Introduction
Hepatocellular carcinoma (HCC) is one of the common
malignant tumors worldwide (1).
Globally, there are more than 500,000 new cases each year and about
1 million HCC-associated cases of mortality (2–6).
Approximately 40–50% of global HCC cases occur in China and HCC is
the second most malignant tumor in China (7–10).
Although there are a number of methods of treatment for HCC, they
are ineffective for achieving sustained remission (11). Invasion, metastasis and postoperative
recurrence are the primary causes leading to the mortality of
patients with HCC (12). The
processes associated with invasion and metastasis of HCC are
complex and involve multiple molecular interactions and
multiple-level cross regulation of signal transduction pathways
(13,14). Therefore, research on the mechanisms
of invasion and metastasis of HCC is important to increase the
clinical curative effects and improve the survival rate of
patients.
Neuroepithelial cell transforming 1 (NET-1), a
member of Ras homolog gene family, was identified in 2000 by Serru
et al (15) and reported to
serve a role in signaling pathways, including ERK1/2 and PI3K/Akt1,
which may be regulated by NET-1 as well as cell adhesion,
proliferation and differentiation (16,17). A
study also demonstrated that the inhibition of NET-1 could suppress
the activation of ERK1/2 and PI3K/Akt1 signaling (18). Previous studies also indicated that
the abnormal expression of NET-1 is associated with numerous types
of cancer, including lung, colorectal, gastric and breast cancer
(19,20). Shen et al (21) reported that NET-1 mRNA is expressed
at very low levels in normal liver tissues and highly expressed in
HCC tissues, suggesting that this protein may serve as a biomarker
in the early diagnosis of liver cancer. Expression of NET-1 is
closely associated with the lymphatic and distant metastasis in
non-small cell lung cancer (22).
One study revealed that inhibition of NET-1 in HCC was associated
with the tumor node metastasis stage (23). Therefore, the authors of the present
study hypothesized that NET-1 may serve an important role in
HCC.
The present study aimed to determine the association
between the expression of NET-1 and HCC. The mRNA expression levels
of NET-1 in HCC cell lines and a normal liver cell line were
compared and the cell line with the highest expression level of
NET-1 was selected. The selected cells were transfected with NET-1
small interfering (si)RNA and si negative control (NC), and the
proliferation rate and apoptosis of cells were determined. The
expression of apoptosis-associated proteins was also determined to
elucidate the molecular mechanism of NET-1 in HCC.
Materials and methods
Cell culture
Human HCC cell lines MHCC97-L and MHCC97-H, and a
normal liver cell line L-02 were obtained from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cell lines stored in −80°C liquid nitrogen was
recovered, inoculated, cultured and digested to obtain single cell
suspension. Cells were routinely cultured in RPMI-1640 supplemented
with 10% heat-inactivated fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified cell incubator with an
atmosphere of 5% CO2 at 37°C.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cell lines using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, 1 ml of TRIzol was added and each
sample was homogenized at 4°C for 10 min. Subsequently, the lysates
were transferred into 1.5 ml Eppendorf (EP) tubes (Eppendorf,
Hamburg, Germany). Following shaking for 15 min, the EP tubes were
centrifuged at 12,000 × g and 4°C for 15 min. The supernatant was
transferred into new EP tubes and mixed with isopycnic isopropanol
for 15 sec. Subsequently, the mixture was centrifuged at 12,000 × g
and 4°C for 10 min, and the supernatant was discarded. The
precipitate was washed with 75% ethanol twice and dried. Then, the
dried precipitate was dissolved in 30 µl DEPC-treated (0.1%) water
(Thermo Fisher Scientific, Inc.) and quantified by a NanoDrop 1000
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, Delaware, USA) and the RNA solution was stored at −80°C
for further use. Genes were amplified using specific
oligonucleotide primers for NET-1 and GAPDH, which was used as the
internal control. The forward and reverse primers are listed in
Table I. The first strand of cDNA
was synthesized by RevertAid First strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) at 42°C for 10 min.
