Introduction
Ovarian cancer remains of the main causes of
cancer-associated mortality. Ovarian cancer is mainly treated by
chemotherapy and/or by surgical interventions (1,2).
Although, the initial responses to chemotherapy are encouraging,
the tumors often reoccur. Additionally, only limited anticancer
agents are available for the treatment of ovarian cancer (3,4).
Naturally occurring compounds have gained considerable attention
for the prevention of various types of cancer (5–7).
Flavonoids consist of a large group of polyphenolics having a
benzo-γ-pyrone skeleton and are widely distributed in the plant
kingdom (8) These are also
frequently found in fruits, grains, green tea and other dietary
supplements (9,10). Numerous biological activities have
been reported for flavonoids, including antioxidant, antitumor,
anti-inflammatory, antiallergenic and hepatoprotective activities
(11,12). It has been found that a flavonoid
rich diet reduces the risk of chronic diseases, particularly
cancer, including prostate and breast cancer, indicating their
potential role as anticancer agents (13,14).
Flavonoids, including flavopiridol, epigallocatechin gallate and
quercetin, have emerged as potent anticancer drug candidates and a
number of these have already entered clinical trials (15).
Apeginin-7-methyl ether is a naturally occurring
flavonoid known to possess several pharmacological properties,
including anti-inflammatory, antioxidant, and cytotoxic properties
(16). Among these, its anticancer
effect has been reported against various human cancer cells
(16). These findings suggest that
apigenin is an ideal bioactive scaffold for the synthesis of a
series of analogues and examination of their structure-activity
associations, and justify its further investigation. In this
context, the present study targeted apeginin-7-methyl ether to
synthesize 1,2,3-triazole analogs. Heterocyclic moieties, including
1,2,3-triazoles, are frequently occurring structural motifs in
various pharmaceuticals, and are reported to exhibit diverse
biological activities, including anti-human immunodeficiency virus
(17), antimicrobial (18) and anticancer effects (19,20). The
addition of such heterocyclic groups can influence the
effectiveness, polarity and aqueous solubility of the parent
compound (21,22). Furthermore, triazoles are stable
against acidic and basic hydrolysis, which is advantageous in
resisting metabolic degradation (23). These also have a high dipole moment
to facilitate hydrogen bond formation and dipole-dipole
interactions while interacting with membrane proteins (24,25).
In view of the preceding discussion, the present
study introduced a triazole moiety at the 4′-OH position of
apeginin-7-methyl ether through a linker to synthesize desired
derivatives using Hugen's 1,3 dipolar cyclo-addition approach. All
the synthesized triazolyl hybrids were evaluated against the SKOV3
ovarian cancer cell line. It was demonstrated that that these
analogs have the potential to induce apoptosis in human ovarian
cancer cell lines. The results facilitate the identification of a
lead compound capable of inhibiting colony formation in SKOV3 cells
and inducing apoptosis via loss of mitochondrial membrane potential
(MMP).
Materials and methods
Chemistry
In the present study, the reagents and solvents were
purchased from Sigma Aldrich; Merck Millipore (Darmstadt, Germany).
TLC (0.25 mm silica gel 60 F254; Merck Millipore) plates were used
to monitor the reaction progress. Compound purification was
performed by column chromatography using silica gel 60–120 mesh.
Bruker DPX 410 and DPX 540 NMR instruments were used to record
1H NMR and 13C NMR spectra, TMS as the
internal standard and CDCl3 as the solvent. The chemical
shifts are expressed in d ppm and coupling constants in Hertz.
Preparation of propargyl
apeginin-7-methylether (compound 2)
In a typical procedure, the apigenin-7-methyl ether
(1 mmol), propargyl bromide (1.1 mmol) and
K2CO3 (1.5 mmol) were added to a round-bottom
flask containing methanol (10 ml). The reaction mixture was then
vigorously stirred on a magnetic stirrer at 80°C until the initial
material had completely disappeared, which was monitored by TLC.
Following completion, the reaction mixture was partitioned with
EtOAc and water three times. The collected organic layers were
concentrated in a vacuum and purified by column chromatography
using silica gel (60–120 mesh) and EtOAc: hexane as eluting
solvents to produce compound 2 at 98% yield.
