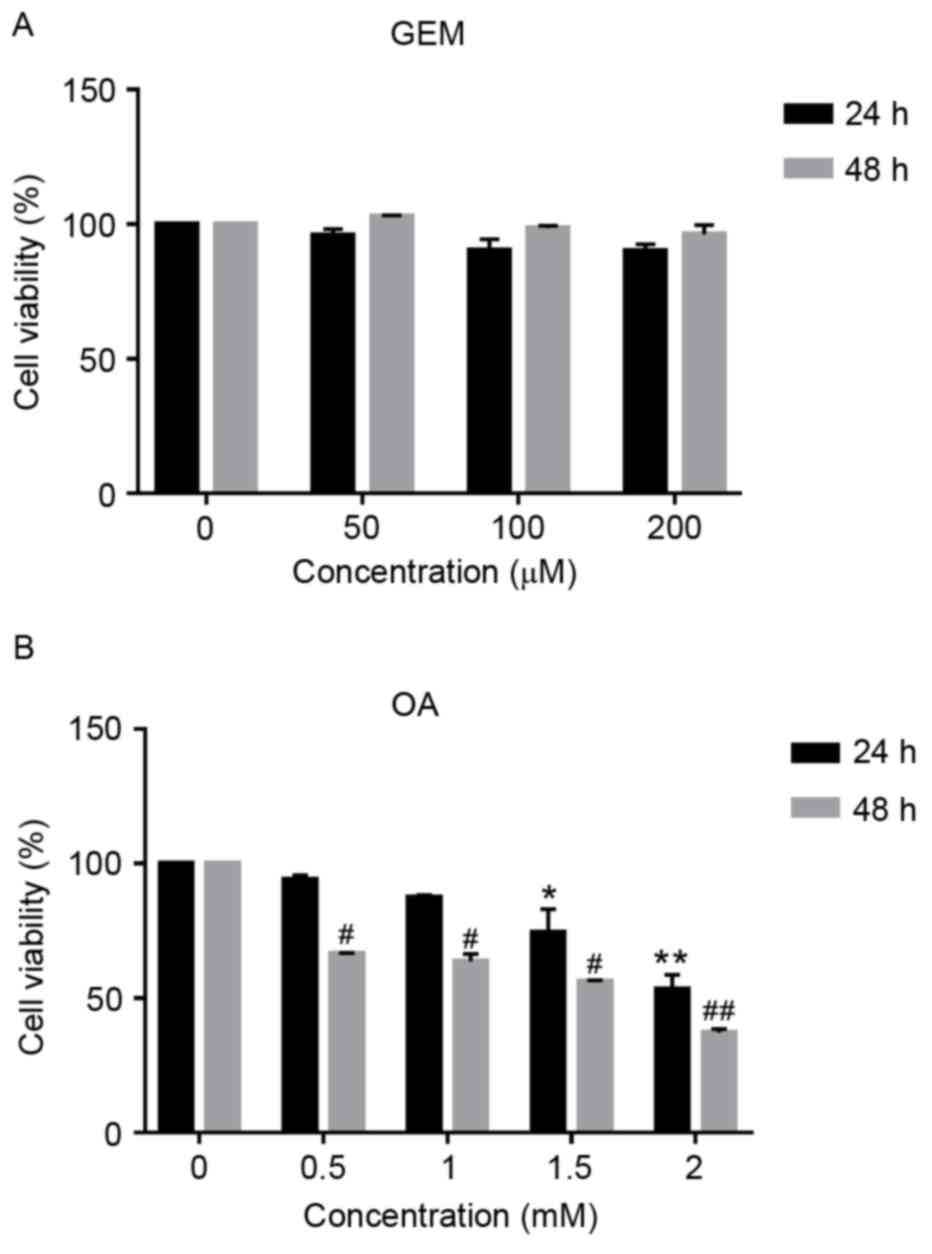
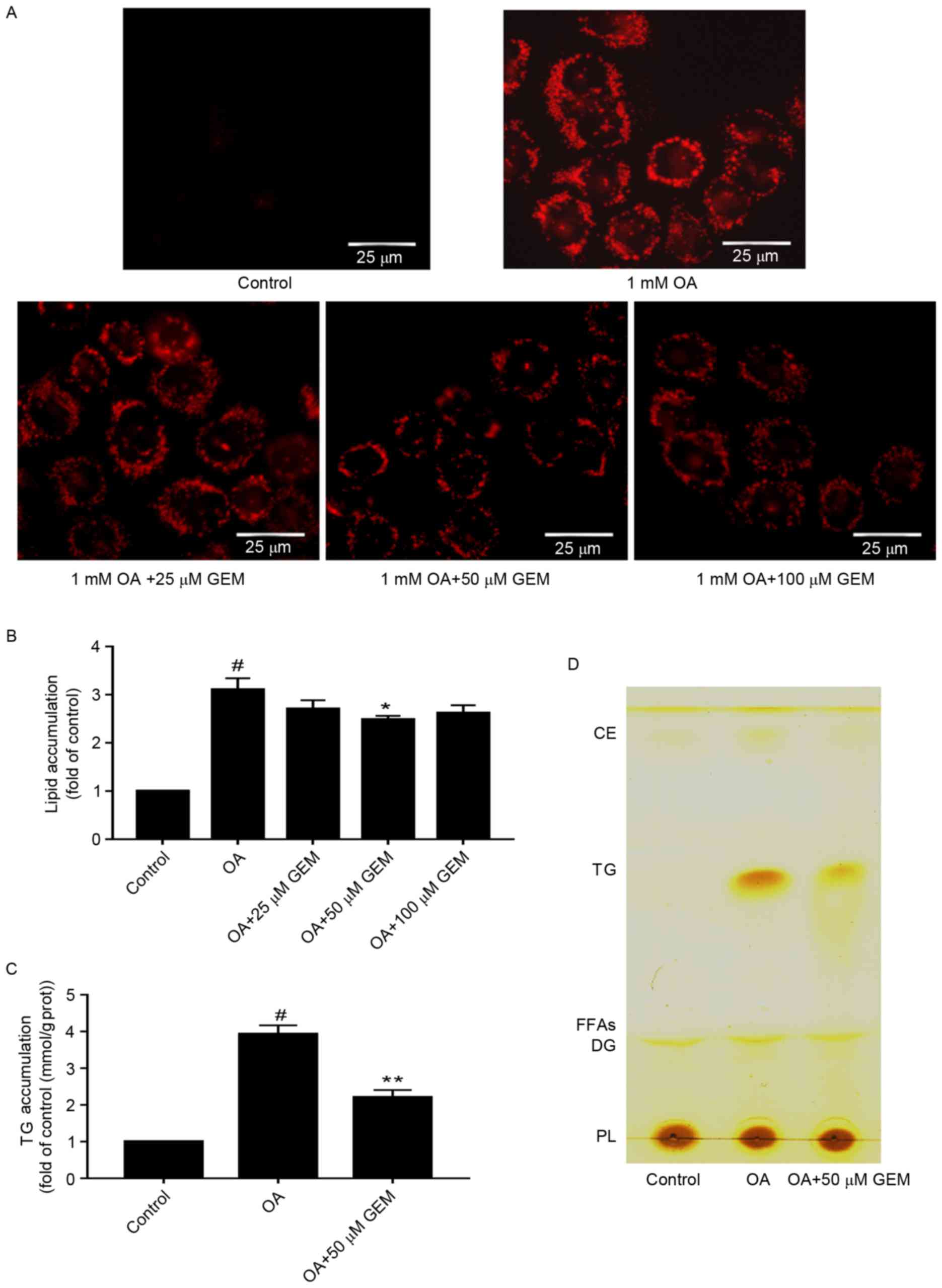
|
1
|
Fruchart JC and Duriez P: Mode of action
of fibrates in the regulation of triglyceride and HDL-cholesterol
metabolism. Drugs Today (Barc). 42:39–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandard S, Müller M and Kersten S:
Peroxisome proliferator-activated receptor alpha target genes. Cell
Mol Life Sci. 61:393–416. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinetti-Gbaguidi G, Fruchart JC and
Staels B: Pleiotropic effects of fibrates. Curr Atheroscler Rep.
