Introduction
Depression is a chronic disease with a high
recurrence rate, seriously affecting people's lives and health
(1). The treatment with
antidepressants has no or poor therapeutic effect on approximately
20–30% patients with depression, so depression will progress into
treatment-resistant depression (TRD) (2). The burden of disease against TRD
patients is 40% higher than that against non-TRD patients (3). Certain progress has been made in
identifying biomarkers of clinical symptoms and affective disorder
of TRD, and therapeutic response to antidepressants (4). Increasingly more studies have
manifested that inflammatory factors are involved in the occurrence
and development of depression, but reliable biomarkers for the
early identification of TRD have not been found yet. In this study,
hypersensitive C-reactive protein (hs-CRP), a clinically-available
factor, was selected to investigate the possibility of inflammatory
factors serving as biomarkers of TRD, so as to realize early
diagnosis and symptomatic treatment.
Materials and methods
Objects
Depression patients treated in the Department of
Psychiatry, Dongfang People's Hospital Affiliated to Xuzhou Medical
University (Xuzhou, China) from May 2012 to December 2016 were
enrolled according to the following inclusion criteria: i) patients
aged 18–60 years. ⅱ) Patients who met the diagnostic criteria of
depressive episode in the 10th edition of International
Classification of Diseases (ICD)-10, and had used two or more kinds
of different antidepressants in full dose for a full course of
treatment (>6 weeks of drug treatment at the therapeutic dose).
Depression patients with no or little therapeutic effect were
enrolled into the TRD group, while those not meeting the TRD
criteria were enrolled into the non-TRD group. ⅲ) Patients with the
Hamilton Depression Scale (HAMD) score of 17 points or above. ⅳ)
Patients who did not take psychotropic drugs within 2 weeks before
enrollment.
Exclusion criteria: i) patients with secondary
depression due to organic disease or other mental diseases. ⅱ)
Patients with a history of head trauma, nervous system disease or
mental disorder caused by psychoactive substances and various
non-addictive substances. ⅲ) Patients with different physical or
immunosuppressive diseases. ⅳ) Pregnant or lactating women; or ⅴ)
patients who took various antibiotics or immunosuppressive agents
within half a year before admission.
Patients in the above two groups signed the informed
consent, and this study was approved by the Ethics Committee of
Dongfang People's Hospital Affiliated to Xuzhou Medical University
(approval no. 2014ZL004).
In the TRD group, there were a total of 103
patients, including 48 males and 55 females aged 25–55 years with
an average of 37.60±5.92 years, and the education was 5–18 years
with an average of 9.51± 2.93 years. In the non-TRD group, there
were a total of 103 patients, including 45 males and 58 females
aged 22–55 years with an average of 38.34±7.18 years, and the
education year was 8–16 years with an average of 10.71±3.39 years.
Sex (p=0.674) and age (p=0.423) were matched between the TRD and
non-TRD groups, and there were no statistically significant
differences.
Scale assessment
At 1 day after admission and at 6 weeks after
treatment, the severity of depression in patients was evaluated
using HAMD-17 by two physicians in the Department of Psychiatry who
had received strict assessment training (Κ=0.83). The total HAMD
score and the scores of five factors were calculated, respectively,
the latter of which included sleep disorder (item 4, 5 and 6),
retardation (item 1, 7, 8 and 14), anxiety/somatization (item 10,
11, 12, 13, 15 and 17), weight (item 16) and cognitive disorder
(item 2, 3 and 9).
Specimen collection and storage
At 2 days after admission and at 6 weeks after
treatment, 5 ml fasting venous blood was drawn from each patient
using the ethylene diamine tetraacetic acid (EDTA) anti-coagulant
tube at 08:00 in the morning, and the blood specimen was
centrifuged at 1,474 × g and 4°C for 15 min within 1 h after blood
collection. After the serum was separated, it was stored in a
refrigerator at −70°C for the detection of hs-CRP.
Determination of hs-CRP
The level of hs-CRP in patients was detected via
immunofluorescence using the immunofluorescence detector and its
supporting reagents (Skyverse, Ltd., Shenzhen, China). The
operation was performed by laboratory staff according to the
operation specifications.