General procedure for the synthesis of
triazolyl derivatives (3a-g)
Compound 3 (1 eq) and respective organic azides (1.1
eq) were added to a round bottom flask containing 15 ml of 1:1
water: ethanol mixture, to which 10 mol% each of sodium ascorbate
and CuSO4.5H2O was added. The reaction
mixture was stirred on a magnetic stirrer at room temperature until
its completion. The crude reaction mixture was then partitioned
using aqueous ethylacetate. The collected ethylacetate layer was
dried over anhydrous magnesium sulphate and subjected to column
chromatography using EtOAc: hexane as eluting solvents to produce
the pure desired products (3a-g) in quantitative yields.
5-hydroxy-2-(4-((1-(2-(hydroxymethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-7-methoxy-4H-chromen-4-one
(3a)
The details of product 3a were as follows: White
crystalline solid, yield: 93%; 1H NMR (400 MHz, CDCl3) δ 7.55 (d,
J=6.3 Hz, 1H), 7.49 (d, J=7.5 Hz, 2H), 7.37 (s, 1H), 7.23–7.17 (m,
3H), 7.01 (d, J=7.4 Hz, 2H), 6.33 (s, 1H), 6.19 (d, J=1.5 Hz, 1H),
6.13 (d, J=1.4 Hz, 1H), 5.16 (s, 2H), 4.76 (s, 2H), 3.81 (s, 3H).
13C NMR (125 MHz,) δ 182.28, 166.02, 163.74, 161.60, 159.47,
140.09, 137.73, 136.17, 132.36, 129.19, 127.52, 127.24, 126.97,
125.85, 125.31, 115.31, 105.68, 104.77, 98.01, 93.64, 63.36, 57.74,
56.03.
5-hydroxy-2-(4-((1-(4-hydroxybenzyl)-1H-1,2,3-triazol-4-yl)
methoxy)phenyl)-7-methoxy-4H-chromen-4-one (3b)
The details of product 3b were as follows: White
solid, yield 89%; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J=7.5 Hz, 2H),
7.42–7.31 (m, 3H), 7.02 (d, J=7.6 Hz, 2H), 6.82 (d, J=7.5 Hz, 2H),
6.30 (s, 1H), 6.21 (d, J=1.4 Hz, 1H), 6.13 (d, J=1.4 Hz, 1H), 5.23
(s, 1H), 3.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 182.28, 166.02,
163.74, 161.60, 161.27, 159.47, 136.00, 133.10, 128.4, 128.06,
127.24, 125.3, 117.03, 115.3, 105.68, 104.77, 98.01, 93.64, 57.74,
56.03.
5-hydroxy-7-methoxy-2-(4-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-4H-chromen-4-one
(3c)
The details of product 3c were as follows: White
solid, yield: 89%; 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J=7.5 Hz,
2H), 7.41–7.31 (m, 3H), 7.02 (d, J=7.5 Hz, 2H), 6.81 (d, J=7.5 Hz,
2H), 6.27 (s, 1H), 6.19 (d, J=1.4 Hz, 1H), 6.09 (d, J=1.4 Hz, 1H),
5.27 (s, 1H), 3.81 (s, 3H), 3.80 (s, 3H).
2-(4-((1-(2-fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-5-hydroxy-7-methoxy-4H-chromen-4-one
(3d)
The details of product 3d were as follows: White
solid, yield: 93%; 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J=7.5 Hz,
2H), 7.48 (m, 1H), 7.39 (s, 1H), 7.15 (m, 1H), 7.10 (m, 1H),
7.07–6.97 (m, 3H), 6.52 (s, 2H), 6.26 (d, J=1.4 Hz, 1H), 6.11 (d,
J=1.6 Hz, 1H), 5.18 (s, 2H), 3.81 (s, 3H).
3-((4-((4-(5-hydroxy-7-methoxy-4-oxo-4H-chromen-2-yl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzonitrile
(3e)
The details of product 3e were as follows: Yellowish
solid, yield: 93%; 1H NMR (400 MHz, CDCl3) δ 7.98 (m, 1H), 7.92 (d,
J=1.5 Hz, 1H), 7.51 (d, J=7.5 Hz, 2H), 7.42–7.32 (m, 3H), 7.03 (d,
J=7.5 Hz, 2H), 6.32 (s, 1H), 6.21 (d, J=1.4 Hz, 1H), 6.14 (d, J=1.4
Hz, 1H), 5.22 (s, 2H), 3.79 (s, 3H).