SYBR® Green Real-Time PCR Master mixes (Takara Bio,
Inc., Otsu, Japan) and a LightCycler® 480 System (Roche
Diagnostics, Basel, Switzerland) were utilized to perform a qPCR
analysis. The following thermocycling conditions were used for the
PCR: 55°C for 30 min, initial denaturation for 15 min at 95°C; 40
cycles of 94°C for 15 sec, 55°C for 30 sec, 72°C for 30 sec. The
expression level was normalized using GAPDH small nuclear RNA and
expression levels were quantified using the
2−ΔΔCq method (23).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
|
| Primer sequences
(5′-3′) |
|---|
|
|
|
|---|
| Gene name | Forward | Reverse |
|---|
| Neuroepithelial
cell transforming 1 |
GAGCCAAGCAATAAAAGAGTTCG |
TGGGACTGTTGACCTGCTAGA |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Western blotting
Cells were seeded into a six-well plate at a density
of 5×105 cells/well. A total of 24 h after seeding, the
medium was discarded and cells were rinsed 3 times with ice-cold
PBS. Subsequently, cells were lysed with radioimmunoprecipitation
assay buffer at 4°C for 15 min and centrifuged at 12,000 × g at 4°C
for 10 min. The precipitation was discarded and the protein extract
in the supernatant was quantified by a BCA kit (Thermo Fisher
Scientific, Inc.). The supernatants were collected and boiled at
95°C with an equal volume of loading buffer for 10 min.
Subsequently, a total of 12 µg of protein was loaded into 4% spacer
and 12% separation gel for SDS-PAGE, and transferred to
polyvinylidene difluoride membranes (Hybond, Inc., Escondido, CA,
USA). The membranes were blocked with 5% skimmed milk dissolved in
Tris-buffered saline Tween-20 (TBST) for 1 h at room temperature.
Subsequently, the membranes were rinsed with TBST twice and
incubated with primary antibodies, including NET-1 (cat. no.
ab5914), Bax (cat. no. ab32503), Cyclin D1 (cat. no. ab134175),
Bcl-2 (cat. no. ab32124), Caspase-3 (cat. no. ab13585), PI3K (cat.
no. ab86714), p-PI3K (cat. no. ab182651), AKT (cat. no. ab8805),
p-AKT (cat. no. ab81283) and GAPDH (cat. no. ab9485; all 1:1,000;
Abcam, Cambridge, MA, USA) dissolved in 5% bovine serum albumin
(Abcam) at room temperature for 1 h. Membranes were then incubated
with the horseradish peroxidase-conjugated secondary antibodies
(cat. no. ab6721; 1:10,000, Abcam) at room temperature for 1 h.
Protein bands were visualized using the EZ-ECL Chemiluminescence
Detection kit for horseradish peroxidase (Biological Industries,
Kibbutz Beit Haemek, Israel).
Cell transfection
A total of 1×103−1×104
cells/well were seeded in 96-well plates. NET-1 overexpression or
control vector plasmids (0.2 µg; both Genentech USA, Inc., South
San Francisco, CA, USA) were transfected into cells using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The NET-1 siRNA or scramble control siNC
(10 pmol) was synthesized and modified chemically by Invitrogen
(Thermo Fisher Scientific, Inc.) using Lipofectamine™ RNAiMAX
(Thermo Fisher Scientific, Inc.). Following 72 h of transfection,
cells were harvested for proliferation and apoptosis assays.
Flow cytometry assay
Apoptosis and cell cycle of MHCC97-H cells were
detected using flow cytometry kit (cat. no. Apobrdu-1KT;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Briefly, MHCC97-H
cells at a logarithmic growth phase were seeded in a 96-well plate
at a density of 2×103 cells/well and maintained in RPMI
1640 medium (cat. no. SH30809.01; Invitrogen; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (cat. no. AD17321268;
Invitrogen; Thermo Fisher Scientific, Inc.) for 16 h at 37°C.