Determination of body mass index
(BMI)
The weight and height of patients were measured at
admission, and the weight of patients in both groups was measured
again after 6 weeks. BMIs of patients in both groups before and
after treatment were calculated according to the formula: BMI =
weight (kg)/height (m)2.
Drug therapy
TRD patients were treated with venlafaxine
sustained-release tablets (trade name: Bolexin sustained-release
tablets) at an initial dose of 37.5 mg/day, and the dose was
increased to 75 mg/day after 4 days and then gradually increased to
75 mg/bid according to the clinical reaction of patients (225
mg/day at the most). The non-TRD patients were treated with
fluoxetine (trade name: Prozac) at an initial dose of 20 mg/day (80
mg/day at the most). Lorazepam was used to improve the sleep of
patients (6 mg/qn at the most) for 6 weeks.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
22.0 software package (IBM Corp., Armonk, NY, USA) was used for
data analysis. Measurement data were presented as means ± SD, the
independent-samples t-test was used for the comparison between the
two groups, and Spearman's correlation analysis was used for the
correlation analysis. χ2 test was performed for
enumeration data. The diagnostic effect of hs-CRP on the TRD
patients was evaluated using the receiver operating characteristic
(ROC) curve, and the value of hs-CRP in the diagnosis of TRD was
investigated via binary logistic regression analysis. P<0.05
suggested that the difference was statistically significant.
Results
Comparison of demographic and clinical
data of patients between the two groups
There were no significant differences in age, sex,
BMI, HAMD score and family history between the two groups of
patients (p>0.05). The education in the TRD group (9.51±2.93
years) was shorter than that in the non-TRD group (10.72±3.39
years), both baseline and post-treatment hs-CRP level in the TRD
group (12.05±5.79 and 9.02±3.71 mg/l) were higher than those in the
non-TRD group (7.85±2.85 and 6.10±2.74 mg/l), and there was a
significant difference in the hs-CRP level (9.02±3.71 mg/l) between
the TRD group after treatment and the non-TRD group before
treatment (7.85±2.85 mg/l) (t=2.827, p=0.005) (Table I).
 | Table I.Comparison of demographic and clinical
data between TRD and non-TRD patients. |
Table I.
Comparison of demographic and clinical
data between TRD and non-TRD patients.
| Variables | TRD | Non-TRD | t/χ2 | P-value |
|---|
| Baseline |
| Age | 37.60±5.92 |
38.3±7.18 | 0.804 | 0.422 |
| Education
yeara | 9.51±2.93 | 10.72±3.39 | 2.728 | 0.007 |
|
hs-CRPa | 12.05±5.79 | 7.85±2.85 | 6.569 | 0.001 |
| BMI | 21.2±2.56 | 21.01±2.01 | 0.727 | 0.468 |
| Total
course of diseasea | 9.1±4.9 | 5.51±2.92 | 6.387 | 0.001 |
| Onset
agea | 25.56±3.21 | 29.10±5.44 | 5.678 | 0.001 |
| HAMD | 28.67±5.71 | 29.95±5.33 | 1.666 | 0.097 |
| Sex | 48/55 | 45/58 | 0.176 | 0.674 |
| Family
history | 20/103 | 18/103 | 0.089 | 0.766 |
| After treatment |
| HAMD | 9.07±2.73 |
8.41±2.41 | 1.87 | 0.063 |
|
hs-CRPa | 9.02±3.71 |
6.10±2.74 | 6.443 | 0.001 |
Correlation analyses of baseline
hs-CRP level with clinical features of TRD patients
The HAMD score (r=0.338, p=0.031),
anxiety/somatization factor score (r=0.465, p=0.015) and sleep
disorder (r=0.387, p=0.029) of the TRD patients were positively
correlated with the hs-CRP level, but the onset age (r=−0.59,
p=0.009) was negatively correlated with the hs-CRP level (Table II).
 | Table II.Correlation of baseline hs-CRP level
with clinical features of TRD patients. |
Table II.