2-(4-((1-butyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-5-hydroxy-7-methoxy-4H-chromen-4-one
(3f)
The details of product 3f were as follows: Yellow
amorphous powder, yield: 93%; 1H NMR (400 MHz, CDCl3) δ 7.52 (d,
J=7.5 Hz, 2H), 7.11 (s, 1H), 7.03 (d, J=7.5 Hz, 2H), 6.30 (s, 1H),
6.25 (d, J=1.4 Hz, 1H), 6.21 (d, J=1.4 Hz, 1H), 5.22 (s, 2H), 3.82
(s, 3H), 2.93 (m, 2H), 1.26–1.37 (m, 4H), 0.91 (t, 3H, J=7.2
Hz).
2-(4-((1-pentyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-5-hydroxy-7-methoxy-4H-chromen-4-one
(3g)
The details of product 3g were as follows: Yellow
amorphous powder, yield: 93%; 1H NMR (400 MHz,
CDCl3) δ 7.50 (d, J=7.4 Hz, 2H), 7.09 (s, 1H), 7.03 (d,
J=7.4 Hz, 2H), 6.31 (s, 1H), 6.27 (d, J=1.4 Hz, 1H), 6.22 (d, J=1.4
Hz, 1H), 5.22 (s, 2H), 3.82 (s, 3H), 2.95 (m, 2H), 1.29–1.38 (m,
6H), 0.92 (t, 3H, J=7.2 Hz).
Antiproliferative assay
The antiproliferation effect of the novel triazole
analogs of apigenin-7-methyl ether against three human ovarian
cancer cell lines, OVCAR-3, Caov-3, and SKOV3, (Type Culture
Collection of Chinese Academy of Sciences, Shanghai, China) was
investigated with a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells were cultured at the density of 1×106
cells/well in 96-well plates for a time period of 12 h at 37°C. The
cells were then subsequently treated with 0–200 µM doses of the
apigenin-7-methyl ether derivatives for 24 h at 37°C. Following
this, 20 µl of MTT solution was added to each well. Prior to the
addition of 500 µl of DMSO, the medium was completely removed. For
solubilizing the MTT formazan crystals, dimethylsulfoxide (500 µl)
was added. The absorbance at 570 nm was measured using an ELISA
plate reader. As the derivative 3d was found to be most active,
only this molecule was used for further experimentation.
Colony formation assay
To investigate the effect of the 3d derivative on
the colony formation potential of SKOV3 cells, the cells were
collected at the exponential growth phase and then counted using a
hemocytometer. The platting of the cells was performed at 200
cells/well, and the plates were then incubated at 37°C for 48 h to
permit the cells to adhere. This was followed by the addition of
various concentrations (0, 5, 10, and 20 µM) of 3d. Following
treatment with 3d, the cells plates were incubated for 6 days at
37°C. Following 6 days of incubation, the cells were washed with
PBS and fixed with methanol. Subsequently, the cells were treated
with crystal violet for 30 min at room temperature and then counted
under a light microscope (magnification, ×200).
Detection of apoptosis
The SKOV3 cells were seeded at the density of
1×106 cells/well in 6-well plates and then treated with
0, 10, 20 and 40 µM 3d for the time period of 24 h at 37°C. This
was immediately followed by 4′,6-diamidino-2-phenylindole (DAPI)
staining at 25°C for 5 min. The cell samples were then examined and
images were captured with a fluorescence microscope (magnification,
×200). To estimate the apoptotic cell populations, the SKOV3 cells
were seeded at a density of 1×106 cells/well in 6-well
plates and treated with varied concentrations (0, 5, 10, and 20 µM)
of 3d for 24 h at 37°C. The cells were then collected and washed
with PBS. The cells were then incubated with Annexin V/FITC and PI
for 15 min and the apoptotic cell populations were estimated by
flow cytometry (BD Biosciences, San Jose, CA, USA). The estimated
percentage of cells in each phase of the cell cycle was quantified
using WinMDI software v2.0 (Informer Technologies, Inc., Los
Angeles, CA, USA).