7:396–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jun M, Foote C, Lv J, Neal B, Patel A,
Nicholls SJ, Grobbee DE, Cass A, Chalmers J and Perkovic V: Effects
of fibrates on cardiovascular outcomes: A systematic review and
meta-analysis. Lancet. 375:1875–1884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keech A, Simes RJ, Barter P, Best J, Scott
R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, et al:
Effects of long-term fenofibrate therapy on cardiovascular events
in 9,795 people with type 2 diabetes mellitus (the FIELD study):
Randomised controlled trial. Lancet. 366:1849–1861. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Design and validation of a histological
scoring system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunt EM: Pathology of nonalcoholic fatty
liver disease. Nat Rev Gastroenterol Hepatol. 7:195–203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ajmal MR, Yaccha M, Malik MA, Rabbani MU,
Ahmad I, Isalm N and Abdali N: Prevalence of nonalcoholic fatty
liver disease (NAFLD) in patients of cardiovascular diseases and
its association with hs-CRP and TNF-α. Indian Heart J. 66:574–579.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Narasimhan S, Gokulakrishnan K,
Sampathkumar R, Farooq S, Ravikumar R, Mohan V and Balasubramanyam
M: Oxidative stress is independently associated with non-alcoholic
fatty liver disease (NAFLD) in subjects with and without type 2
diabetes. Clin Biochem. 43:815–821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan XY, Zhang L, Fan JG and Qiao L: NAFLD
leads to liver cancer: Do we have sufficient evidence? Cancer Lett.
345:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shifflet A and Wu YG: Non-alcoholic
steatohepatitis: An overview. J Formos Med Assoc. 108:4–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musso G, Gambino R and Cassader M: Recent
insights into hepatic lipid metabolism in non-alcoholic fatty liver
disease (NAFLD). Prog Lipid Res. 48:1–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui W, Chen SL and Hu KQ: Quantification
and mechanisms of oleic acidinduced steatosis in HepG2 cells. Am J
Transl Res. 2:95–104. 2010.PubMed/NCBI
|
|
15
|
Wang S, Kuang X, Fang ZJ, Huang Z and Shi
P: Effect of oleic acid on the levels of eight metal ions in human
hepatoma SMMC-7721 cells. Biol Trace Elem Res. 159:445–450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramírez-Zacarías JL, Castro-Muñozledo F
and Kuri-Harcuch W: Quantitation of adipose conversion and
triglycerides by staining intracytoplasmic lipids with Oil red O.
Histochemistry. 6:493–497. 1992. View Article : Google Scholar
|
|
17
|
Bergman AC, Benjamin T, Alaiya A, Waltham
M, Sakaguchi K, Franzén B, Linder S, Bergman T, Auer G, Appella E,
et al: Identification of gel-separated tumor marker proteins by
mass spectrometry. Electrophoresis. 21:679–686. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradford MM: A rapid and sensitive method
for quantitation of microgram quantities of protein utilizing the
principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown MS and Goldstein JL: The SREBP
pathway: Regulation of cholesterol metabolism by proteolysis of a
membrane-bound transcription factor. Cell. 89:331–340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang H, McIntosh AL, Martin AG, Petrescu
AD, Landrock KK, Landrock D, Kier AB and Schroeder F: Inhibitors of
fatty acid synthesis induce PPAR α-regulated fatty acid β-oxidative
genes: Synergistic roles of L-FABP and glucose. PPAR Res.
2013:804–865. 2013. View Article : Google Scholar
|
|
22
|
Bishop-Bailey D: Peroxisome
proliferator-activated receptors in the cardiovascular system. Br J
Pharmacol. 129:823–834. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith WJ, Wang J, Dang AQ, Reeves C, Bibbs
D and Faas FH: Gemfibrozil lowers plasma lipids and increases
polyunsaturated fatty acid content and oxidative susceptibility of
lipoproteins in hypertriglyceridemia. Clin Chim Acta. 322:77–84.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uchida A, Slipchenko MN, Cheng JX and
Buhman KK: Fenofibrate, a peroxisome proliferator-activated
receptor α agonist, alters triglyceride metabolism in enterocytes
of mice. Biochim Biophys Acta. 1811:170–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peters JM, Rusyn I, Rose ML, Gonzalez FJ
and Thurman RG: Peroxisome proliferator-activated receptor alpha is
restricted to hepatic parenchymal cells, not Kupffer cells:
Implications for the mechanism of action of peroxisome
proliferators in hepatocarcinogenesis. Carcinogenesis. 21:823–826.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernández-Rojo MA, Restall C, Ferguson C,
Martel N, Martin S, Bosch M, Kassan A, Leong GM, Martin SD, McGee
SL, et al: Caveolin-1 orchestrates the balance between glucose and
lipid-dependent energy metabolism: Implications for liver
regeneration. Hepatology. 55:1574–1584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogata M, Tsujita M, Hossaina MA, Akita N,
Gonzalez FJ, Staels B, Suzuki S, Fukutomi T, Kimura G and Yokoyama
S: On the mechanism for PPAR agonists to enhance ABCA1 gene
expression. Atherosclerosis. 205:413–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mimeault C, Trudeau VL and Moon TW:
Waterborne gemfibrozil challenges the hepatic antioxidant defense
system and down-regulates peroxisome proliferator activated
receptor beta (PPARbeta) mRNA levels in male goldfish (Carassius
auratus). Toxicology. 228:140–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Passeri MJ, Cinaroglu A, Gao C and Sadler
KC: Hepatic steatosis in response to acute alcohol exposure in
zebrafish requires sterol regulatory element binding protein
activation. Hepatology. 49:443–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Tai Y, Zhou J, Gu W, Bai Z, Zhou T,
Zhong Z, McCue PA, Sang N, Ji JY, et al: Repression of endometrial
tumor growth by targeting SREBP1 and lipogenesis. Cell Cycle.