Following cell transfection with control plasmids, control siRNA or
NET-1-siRNA for 72 h, the cells were rinsed twice with PBS and
counted. A total of 5–10×104 cells were collected and
centrifuged at 2,000 × g for 5 min at 4°C. Subsequently, cells were
resuspended with and incubated for additional 10 min at 37°C.
Centrifugation at 2,000 × g for 5 min at 4°C was performed and the
cells were resuspended in PBS containing 10 µl propidium iodide in
the dark for 30 min at room temperature. Finally, apoptosis was
measured using a flow cytometer and CellQuest software (version
3.3; BD Biosciences, San Jose, CA, USA).
Cell proliferation assay
Cells were seeded into 96-well plates at a density
of 5×104 cells/well the day prior to transfection.
Following transfection, cells were seeded in a 96-well plate at a
density of 2×103 cells/well. Proliferation of cells was
determined using Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's protocol at 12, 24 and 48 h of culture. The optical
density (OD) was measured at a wavelength of 450 nm.
Statistical analysis
Data were analyzed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). All data are presented as the
mean ± standard deviation. All experiments were performed in
triplicate. Groups were compared using one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Screening for HCC cells with high
expression of NET-1
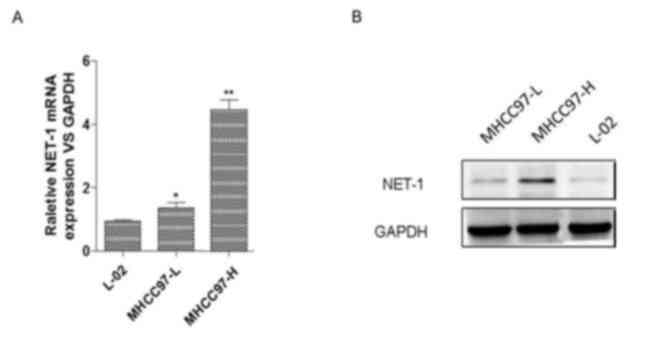
To study the effect of NET-1 on HCC, the present
study determined the relative mRNA and protein expression levels of
NET-1 in HCC cell lines MHCC97-L and MHCC97-H and in a normal liver
cell line L-02 using RT-qPCR and western blotting, respectively.
The results of the RT-qPCR assay indicated that the expression
levels of NET-1 were significantly elevated in MHCC97-L and
MHCC97-H cells compared with the L-02 cell line. Specifically, the
MHCC97-H cell line exhibited the highest expression of NET-1 among
these cell lines (Fig. 1A).
Furthermore, western blotting indicated that protein expression of
NET-1 increased in MHCC97-L and MHCC97-H cells compared with the
L-02 cell line, and MHCC97-H exhibited the highest expression level
among these cell lines (Fig. 1B).
Therefore, MHCC97-H cells were selected for further analysis.
Knockdown of NET-1 inhibited the
proliferation of HCC cells
Following transfection with si-NET-1, the mRNA and
protein expression of NET-1 was successfully downregulated in cells
compared with the control (Fig. 2A and
B). The OD value of MHCC97-H cells was determined by CCK-8. The
viability of MHCC97-H cells in the si-NET-1 group was significantly
decreased after 12, 24 and 48 h compared with the control groups
(Fig. 2C), suggesting the inhibition
of NET-1 could inhibit the proliferation of HCC.
Knockdown of NET-1 promotes HCC cell
apoptosis
To study the effect of NET-1 on HCC, the apoptotic
rate and cell cycle of MHCC97-H cells were determined using flow
cytometry. The apoptotic percent of MHCC97-H cells increased
following the knockdown of NET-1 compared with the control and
si-NC groups (Fig. 3A). Furthermore,
cell cycle of MHCC97-H cells was arrested at the G1/S phase
following transfection with NET-1 siRNA (Fig. 3B).