Determination of ROS and MMP
The SKOV3 cells were seeded at a density of
2×105 cells/well in a 6-well plate and incubated at 37°C
for 24 h and treated with 0, 5, 10, and 20 µM of 3d for 24 h at
37°C in 5% CO2 and 95% air. Subsequently, the cells from
all samples were collected, washed twice PBS and resuspended in 500
µl of DCFH-DA (10 µM) for ROS estimation and DiOC6 (1
µmol/l) for MMP at 37°C in a dark room for 30 min. The samples were
then examined immediately using a flow cytometer and BD FACSuite
software v1.0 (BD Biosciences, San Jose, CA, USA).
Western blot analysis
Protein expression was determined by western blot
analysis. Briefly, the cells were lysed in a lysis buffer (20 mM
HEPES, 350 mM NaCl, 20% glycerol, 1% Nonidet P 40, 1 mM
MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2
mM protease inhibitor cocktail and 10% phosphatase inhibitor
cocktail). The proteins present in the cell extracts were
quantified using a BCA assay and proteins (50 µg/lane) from each
sample were resolved by SDS-PAGE on a 10% gel. This was followed by
transference onto a nitrocellulose membrane. The membrane was then
treated with non-fat milk (5%) in PBS, and then incubated with a
suitable primary antibody: B-cell lymphoma 2-associated X protein
(Bax; cat. no. sc-6236) and Bcl-2 (cat no. sc-509) purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) overnight at 4°C
(dilution 1:1,000), followed by incubation with horseradish
peroxidase-conjugated (cat. no. 9003-99-0) and anti-rabbit
secondary antibody (cat. no. sc-2372) (dilution 1:1,000) for 1 h at
room temperature. The western blots were then observed in an ECL
western blot analysis system (GE Healthcare Life Sciences,
Chalfont, UK).
Statistical analysis
The experiments were repeated three times and
results are presented as the mean ± standard deviation. The
significance was determined, compared with the untreated control,
using one way analysis of variance and Tukey's test with GraphPad
prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.01 was considered to indicate a statistically significant
difference.
Results
Synthesis of novel triazole analogs of
apigenin-7-methyl ether
In the present study, apeginin-7-methylether
(compound 1) was isolated from the ethanolic extract of leaves of
Aquilaria sinensis. The isolated natural product (compound
1) was subjected to propargylation using propargyl bromide in
presence of base K2CO3 to give compound 2.
Compound 2 was then reacted with different substituted organic
azides under click chemistry conditions (Fig. 1) to produce desired 1,2,3-triazole
products in quantitative yields. In the 1H NMR, products
were easily identified by a characteristic singlet for H-5 in the
1,2,3-triazole moiety, which appeared as singlet downfield (~7.5
ppm) with other aromatic protons. All the prepared triazolyl
analogs were characterized by 1H NMR, 13CNMR
and MS spectroscopic analysis.
Anticancer effects of synthesized
derivatives on ovarian cancer cell lines
To investigate the antiproliferative role of
synthesized compounds on three human ovarian cancer cell lines
(OVCAR-3, Caov-3, and SKOV3), the cells were treated with different
concentrations of the synthesized compounds and the IC50
was determined for all compounds (Table
I). Compound 3d exhibited a potent antiproliferative effect
against SKOV3 cells in a dose-dependent manner with an
IC50 of 10 µM (Fig. 2).
In the formazan crystal assay, it was revealed that administering
3d to cells reduced the number of formazan crystals in a
concentration-dependent manner (Fig.
3). As 3d exhibited highest activity against the SKOV3 cells,
this cell line was used for further experimentation.
 | Table I.IC50 values of novel
triazole analogs of apigenin-7-methyl against ovarian cancer cell
lines, determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assay. |
Table I.