Correlation of baseline hs-CRP level
with clinical features of TRD patients.
| Variables | Pearson's correlation
coefficient (r) | P-value |
|---|
| Total HAMD
scorea | 0.338 | 0.031 |
|
Anxiety/somatizationa | 0.465 | 0.015 |
| Sleep
disordera | 0.387 | 0.029 |
| Weight | −0.083 | 0.168 |
| Cognitive
disorder | 0.264 | 0.133 |
| Retardation | 0.261 | 0.077 |
| Age | −0.156 | 0.076 |
| Education year | −0.23 | 0.067 |
| BMI | 0.07 | 0.315 |
| Total course of
disease | 0.135 | 0.054 |
| Onset
agea | −0.59 | 0.009 |
Logistic regression analyses of
correlation of clinical factors with TRD risk
Logistic regression analyses manifested that HAMD
and hs-CRP were included into the regression equation [β=0.087,
SE=0.035, p=0.012, odds ratio (OR) = 1.091, 95% confidence interval
(CI) = 1.019–1.269; β=0.223, SE=0.052, p=0.001, OR=2.834, 95%
CI=1.723–4.886), indicating that hs-CRP is a risk factor for TRD
(Table III).
 | Table III.Logistic regression analyses. |
Table III.
Logistic regression analyses.
| Variables | β | SE | Wald | P-value | Exp (β) | 95% CI |
|---|
| HAMDa | 0.087 | 0.035 | 6.271 | 0.012 | 1.091 | 1.019–1.269 |
| Total course of
disease | 0.343 | 0.068 | 25.333 | 0.001 | 1.71 | 0.621–3.811 |
| Onset age | 0.242 | 0.047 | 26.102 | 0.001 | 1.274 | 0.561–2.398 |
| Family history | 0.035 | 0.466 | 0.006 | 0.94 | 1.036 | 0.415–2.581 |
| Education year | 0.09 | 0.061 | 2.227 | 0.136 | 1.094 | 0.972–1.231 |
| hs-CRPa | 0.223 | 0.052 | 18.235 | 0.001 | 2.834 | 1.723–4.886 |
| Constant | 0.526 | 1.826 | 9.168 | 0.002 | 0.004 |
|
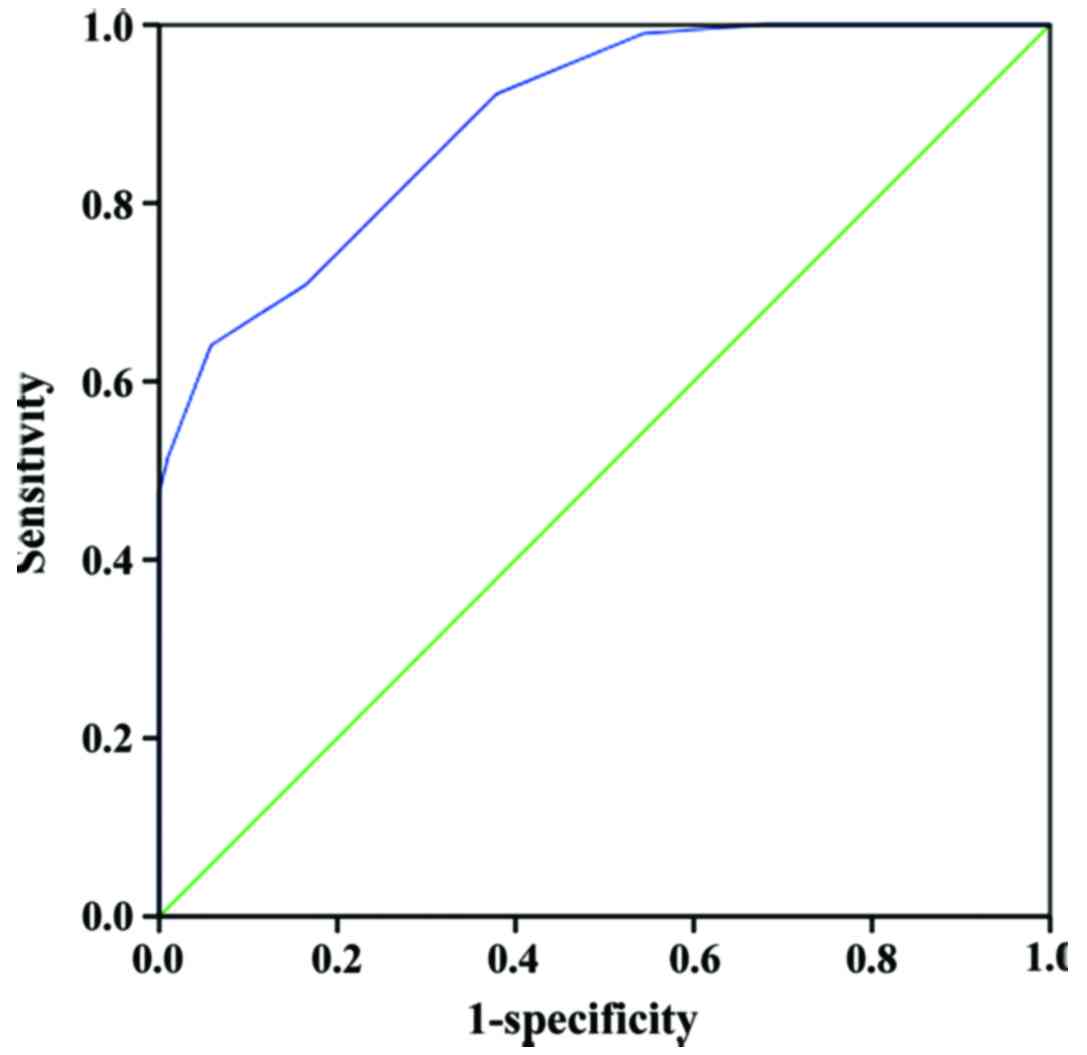
Diagnostic effect of hs-CRP on
TRD
The larger the area under the ROC curve, the better
the diagnostic effect. The sensitivity and specificity of hs-CRP in
the diagnosis of TRD are shown in Fig.
1, and the area under the ROC curve was 0.893 (p=0.001, 95%
CI=0.852–0.933), indicating that hs-CRP has high accuracy in the