11:2348–2358. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stone SJ, Myers HM, Watkins SM, Brown BE,
Feingold KR, Elias PM and Farese RV Jr: Lipopenia and skin barrier
abnormalities in DGAT2-deficient mice. J Biol Chem.
279:11767–11776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sumida Y, Niki E, Naito Y and Yoshikawa:
Involvement of free radicals and oxidative stress in NAFLD/NASH.
Free Radical Res. 47:869–880. 2004. View Article : Google Scholar
|
|
33
|
Horton JD, Bashmakov Y, Shimomura I and
Shimano H: Regulation of sterol regulatory element binding proteins
in livers of fasted and refed mice. Proc Natl Acad Sci USA.
95:5987–5992. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kok BP, Dyck JR, Harris TE and Brindley
DN: Differential regulation of the expressions of the PGC-1α splice
variants, lipins, and PPARα in heart compared to liver. J Lipid
Res. 54:1662–1677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Péterfy M, Phan J, Xu P and Reue K:
Lipodystrophy in the fld mouse results from mutation of a new gene
encoding a nuclear protein. Lipin Nat Genet. 27:121–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yen CLE, Stone SJ, Koliwad S, Harris C and
Farese RV Jr: Thematic review series: Glycerolipids. DGAT enzymes
and triacylglycerol biosynthesis. J Lipid Res. 49:2283–2301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li C, Li L, Lian J, Watts R, Nelson R,
Goodwin B and Lehner R: Roles of Acyl-CoA: Diacylglycerol
acyltransferases 1 and 2 in triacylglycerol synthesis and secretion
in primary hepatocytes. Arterioscl Throm Vas. 35:1080–1091. 2015.
View Article : Google Scholar
|
|
38
|
Ibrahimi A and Abumrad NA: Role of CD36 in
membrane transport of long-chain fatty acids. Curr Opin Clin Nutr.
5:139–145. 2002. View Article : Google Scholar
|
|
39
|
Kennedy DJ, Kuchibhotla S, Westfall KM,
Silverstein RL, Morton RE and Febbraio M: A CD36-dependent pathway
enhances macrophage and adipose tissue inflammation and impairs
insulin signalling. Cardiovasc Res. 89:604–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ouwens DM, Diamant M, Fodor M, Habets DDJ,
Pelsers MMAL, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R,
Rietdijk A, et al: Cardiac contractile dysfunction in
insulin-resistant rats fed a high-fat diet is associated with
elevated CD36-mediated fatty acid uptake and esterification.
Diabetologia. 50:1938–1948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koonen DP, Jacobs RL, Febbraio M, Young
ME, Soltys CL, Ong H, Vance DE and Dyck JR: Increased hepatic CD36
expression contributes to dyslipidemia associated with diet-induced
obesity. Diabetes. 56:2863–2871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miquilena-Colina ME, Lima-Cabello E,
Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M,
Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B,
Aspichueta P, et al: Hepatic fatty acid translocase CD36
upregulation is associated with insulin resistance,
hyperinsulinaemia and increased steatosis in non-alcoholic
steatohepatitis and chronic hepatitis C. Gut. 60:1394–1402. 2011.
View Article : Google Scholar : PubMed/NCBI
|