IC50 values of novel
triazole analogs of apigenin-7-methyl against ovarian cancer cell
lines, determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assay.
| Derivative | SKOV3 (µM) | OVCAR-3 (µM) | Caov-3 (µM) |
|---|
| 1 | 29 | 30 | 30 |
| 2 | 20 | 25 | 20 |
| 3a | 18 | 20 | 20 |
| 3b | 17 | 20 | 20 |
| 3c | 25 | 30 | 25 |
| 3d | 10 | 15 | 20 |
| 3e | 40 | 40 | 40 |
| 3f | 40 | 30 | 25 |
| 3g | 40 | 40 | 30 |
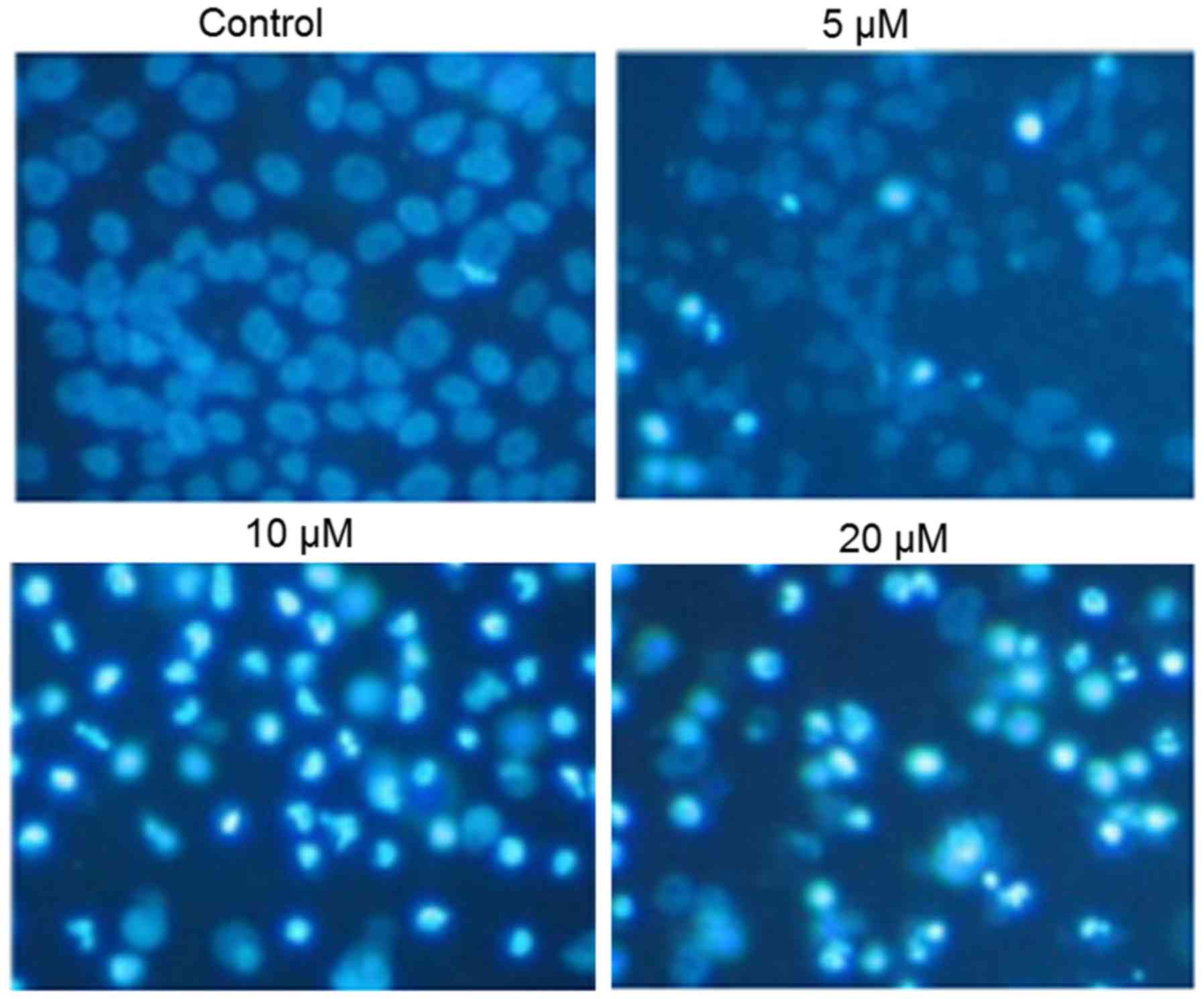
Compound 3d induces apoptosis in SKOV3
ovarian cancer cells
Following treatment with the different
concentrations of compound 3d, apoptosis was detected by DAPI
staining. The results indicated that compound 3d caused apoptosis
in a concentration-dependent manner, as evident from the increased
density of white-colored nuclei (Fig.