diagnosis of TRD. The level of hs-CRP of 10.5 mg/l was the best
critical value for the diagnosis of TRD with sensitivity of 0.922
and specificity of 0.721 (Fig.
1).
Discussion
Results of this study manifested that the total
course of disease of the TRD patients was longer than that of the
non-TRD patients, and the onset age was lower than that of the
non-TRD patients before treatment. Conway et al (5) found that the course of disease of TRD
patients is longer, suggesting that the disease of such patients is
more likely to be chronic. According to the study of Juruena et
al (6), it was also confirmed
that the longer the single course of TRD in patients is, the worse
the recovery of social function will be. Moreover, Vandoolaeghe
et al (7) also found that the
onset age of the TRD patients is lower. The above conclusions are
consistent with results in this study, suggesting that the samples
selected in this study have certain homogeneity with those selected
by other clinical research organizations. In addition, this study
demonstrated that the education of the TRD patients was shorter
than that of the non-TRD patients, suggesting that the cognitive
function of TRD patients may be poorer than that of non-TRD
patients, which is consistent with the study result of Bodnar et
al (8). Besides, Kiosses et
al (9) showed that various
cognitive impairments occur in TRD patients, indirectly proving the
results of this study.
Research has displayed that both under- and
overweight affect the correlation between hs-CRP and depression
(10). According to a meta-analysis
of a cross-sectional study, the correlation between depression and
hs-CRP is significantly reduced after BMI is matched, but this
correlation has not been determined yet in the longitudinal study
after BMI is matched. To reduce the influence of BMI on the
correlation between them, two groups of patients with no
significant difference in BMI were selected in this study.
