessment
Throughout the study period, 95 children with
congenital heart diseases were admitted to the CICU at Shanghai
Children's Medical Center following cardiac surgery and 48 of the
patients met the inclusion criteria were suspected of having VAP.
Among them, 31 were diagnosed with VAP following the positive
detection of bacteria using PCR assays and 17 were not. The
baseline characteristics of the 48 patients are shown in Table I. The duration of mechanical
ventilation, as well as the CICU and hospital length of stay were
significantly increased in the VAP group compared with that in the
non-VAP control group (P<0.01). In addition, the mCPIS,
PO2/FiO2 (mmHg), PCT and CRP expression level
were not significantly different in the VAP group compared with
that in the non-VAP control group (P>0.05).
 | Table I.Patient characteristics of the study
groups. |
Table I.
Patient characteristics of the study
groups.
| Characteristics | Patients with VAP
(n=31) | Patients without VAP
(n=17) | P-value |
|---|
| Age (days) | 42 (1–2,738) | 49 (1–3,492) | 0.82 |
| Weight (kg) | 3.5 (1.9–18.2) | 3.6 (2.1–23) | 0.78 |
| Male sex | 26 (54.2%) | 25 (52.1%) | 0.73 |
| PRISM score | 12 (4–27) | 11 (2–20) | 0.25 |
| RACHS-1 score | 4 (2–6) | 4 (2–5) | 0.62 |
| mCPIS | 5 (3–8) | 4 (2–8) | 0.54 |
|
PO2/FiO2 (mmHg) | 230 (135–305) | 252 (153–320) | 0.75 |
| PCT expression
level | 5.2 (0.9–12) | 5 (0.8–11) | 0.63 |
| CRP expression
level | 22 (8–89) | 25 (6–92) | 0.35 |
| Use of CPB | 29 (93.5%) | 15 (88.2%) | 0.67 |
| Duration of CPB
(min) | 97 (0–172) | 94 (0–156) | 0.42 |
| Aortic cross clamp
time (min) | 62 (0–98) | 65 (0–109) | 0.23 |
| Duration of
mechanical ventilation (days) | 7 (3–17) | 3 (1–7) | <0.01 |
| CICU length of stay
(days) | 15 (5–30) | 7 (4–15) | <0.01 |
| Hospital length of
stay (days) | 28 (8–210) | 13 (7–26) | <0.01 |
Bacteria detection by qPCR and
microbiological culture
Of the 48 samples, the positive culture rate was
39.6% (19/48). From the culture experiments, a total of 21
pathogens were identified after culturing for 72 h, and >1
pathogen was detected in 2 samples (data not shown). Of the 48
samples, 31 (64.6%) were qPCR positive, confirming the diagnosis of
VAP. The qPCR positive samples were defined as the VAP group,
whilst the 17 qPCR negative samples were defined as the non-VAP
control group. A total of 44 pathogens from 31 samples were
detected in just 24 h of the samples being obtained from patients
with suspected VAP. A total of 9 samples had a mixed pathogen
infection. Of the 44 pathogens, 8 were Acinetobacter
baumannii, 2 were Haemophilus influenza, 4 were
Escherichia coli, 3 were Klebsiella pneumonia, 3 were
Enterobacter cloacae, 3 were Streptococcus
pneumoniae, 6 were Staphylococcus aureus, 2 were
Enterococcus faecium, 2 were Stenotrophomonas
maltophilia, 2 were Pseudomonas aeruginosa, 3 were
Mycoplasma pneumoniae and 6 were Candida albicans.
The qPCR results of the BALF samples yielded the best sensitivity
and specificity to diagnose VAP, differentiating true infections
from inflammation or colonisation.