4). The apoptotic cell populations were further estimated by
annexin V/PI staining and it was observed that the apoptotic cell
populations increased from 0.05% in the control to 39.62% at 20 µM
concentrations of 3d (Fig. 5). In
addition, this was associated with the increase in the expression
of Bax and a decrease in the expression of Bcl-2 (Fig. 6).
Compound 3d triggers ROS activation in
SKOV3 ovarian cancer cells
The potential of 3d to induce apoptosis, as observed
through DAPI staining, indicated that 3d may trigger the production
of intracellular ROS. Therefore, the present study estimated the
ROS level at different concentrations of 3d for 48 h. The results
showed that the intracellular ROS levels of the treated cells
increased up to 255%, compared with the untreated cells (Fig. 7). This result suggested that compound
3d is an effective molecule for stimulating the generation of ROS
in SKOV3 cells.
Compound 3d reduces MMP
The generation of ROS causes mitochondrial
mutilation and disrupts the outer mitochondrial potential,
ultimately leading to the discharge of death-promoting proteins
(16). Therefore, the present study
investigated whether compound 3d decreased the MMP in the SKOV3
cells administrated with various doses (0–20 µM). The compound
3d-administrated SKOV3 cells showed a considerable decrease in MMP
in a dose-dependent manner. The MMP decreased up to 63% at 20 µM of
compound 3d, compared with that in the untreated control (Fig. 8).
Discussion
Of types of gynecological cancer, ovarian cancer is
one of the main causes of cancer-associated mortality around the
world. Despite preliminary responses to chemotherapy, the tumors
consistently relapse (1,2). Apeginin-7-methyl ether is a naturally
occurring flavonoid reported to possess several biological
activities, including antioxidant, anti-inflammatory and antitumor
activities (15). Among these, its
anticancer effect has been reported against various human cancer
cells. These findings suggest that apigenin is an ideal bioactive
scaffold for the synthesis of a series of analogues and examination
of their structure-activity associations, and justifies its further
investigation. In this context, the present study targeted
apeginin-7-methyl ether to synthesize 1,2,3-triazole analogs. All
derivatives exhibited potential growth inhibitory effects on the
three ovarian cancer cell lines, as evident from the proliferation
assay, however 3d exhibited the most potent activity against the
SKOV3 cancer cell line. As it has been shown previously, several
anticancer drugs trigger antiproliferative effects through the
induction of apoptosis (26,27). For example, the anticancer drugs
cisplatin, taxol and 5-fluorouracil (28–34) have
been shown to activate apoptotic pathways and cause DNA damage
(35). To assess whether compound 3d
triggers apoptosis in SKOV3 cells, the treated cells were subjected
to DAPI staining. The results revealed that compound 3d induced
apoptotic damage in a concentration-dependent manner. In addition
to this, it was observed that the compound 3d-treated cells showed
that ROS promoted a reduction in MMP (33). Therefore, these results indicated
that compound 3d may trigger apoptosis by the accretion of
intracellular ROS and lessening of MMP. These results are well
supported by earlier studies wherein a number of anticancer drugs
have been shown to cause cancer cell death partly by the generation
of high levels of ROS (35). In
addition, the role of mitochondria in ROS is key (36). For example, capsaicin disrupts MMP
and modulates oxidative stress, resulting in apoptosis of
pancreatic cancer cells (37).
Therefore, the inhibitory effect of compound 3d on ovarian cancer
cells may prove beneficial in the treatment and management of
ovarian cancer.
In conclusion, a small series of
apeginin-7-methylether derived 1,2,3-triazole hybrids were
synthesized using an alkyne azide cyclo-addition reaction. All the
prepared triazolyl analogs were evaluated against the SKOV3 human
ovarian cancer cell line, however, the biological data revealed
that compound 3d exhibited the lowest IC50 and exerted
its anticancer activity through the induction of apoptosis through
ROS-mediated alterations in MMP. The present study confirmed that
potential anticancer agents can be synthesized from flavonoids.
Acknowledgements
Not applicable.
Funding
The current study was supported by The Affiliated
Hospital of Taishan Medical University (Taishan, China; grant no.