In recent years, studies on the correlation of
depression with inflammatory factors have emphasized the immune
activation in depression patients, and a variety of cytokines are
produced, including interleukin (IL)-1, IL-6, tumor necrosis
factor-α (TNF-α) and interferon-β (IFN-β), after immune activation.
Matrisciano et al (11)
revealed that IL-2, IL-6 and TNF-γ levels in patients with
first-episode depression are significantly higher than those in the
normal control group. Maes (12)
also proposed the depression immune response hypothesis that
depression is related to the activation of immune system, which is
a kind of psychoneural immune disorder, and the peripheral immune
activation, through releasing pro-inflammatory cytokines, leads to
the changes in various behavior, neuroendocrine and
neurobiochemistry related to the depression. In this study, it was
also manifested that the hs-CRP levels were increased before and
after treatment in depression patients, and it was higher in the
TRD patients than that in the non-TRD patients, suggesting that the
immune activation in TRD patients is stronger, and the hs-CRP level
is still increased (normal level of hs-CRP: 0–5 mg/l) after
clinical symptoms are greatly improved. The above conclusion
demonstrates that hs-CRP is associated with depressive state, and
also may possibly be used as a specific factor of depression.
According to clinical research, the levels of hs-CRP, IL-1, IL-6,
IFN-γ and TNF-α are increased in the blood of depression patients
(13), which is possibly related to
the excessive activation of hypothalamic-pituitary-adrenal (HPA)
axis. The IL-6 level in the plasma is higher in patients receiving
ineffective treatment with selective serotonin reuptake inhibitor
(SSRI) and serotonin-norepinephrine reuptake inhibitor (SNRI)
compared with that in patients receiving effective treatment
(14). The increased levels of
inflammatory factors upregulate the levels of 5-HT transporters and
dopamine transporter, reduce neurogenesis, long-term potentiation
(LTP) and 5-HT activity, increase excitotoxicity, activate HPA
axis, improve release of glucocorticoids, regulate neuronal
activity, lead to cognitive impairment (15).
The hs-CRP concentration in the blood can reflect
the efficacy of antidepressants. The higher the concentration of
hs-CRP is, the higher the inflammatory level and the stronger the
response to drugs will be (16).
Gene polymorphisms of inflammatory factors are also related to the
sensitivity of patients to antidepressants (17), and the rs2279115C allele of B
lymphocyte anti-apoptotic protein 2 is significantly associated
with the response of male patients with depression to
antidepressants (18). Some studies
have shown that different clinical manifestations of depression
will also affect the correlations of inflammatory factors with
depression (19,20), and the relationship of hs-CRP with
depression accompanied by somatic symptoms is higher than that with
depression patients accompanied by other clinical symptoms
(21,22). This study also displayed that the
anxiety/somatization score in TRD patients had a significant
correlation with the baseline hs-CRP level. In addition, it was
found that there was an obvious correlation between sleep disorder
score and baseline hs-CRP level. The possible reason is that the
long-term sleep disorder can result in neuroendocrine and immune
system dysfunction, thus increasing the hs-CRP level. Moreover,
results of this study manifested that there was a significant
negative correlation between onset age and baseline hs-CRP level in
TRD patients, indicating that the lower the onset age is, the more
severe the inflammatory factor system disorder will be. Chang et
al (23) revealed that hs-CRP
can act as an effective biomarker for affective disorders. Another
study manifested that hs-CRP has remarkable correlations with the
severity and unique subtypes of depression patients, especially in
female patients (24). The increased
hs-CRP level in some depression patients indicates that the hs-CRP
level can be used as a predictor of antidepression effect.
According to a prospective study, depression patients with the
hs-CRP level of <1 mg/l have good response to escitalopram, an
SSRI, while those with the hs-CRP level of >1 mg/l have good
response to tricyclic drugs (25).
Moreover, can the difference in the baseline hs-CRP level between
TRD and non-TRD patients be used as a marker to distinguish them?