Detection and comparison of sTREM-1
and diagnostic value of VAP
sTREM-1 protein concentration was detected in all 48
patients on the day that VAP was suspected. sTREM-1 protein
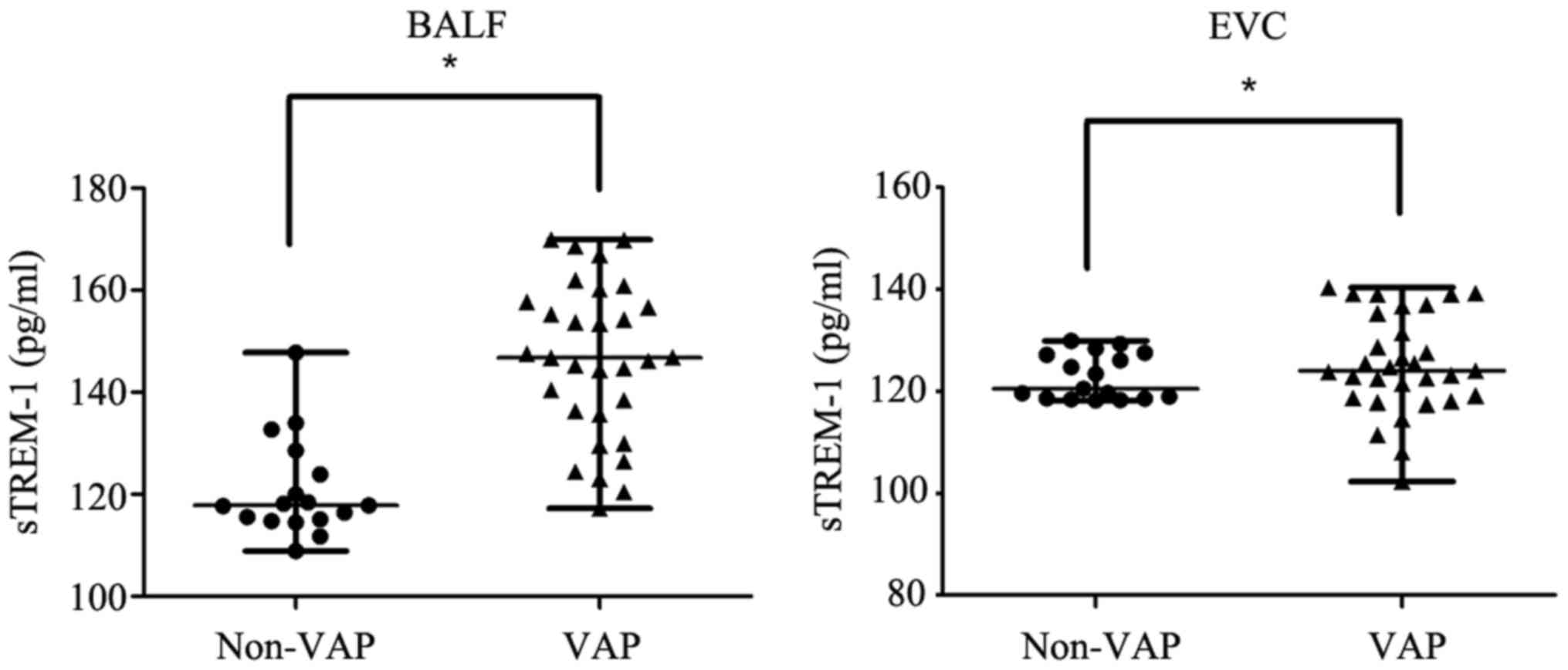
expression in BALF of the VAP group (median, 146.21 pg/ml; range,
117.26–169.91 pg/ml) was significantly higher compared with the
Non-VAP group (median, 118.06 pg/ml; range, 108.89–147.76 pg/ml;
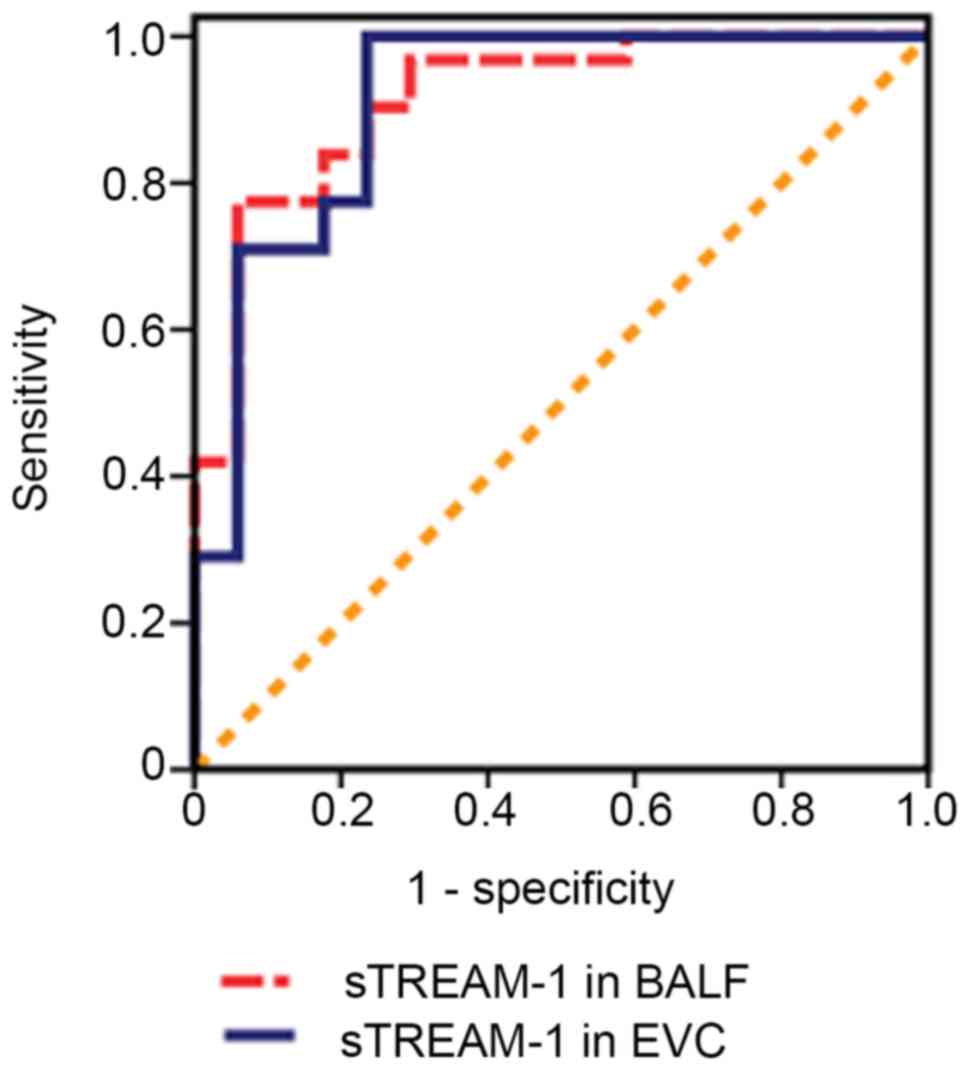
P<0.05; Fig. 1). The cut-off
value of sTREM-1 in BALF on the day of VAP diagnosis was 134.80
pg/ml, which had a sensitivity of 77.5% and a specificity of 93.8%
for the diagnosis of VAP [area under the ROC curve, 0.91; 95%
confidence intervals (CI), 0.83–0.99; Fig. 2]. sTREM-1 protein expression in EVC
of the VAP group (median, 125.29 pg/ml; range, 102.31–140.34 pg/ml)
was significantly higher compared with the Non-VAP group (median,
120.48 pg/ml; range, 118.21–129.91 pg/ml; P<0.05; Fig. 1). The cut-off value for sTREM-1 in
EVC was 109.75 pg/ml, which had a sensitivity of 93.2% and a
specificity of 76.5% for the diagnosis of VAP (area under the ROC
curve, 0.89; 95% CI, 0.79–0.98; Fig.
2).
Discussion
VAP is a major cause of morbidity and mortality
following cardiac surgery worldwide, particularly in children
(5). Despite advances in
diagnostics, it is still challenging to diagnose VAP early, and the
aetiology and therapy is empirical (4). As systemic inflammatory response
syndrome and VAP have similar characteristics in early development,
patients are treated for VAP, which requires treatment with
antibiotics, which is excessive and unnecessary for patients with
systemic inflammatory response syndrome (24). This excessive and unnecessary use of
antibiotics may lead to increased bacterial resistance and
increased costs, which highlights the importance of an early and
accurate diagnosis of VAP (25). As
a result, the current study investigated biological markers of
infection, such as sTREM-1, to improve the accuracy of the
diagnosis of VAP.