TMU-126/2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ, ZD, YY and DM performed all the experiments. FR,
HY and AC collected the materials and provided instrumental
suggestion for the present study. AC designed the study.
Ethics approval and consent to
participate
Not applicable.
Petient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Leary A, Auclin E, Pautier P and Lhommé C:
The PI3K/Akt/mTOR pathway in ovarian cancer: Biological rationale
and therapeutic opportunities. Ovarian Cancer-A Clinical and
Translational Update. 275–302. 2013.
|
|
2
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Can.
9:550–562. 2009. View
Article : Google Scholar
|
|
5
|
Hafeez BB, Siddiqui IA, Asim M, Malik A,
Afaq F, Adhami VM, Saleem M, Din M and Mukhtar H: A dietary
anthocyanidin delphinidin induces apoptosis of human prostate
cancer PC3 cells in vitro and in vivo: Involvement of nuclear
factor-kappa B signaling. Cancer Res. 68:8564–8572. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hafeez BB, Fischer JW, Singh A, Zhong W,
Mustafa A, Meske L, Sheikhani MO and Verma AK: Plumbagin inhibits
prostate carcinogenesis in intact and castrated PTEN knockout mice
via targeting PKCε, Stat3, and epithelial-to-mesenchymal transition
markers. Cancer Prev Res (Phila). 8:375–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lall RK, Adhami VM and Mukhtar H: Dietary
flavonoid fisetin for cancer prevention and treatment. Mol Nutr
Food Res. 60:1396–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gülçin I: Antioxidant activity of caffeic
acid (3,4-dihydroxycinnamic acid). Toxicology. 217:213–220. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cook NC and Samman S:
Flavonoids-chemistry, metabolism, cardioprotective effects and
dietary sources. J NutBiochem. 7:66–76. 1996.
|
|
10
|
Rice-Evans CA, Miller NJ, Bolwell PG,
Bramley PM and Pridham JB: The relative antioxidant activities of
plant derived polyphenolic flavonoids. Free Radic Res. 22:375–383.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke
FC, Huang YT and Lee MT: The antitumor activities of flavonoids. In
vivo. 19:895–909. 2005.PubMed/NCBI
|
|
12
|
Ren W, Qiao Z, Wang H, Zhu L and Zhang L:
Flavonoids: promising anticancer agents. Med Res Rev. 23:519–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ravindranath MH, Muthugounder S, Presser N
and Viswanathan S: Anticancer therapeutic potential of soy
isoflavone, genistein. Adv Exp Med Biol. 546:121–165. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HK: The therapeutic potential of
flavonoids. Expert Opin Investig Drugs. 9:2103–2119. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasr Bouzaiene N, Chaabane F, Sassi A,
Chekir-Ghedira L and Ghedira K: Effect of apigenin-7-glucoside,
genkwanin and naringenin on tyrosinase activity and melanin
synthesis in B16F10 melanoma cells. Life Sci. 144:80–85. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Androutsopoulos VP, Ruparelia K, Arroo RR,
Tsatsakis AM and Spandidos DA: CYP1-mediated antiproliferative
activity of dietary flavonoids in MDA-MB-468 breast cancer cells,
Toxicology. 264:162–170. 2009.PubMed/NCBI
|
|
17
|
Alvarez R, Velázquez S, San-Félix A,
Aquaro S, De Clercq E, Perno CF, Karlsson A, Balzarini J and
Camarasa MJ:
1,2,3-Triazole-[2′,5′-bis-O-(tert-butyldimethylsilyl)-beta-D-ribofuranosyl]-3′-spiro-5″-(4″-amino-1″,2″-oxathiole
2″,2″-dioxide) (TSAO) analogues: synthesis and anti-HIV-1 activity.
J Med Chem. 37:4185–4194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Genin MJ, Allwine DA, Anderson DJ,
Barbachyn MR, Emmert DE, Garmon SA, Graber DR, Grega KC, Hester JB,
Hutchinson DK, et al: Substituent effects on the antibacterial
activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones
with expanded activity against the fastidious gram-negative
organisms Haemophilus influenzae and Moraxella catarrhalis. J Med
Chem. 43:953–970. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majeed R, Sangwan PL, Chinthakindi PK,
Khan I, Dangroo NA, Thota N, Hamid A, Sharma PR, Saxena AK and Koul
S: Synthesis of 3-O-propargylated betulinic acid and its
1,2,3-triazoles as potential apoptotic agents. Eur J Med Chem.