In this study, the baseline hs-CRP level in the TRD patients was
significantly higher than that in the non-TRD patients, and the
same was true after treatment, displaying a large difference
between them. In other words, there was little overlap between the
the low hs-CRP level in TRD patients after treatment and the high
baseline hs-CRP level in the non-TRD patients, so hs-CRP could
serve as a biomarker of TRD in this study. In addition, logistic
regression analyses and ROC curve in this study illustrated that
hs-CRP was associated with TRD, and it was concluded that 10.5 mg/l
hs-CRP was the best critical value for the diagnosis of TRD with
sensitivity of 0.922 and specificity of 0.721, indicating that the
diagnostic possibility of TRD is larger when the level of hs-CRP is
>10.5 mg/l, with the diagnostic coincidence rate of 0.893.
Therefore, hs-CRP is more likely to be a reference index for
distinguishing TRD from non-TRD, which has important reference
value for early identification and individualized treatment of TRD
patients.
In conclusion, the educational level was lower, the
first-onset age was lower, and both baseline and post-treatment
hs-CRP level after treatment were higher in the TRD group than
those in the non-TRD patients, so hs-CRP can serve as one of the
diagnostic bases of TRD. However, the follow-up time was short and
the sample size was small in this study, so the follow-up time
should be extended and the sample size should be expanded to
further reveal the correlation between hs-CRP and TRD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ and HZ were responsible for scale assessment and
drug therapy. DG and LQ were responsible for the collection and
analysis of patient data. JQ, XZ and HZ determined hs-CRP and BMI.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Dongfang People's Hospital Affiliated to Xuzhou Medical University
(Xuzhou, China). Patients who participated in this study had
complete clinical data. Signed informed consents were obtained from
the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wiles N, Thomas L, Abel A, Barnes M,
Carroll F, Ridgway N, Sherlock S, Turner N, Button K, Odondi L, et
al: Clinical effectiveness and cost-effectiveness of cognitive
behavioural therapy as an adjunct to pharmacotherapy for
treatment-resistant depression in primary care: The CoBalT
randomised controlled trial. Health Technol Assess. 18:1–167,
vii-viii. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swaab DF, Fliers E, Hoogendijk WJ, Veltman
DJ and Zhou JN: Interaction of prefrontal cortical and hypothalamic
systems in the pathogenesis of depression. Prog Brain Res.
126:369–396. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Waarde JA, Scholte HS, van Oudheusden
LJ, Verwey B, Denys D and van Wingen GA: A functional MRI marker
may predict the outcome of electroconvulsive therapy in severe and
treatment-resistant depression. Mol Psychiatry. 20:609–614. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider B and Prvulovic D: Novel
biomarkers in major depression. Curr Opin Psychiatry. 26:47–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conway CR, Gebara MA, Walker MC,
Lessov-Schlaggar CN, Janski AM, Chibnall JT, Cristancho P, Sheline
YI, Gott BM and Svrakic DM: Clinical characteristics and management
of treatment-resistant depression. J Clin Psychiatry. 76:1569–1570.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Juruena MF, Pariante CM, Papadopoulos AS,
Poon L, Lightman S and Cleare AJ: The role of mineralocorticoid
receptor function in treatment-resistant depression. J
Psychopharmacol. 27:1169–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vandoolaeghe E, Maes M, Vandevyvere J and
Neels H: Hypothalamic-pituitary-thyroid-axis function in treatment
resistant depression. J Affect Disord. 43:143–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bodnar A, Krzywotulski M, Lewandowska A,
Chlopocka- Wozniak M, Bartkowska-Sniatkowska A, Michalak M and
Rybakowski JK: Electroconvulsive therapy and cognitive functions in
treatment-resistant depression. World J Biol Psychiatry.
17:159–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiosses DN, Ravdin LD, Gross JJ, Raue P,
Kotbi N and Alexopoulos GS: Problem adaptation therapy for older
adults with major depression and cognitive impairment: A randomized
clinical trial. JAMA Psychiatry. 72:22–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Al-Sayegh H, Jabrah R, Wang W, Yan
F and Zhang J: Association between C-reactive protein and
depression: Modulated by gender and mediated by body weight.