One aspect of the current study was the use of PCR
for the diagnosis of VAP. Cultures lack the sensitivity to identify
all the bacteria in samples for multiple reasons, including
previous antibiotic administration, sampling error and fastidious
bacteria (26,27); whereas PCR amplification may
supplement cultures when detecting pathogens (16). In the present study, more samples and
more pathogens were detected by the PCR assay compared with
traditional culturing, and the positive rates were 64.6 and 39.6%,
respectively. The results imply that the false negative rate was
much higher when the culture method was employed compared with the
PCR assay, and the PCR assay was more sensitive for the detection
of pathogens compared with traditional culturing.
Several previous studies have reported the
diagnostic effects of sTREM-1 protein concentration evaluation in
VAP. Yu et al (14)
demonstrated that the sTREM-1 concentration was unregulated in EBC
and BALF of patients with VAP, allowing the differentiation of
patients with ischaemic stroke and VAP. Matsuno and Carlotti
(13) demonstrated a similar result
for the sTREM-1 concentration in the mBALF of children with and
without VAP following congenital heart surgery. In the present
study, a significant increase in the sTREM-1 concentration was
observed in the BALF sample of the VAP group; the cut-off value was
134.8 pg/ml, with a sensitivity of 77.5% and a specificity of 93.8%
for the diagnosis of VAP. In addition, the levels of sTREM-1 in the
EVC were significantly higher in the VAP group; the cut-off value
was 109.75 pg/ml, with a sensitivity of 93.2% and a specificity of
76.5% for the diagnosis of VAP. The results of the current study
demonstrated that measuring the sTREM-1 in the BALF and EVC were
useful for diagnosing VAP following the result of a PCR assay in
this population. However, a previous study revealed that measuring
the sTREM-1 protein concentration in the mBALF did not discriminate
patients with VAP from those without VAP following cardiac surgery
in children (28). In the
aforementioned study, mBALF was collected in a plastic container
located at the centre of the exhaled portion of the ventilator
tubing, which was far away from the patient's natural airway. Thus,
the detection of sTREM-1 protein concentration and analysis of
mBALF were inevitably affected by the bacterial colonization in the
artificial airway. The difference between the two studies is that
bronchoscopy was used for the BALF in the present study and the
samples were obtained from deep within the pulmonary alveolus. The
detection of sTREM-1 in BALF through the collection of BALF from
the pulmonary alveolus was a more effective method as it was less
likely to be influenced by an extrapulmonary infection.
Additionally, the use of PCR for diagnosis of pneumonia has been
demonstrated to be more sensitive in several studies (29,30). In
the present study, PCR was used for the pathogenic diagnosis of VAP
and more pathogens were detected using that method compared with
culture methods, reducing the rate of missed diagnoses.
One of the limitations of the current study is the
lack of measurements of sTREM-1 concentrations prior to surgery to
determine the patients' baseline levels. Another limitation is the
lack of detection of viruses; the detection of viruses and bacteria
by PCR will be performed in a future study.
To the best of our knowledge, the current study is
the first to evaluate the diagnostic value of sTREM-1 and a PCR
assay in the diagnosis of VAP following cardiac surgery. The
detection of sTREM-1 protein concentration in the BALF and EVC
samples may be useful for the diagnosis of VAP following paediatric
heart surgery. The PCR assay defined the diagnosis and pathogens
for VAP early in children undergoing cardiac surgery.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81602818) and
Science and Technology Commission of Shanghai Municipality (grant
no. 15411967100).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL, LZ and ZX designed the study. YL, QC and MZ
collected the data. CL and XG analyzed the data. CL prepared the
manuscript. CL, LZ and QC revised the manuscript. All authors read
and approved the final manuscript
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai Jiaotong University School of Medicine and
written informed consent was obtained from the patients'
parents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guardia Camí MT, Jordan García I and Urrea
Ayala M: Nosocomial infections in pediatric patients following
cardiac surgery. An Pediatr (Barc). 69:34–38. 2008.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roeleveld PP, Guijt D, Kuijper EJ,
Hazekamp MG, de Wilde RB and de Jonge E: Ventilator-associated
pneumonia in children after cardiac surgery in The Netherlands.