Knockdown of NET-1 influences the
expression of apoptosis-associated proteins and the activity of the
PI3K/AKT signaling pathway
To further reveal the underlying mechanism of NET-1
in HCC, expression levels apoptosis-associated proteins were
determined by western blotting. The expression levels of Bax and
cyclinD1 in MHCC97-H cells decreased following the knockdown of
NET-1, while the expression of Bcl-2 and caspase-3 increased
(Fig. 4A). The activity of the
PI3K/AKT signaling pathway was also determined when PI3K expression
was reduced by the NET-1 siRNA. There was no apparent difference
identified in the activity of PI3K, however, the expression of
p-AKT decreased following transfection with si-NET-1 (Fig. 4B).
 | Figure 4.Knockdown of NET-1 influences the
expression of apoptosis-associated proteins and the activity of the
PI3K/AKT signaling pathway. (A) Protein expression of Bax,
cyclinD1, Bcl-2 and caspase-3. (B) Protein expression of p-PI3K,
PI3K, p-AKT and AKT. (C) Quantification of the protein levels of
Bax, cyclinD1, Bcl-2 and caspase-3. (D) Quantification of the
protein levels of p-PI3K and p-AKT. *P<0.05 vs. control;
#P<0.05 vs. siNC. Bax, apoptosis regulator Bax;
Bcl-2, apoptosis regulator Bcl-2; PI3K, phosphoinositide 3-kinase;
AKT, protein kinase B; siNC, small interfering RNA negative
control; siRNA, small interfering RNA targeting neuroepithelial
cell transforming 1. |
Discussion
HCC is the most common type of primary liver cancer
and has been reported to be the fifth most common cancer worldwide
(10). The incidence of HCC has
increased worldwide and this disease is characterized by geographic
risk factor and diagnosis differences (24). There remains no standard effective
therapy for patients with HCC. This type of carcinoma is associated
with a high degree of vascular invasion and metastasis, and poor
prognosis (25). Numerous factors
contribute to the invasion and metastasis of HCC. Twist-related
protein 1 is a regulator of EMT-mediated invasion and metastasis,
which affects the expression of E-cadherin (26). As a pro-inflammatory cytokine,
interleukin (IL)-17A is frequently involved in the pathology of
inflammatory diseases and regulation of tumor microenvironment
(27–29). A previous study reported that IL-17A
promoted the metastasis of HCC (30). As a tumor suppressor, microRNA-122
was reported to regulate the intrahepatic metastasis of HCC
(31). It has also been demonstrated
that NET-1 exhibits higher expression levels in HCC cells compared
with normal liver cells, suggesting that NET-1 may serve a role in
HCC (21).
In the present study, the mechanism of NET-1 in the
invasion and metastasis of HCC was investigated in vitro.
Relative mRNA expression of NET-1 was determined using RT-qPCR in
MHCC97-H and MHCC97-L cells with different metastasis potentials
(32,33) and normal liver cell line L-02. The
results indicated that the expression of NET-1 was upregulated in
HCC cell lines compared with the normal liver cell line, which may
contribute to the metastasis and invasion of HCC. The MHCC97-H cell
line exhibited the highest expression level of NET-1 and was
therefore selected for subsequent experiments. NET-1 was knocked
down in MHCC97-H cells and proliferation, cell cycle progression
and apoptosis were determined. The results indicated that si-NET-1
could decrease the proliferation of MHCC97-H cells. Furthermore,
the apoptotic percent of MHCC97-H cells was elevated following the
knockdown of NET-1. In addition, cell cycle was arrested at the
G1/S phase in the si-NET-1 group of MHCC97-H cells. Shen et
al (21) demonstrated that the
expression of NET-1 was associated with the proliferation,
metastasis and clinical stages of HCC. Chen et al (34) reported a strong correlation between
the expression level of NET-1 and HCC pathological grading.
Therefore, in the present study it was hypothesized that NET-1 may
serve a role in promoting proliferation and suppressing apoptosis
of HCC.
To further elucidate the molecular mechanisms of
NET-1, the expressions levels of Bax, cyclinD1, Bcl-2 and caspase-3
were determined. The expression levels of Bax and cyclinD1
decreased in the si-NET-1 MHCC97-H cells, while the expression
levels of Bcl-2 and caspase-3 increased compared with the controls.