63:782–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mack DJ, Weinrich ML, Vitaku E and
Njarðarson JT: Top 200 Brand Name Drugs by US Retail Sales in 2010.
J Chem Ed. 87:13482010.
|
|
21
|
Waring MJ: Lipophilicity in drug
discovery. Expert Opin Drug Discov. 5:235–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lipinski CA, Lombardo F, Dominy BW and
Feeney PJ: Experimental and computational approaches to estimate
solubility and permeability in drug discovery and development
settings. Adv Drug Deliv Rev. 46:3–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferreira SB, Sodero AC, Cardoso MF, Lima
ES, Kaiser CR, Silva FP and Ferreira VF: Synthesis, biological
activity, and molecular modeling studies of 1H-1,2,3-triazole
derivatives of carbohydrates as alpha-glucosidases inhibitors. J
Med Chem. 53:2364–2375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Whiting M, Muldoon J, Lin YC, Silverman
SM, Lindstrom W, Olson AJ, Kolb HC, Finn MG, Sharpless KB, Elder JH
and Fokin VV: Inhibitors of HIV-1 protease by using in situ click
chemistry. Angew Chem Int Ed Engl. 45:1435–1439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lauria A, Delisi R, Mingoia F, Terenzi A,
Martorana A, Barone G and Almerico AM: 1,2,3-Triazole in
heterocyclic compounds, endowed with biological activity, through
1,3-dipolar cycloadditions. Eur J Org Chem. 16:3289–3306. 2014.
View Article : Google Scholar
|
|
26
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiang JH, Yang JS, Ma CY, Yang MD, Huang
HY, Hsia TC, Kuo HM, Wu PP, Lee TH and Chung JG: Danthron, an
anthraquinone derivative, induces DNA damage and caspase
cascades-mediated apoptosis in SNU-1 human gastric cancer cells
through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maitra R, Porter MA, Huang S and Gilmour
BP: Inhibition of NFkappaB by the natural product Withaferin A in
cellular models of Cystic Fibrosis inflammation. J Inflamm (Lond).
6:152009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hissin PJ and Hilf R: A fluorometric
method for determination of oxidized and reduced glutathione in
tissues. Anal Biochem. 74:214–226. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chipuk JE, Bouchier-Hayes L and Green DR:
Mitochondrial outer membrane permeabilization during apoptosis: The
innocent bystander scenario. Cell Death Differ. 13:1396–1402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azuma M, Tamatani T, Ashida Y, Takashima
R, Harada K and Sato M: Cisplatin induces apoptosis in oral
squamous carcinoma cells by the mitochondria-mediated but not the
NF-kappaB-suppressed pathway. Oral Oncol. 39:282–289. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoneda K, Yamamoto T and Osaki T: p53- and
p21-independent apoptosis of squamous cell carcinoma cells induced
by 5-fluorouracil and radiation. Oral Oncol. 34:529–537. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abal M, Andreu JM and Barasoain I:
Taxanes: Microtubule and centrosome targets, and cell cycle
dependent mechanisms of action. Curr Canc Drug Targs. 3:193–203.
2003. View Article : Google Scholar
|
|
34
|
Ferreira CG, Epping M, Kruyt FA and
Giaccone G: Apoptosis: Target of cancer therapy. Clin Cancer Res.
8:2024–2034. 2002.PubMed/NCBI
|
|
35
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Met Rev. 23:367–387. 2004.
View Article : Google Scholar
|
|
36
|
Ding H, Han C, Guo D, Chin YW, Ding Y,
Kinghorn AD and D'Ambrosio SM: Selective induction of apoptosis of
human oral cancer cell lines by avocado extracts via a ROS-mediated
mechanism. Nutr Cancer. 61:348–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kowaltowski AJ, de Souza-Pinto NC,
Castilho RF and Vercesi AE: Mitochondria and reactive oxygen
species. Free Radic Biol Med. 47:333–343. 2009. View Article : Google Scholar : PubMed/NCBI
|