Intensive Care Med. 37:1656–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tarnok A and Schneider P: Pediatric
cardiac surgery with cardiopulmonary bypass: Pathways contributing
to transient systemic immune suppression. Shock. 16 Suppl
1:S24–S32. 2001. View Article : Google Scholar
|
|
4
|
Bassetti M, Taramasso L, Giacobbe DR and
Pelosi P: Management of ventilator-associated pneumonia:
Epidemiology, diagnosis and antimicrobial therapy. Expert Rev Anti
Infect Ther. 10:585–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melsen WG, Rovers MM, Groenwold RH,
Bergmans DC, Camus C, Bauer TT, Hanisch EW, Klarin B, Koeman M,
Krueger WA, et al: Attributable mortality of ventilator-associated
pneumonia: A meta-analysis of individual patient data from
randomised prevention studies. Lancet Infect Dis. 13:665–671. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brix-Christensen V: The systemic
inflammatory response after cardiac surgery with cardiopulmonary
bypass in children. Acta Anaesthesiol Scand. 45:671–679. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venkatachalam V, Hendley JO and Willson
DF: The diagnostic dilemma of ventilator-associated pneumonia in
critically ill children. Pediatr Crit Care Med. 12:286–296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fabregas N, Ewig S, Torres A, El-Ebiary M,
Ramirez J, de La Bellacasa JP, Bauer T and Cabello H: Clinical
diagnosis of ventilator associated pneumonia revisited: Comparative
validation using immediate post-mortem lung biopsies. Thorax.
54:867–873. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pontrelli G, De Crescenzo F, Buzzetti R,
Calò Carducci F, Jenkner A, Amodio D, De Luca M, Chiurchiu S,
Davies EH, Simonetti A, et al: Diagnostic value of soluble
triggering receptor expressed on myeloid cells in paediatric
sepsis: A systematic review. Ital J Pediatr. 42:442016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazzucchelli I, Garofoli F, Ciardelli L,
Borghesi A, Tzialla C, Di Comite A, Angelini M, Tinelli C, Merlini
G and Stronati M: Diagnostic performance of triggering receptor
expressed on myeloid cells-1 and CD64 index as markers of sepsis in
preterm newborns. Pediatr Crit Care Med. 14:178–182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palazzo SJ, Simpson T and Schnapp LM:
Triggering receptor expressed on myeloid cells type 1 as a
potential therapeutic target in sepsis. Dimens Crit Care Nurs.
31:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Determann RM, Millo JL, Gibot S, Korevaar
JC, Vroom MB, van der Poll T, Garrard CS and Schultz MJ: Serial
changes in soluble triggering receptor expressed on myeloid cells
in the lung during development of ventilator-associated pneumonia.
Intensive Care Med. 31:1495–1500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuno AK and Carlotti AP: Role of
soluble triggering receptor expressed on myeloid cells-1 for
diagnosing ventilator-associated pneumonia after cardiac surgery:
An observational study. BMC Cardiovasc Disord. 13:1072013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Zhu C, Liu C, Gao Y, Yin R and Cao
J: Diagnostic performance of soluble triggering receptor expressed
on myeloid cells-1 in ventilator-associated pneumonia of patients
with ischemic stroke. Can J Infect Dis Med Microbiol.
2017:95136902017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy J, O' Rourke S, Corcoran M, O'
Sullivan N, Cunney R and Drew R: Evaluation of the clinical utility
of a real-time PCR assay for the diagnosis of streptococcus
pneumoniae bacteremia in children: A retrospective diagnostic
accuracy study. Pediatr Infect Dis J. 37:153–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YS, Liu PY, Huang YF, Chen CS, Chiu
LH, Huang NY, Hsieh KS and Chen YS: Comparison of diagnostic tools
with multiplex polymerase chain reaction for pediatric lower
respiratory tract infection: A single center study. J Microbiol
Immunol Infect. 46:413–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ngo TT, Hoang VT, Tran TL, Trinh VS, Tran
T, Dao TQ, Phan QH, Meyer CG and Le HS: Clinical utility of an
optimised multiplex real-time PCR assay for the identification of
pathogens causing sepsis in vietnamese patients. Int J Infect Dis.