Psychiatry Res. 219:103–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matrisciano F, Bonaccorso S, Ricciardi A,
Scaccianoce S, Panaccione I, Wang L, Ruberto A, Tatarelli R,
Nicoletti F, Girardi P, et al: Changes in BDNF serum levels in
patients with major depression disorder (MDD) after 6 months
treatment with sertraline, escitalopram, or venlafaxine. J
Psychiatr Res. 43:247–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maes M: Evidence for an immune response in
major depression: A review and hypothesis. Prog
Neuropsychopharmacol Biol Psychiatry. 19:11–38. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dowlati Y, Herrmann N, Swardfager W, Liu
H, Sham L, Reim EK and Lanctôt KL: A meta-analysis of cytokines in
major depression. Biol Psychiatry. 67:446–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshimura R, Hori H, Ikenouchi-Sugita A,
Umene-Nakano W, Ueda N and Nakamura J: Higher plasma interleukin-6
(IL-6) level is associated with SSRI- or SNRI-refractory
depression. Prog Neuropsychopharmacol Biol Psychiatry. 33:722–726.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krishnadas R and Cavanagh J: Depression:
An inflammatory illness? J Neurol Neurosurg Psychiatry. 83:495–502.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raison CL, Rutherford RE, Woolwine BJ,
Shuo C, Schettler P, Drake DF, Haroon E and Miller AH: A randomized
controlled trial of the tumor necrosis factor antagonist infliximab
for treatment-resistant depression: The role of baseline
inflammatory biomarkers. JAMA Psychiatry. 70:31–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong ML, Dong C, Maestre-Mesa J and
Licinio J: Polymorphisms in inflammation-related genes are
associated with susceptibility to major depression and
antidepressant response. Mol Psychiatry. 13:800–812. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Wu Z, Hong W, Wang Z, Peng D,
Chen J, Yuan C, Yu S, Xu L and Fang Y: Influence of BCL2 gene in
major depression susceptibility and antidepressant treatment
outcome. J Affect Disord. 155:288–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart JC, Zielke DJ, Hawkins MA,
Williams DR, Carnethon MR, Knox SS and Matthews KA: Depressive
symptom clusters and 5-year incidence of coronary artery
calcification: The coronary artery risk development in young adults
study. Circulation. 126:410–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michal M, Wiltink J, Kirschner Y, Wild PS,
Münzel T, Ojeda FM, Zeller T, Schnabel RB, Lackner K, Blettner M,
et al: Differential associations of depressive symptom dimensions
with cardio-vascular disease in the community: Results from the
Gutenberg health study. PLoS One. 8:e720142013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deverts DJ, Cohen S, DiLillo VG, Lewis CE,
Kiefe C, Whooley M and Matthews KA: Depressive symptoms, race, and
circulating C-reactive protein: The Coronary Artery Risk
Development in Young Adults (CARDIA) study. Psychosom Med.
72:734–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duivis HE, Vogelzangs N, Kupper N, de
Jonge P and Penninx BW: Differential association of somatic and
cognitive symptoms of depression and anxiety with inflammation:
Findings from the Netherlands Study of Depression and Anxiety
(NESDA). Psychoneuroendocrinology. 38:1573–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang HH, Wang TY, Lee IH, Lee SY, Chen
KC, Huang SY, Yang YK, Lu RB and Chen PS: C-reactive protein: A
differential biomarker for major depressive disorder and bipolar II
disorder. World J Biol Psychiatry. 18:63–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Köhler-Forsberg O, Buttenschøn HN, Tansey
KE, Maier W, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer
A, Rietschel M, et al: Association between C-reactive protein (CRP)
with depression symptom severity and specific depressive symptoms
in major depression. Brain Behav Immun. 62:344–350. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uher R, Tansey KE, Dew T, Maier W, Mors O,
Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, et al: An
inflammatory biomarker as a differential predictor of outcome of
depression treatment with escitalopram and nortriptyline. Am J
Psychiatry. 171:1278–1286. 2014. View Article : Google Scholar : PubMed/NCBI
|