67:122–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jordana-Lluch E, Gimenez M, Quesada MD,
Ausina V and Martro E: Improving the diagnosis of bloodstream
infections: PCR coupled with mass spectrometry. Biomed Res Int.
2014:5012142014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vutukuru MR, Sharma DK, Ragavendar MS,
Schmolke S, Huang Y, Gumbrecht W and Mitra N: A rapid, highly
sensitive and culture-free detection of pathogens from blood by
positive enrichment. J Microbiol Methods. 131:105–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du Rand IA, Barber PV, Goldring J, Lewis
RA, Mandal S, Munavvar M, Rintoul RC, Shah PL, Singh S, Slade MG,
et al: British thoracic society guideline for advanced diagnostic
and therapeutic flexible bronchoscopy in adults. Thorax. 66 Suppl
3:iii1–iii21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balakrishnan G, Aitchison T, Hallworth D
and Morton NS: Prospective evaluation of the Paediatric Risk of
Mortality (PRISM) score. Arch Dis Child. 67:196–200. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mildh L, Pettilä V, Sairanen H and
Rautiainen P: Predictive value of paediatric risk of mortality
score and risk adjustment for congenital heart surgery score after
paediatric open-heart surgery. Interact Cardiovasc Thorac Surg.
6:628–631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lauzier F, Ruest A, Cook D, Dodek P,
Albert M, Shorr AF, Day A, Jiang X and Heyland D: The value of
pretest probability and modified clinical pulmonary infection score
to diagnose ventilator-associated pneumonia. J Crit Care. 23:50–57.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salehifar E, Tavakolian Arjmand S, Aliyali
M, Abedi S, Sharifpour A, Alipour A, Ala S, Eslami G, Bozorgi F,
Mahdavi MR and Walley KR: Role of C-reactive protein and tumor
necrosis factor-alpha in differentiating between
ventilator-associated pneumonia and systemic inflammatory response
syndrome without infectious etiology. Tanaffos. 15:205–212.
2016.PubMed/NCBI
|
|
25
|
Klompas M, Branson R, Eichenwald EC,
Greene LR, Howell MD, Lee G, Magill SS, Maragakis LL, Priebe GP,
Speck K, et al: Strategies to prevent ventilator-associated
pneumonia in acute care hospitals: 2014 update. Infect Control Hosp
Epidemiol. 35 Suppl 2:S133–S154. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiu YH, Chen TJ, Chen CT and Lu CC:
Positive blood cultures in pediatric emergency department patients:
Epidemiological and clinical characteristics. Acta Paediatr Taiwan.
46:11–16. 2005.PubMed/NCBI
|
|
27
|
Shin JH, Song SA, Kim MN, Lee NY, Kim EC,
Kim S, Koo SH, Ryoo NH, Kim JS and Cho JH: Comprehensive analysis
of blood culture performed at nine university hospitals in Korea.
Korean J Lab Med. 31:101–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schindler MB and Cox PN: A simple method
of bronchoalveolar lavage. Anaesth Intensive Care. 22:66–68.
1994.PubMed/NCBI
|
|
29
|
Siow WT, Koay ES, Lee CK, Lee HK, Ong V,
Ngerng WJ, Lim HF, Tan A, Tang JW and Phua J: The use of polymerase
chain reaction amplification for the detection of viruses and
bacteria in severe community-acquired pneumonia. Respiration.
92:286–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carrol ED, Mankhambo LA, Guiver M, Banda
DL; IPD Study Group, ; Denis B, Dove W, Jeffers G, Molyneux EM,
Molyneux ME, et al: PCR improves diagnostic yield from lung
aspiration in Malawian children with radiologically confirmed
pneumonia. PLoS One. 6:e210422011. View Article : Google Scholar : PubMed/NCBI
|