As a pro-apoptotic member of the Bcl-2 family, Bax shares highly
conserved domains with Bcl-2 and serves a role in regulating
programmed cell death (35).
Dysfunction of the p53/Bax/caspase-3 apoptosis signaling pathway
promotes carcinogenesis (36).
Furthermore, a balance between Bax and Bcl-2 is also involved in
cancer therapeutic resistance (37),
as well as proliferation, invasion, adhesion and metastasis of
cancer cells (38). In a human
breast cancer line, overexpression of Bcl-2 enhanced the metastatic
ability (39). Cyclin D1 is a
proto-oncogene abnormally overexpressed in several cancers,
including breast and prostate cancers, which promotes cell
proliferation via activation of cyclin-dependent kinases (40). Cyclin D1 may act as a subunit of a
holoenzyme to phosphorylate and inactivate the retinoblastoma
protein, and promote cell cycle progression to the G2
phase of the cell cycle (41).
Apoptosis is an important mechanism of cell death regulation which
serves a role in eliminating infected, damaged and other
undesirable cells from tissues (42,43).
Caspase-3 is the main executor of apoptosis in cells (44). During programmed cell death,
activation of caspase-3 leads to proteolysis of DNA repair proteins
and cytoskeletal proteins to alter the morphology and DNA of cells
(45). Dysregulation of caspase-3
was reported in several malignancies (46–48) and
overexpression of this protein was reported in HCC (49).
To further explore the molecular mechanism of NET-1
in HCC, the activity of the PI3K/AKT signaling pathway was
determined. The results indicated that there was no apparent
difference identified in the expression of PI3K, however, the
expression of AKT was downregulated following knockdown of NET-1.
The PI3K/AKT signaling pathway serves an important role in
mediating survival signals in a number of neuronal cell types
(50). AKT and AKT-dependent
signaling pathways, including glycogen synthase kinase-3β (51), PI3K (52) and mitogen-activated protein kinase
(53) signaling pathways serve
critical roles in the pathogenesis of degenerative diseases and
cancers (51), including apoptosis,
metabolism, cell proliferation and cell growth (50). Epidemiological and experimental
studies reported that abnormally activated PI3K/AKT pathway is
involved in the initiation and maintenance of cancer (52–55). In
addition, the PI3K/AKT signaling pathway has also been confirmed to
participate in leptin-mediated promotion of invasion and migration
of HCC (56). Therefore, these
studies verified the reasons why NET-1 promotes proliferation and
inhibits apoptosis of HCC cells.
In conclusion, inhibition of NET-1 can suppress
proliferation and promote apoptosis of HCC cells by activating the
PI3K/AKT signaling pathway and increasing the expression levels of
apoptosis-associated proteins.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Key
Development Projects in Shandong (grant no. 2018GSF118191) and
Shandong Medical Science and Technology Development Program (grant
no. 2017WS321).
Availability of data and materials
All data and materials in the present study were
available when proper request to the authors.
Authors' contributions
XS conceived of and designed the present study,
collected and consolidated the data, analyzed and interpreted the
data, and wrote the manuscript. MW conceived of and designed the
current study, and collected and consolidated the data. FZ
conceived of and designed the current study, analyzed and
interpreted the data, and wrote the manuscript. XK conceived of and
designed the current study, analyzed and interpreted the data, and
wrote the manuscript.
Ethics approval and consent to
participate
Ethical approval for cell culturing was given by the
Medical Ethics Committee of Linyi People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Keeffe EB: Risk score for development of
HCC: Ready for use in practice? Lancet Oncol. 12:517–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furihata T, Sawada T, Kita J, Iso Y, Kato
M, Rokkaku K, Shimoda M and Kubota K: Serum alpha-fetoprotein level
per tumor volume reflects prognosis in patients with hepatocellular
carcinoma after curative hepatectomy. Hepatogastroenterology.
55:1705–1709. 2008.PubMed/NCBI
|
|
3
|
Kütting F, Schubert J, Franklin J, Bowe A,
Hoffmann V, Demir M, Pelc A, Nierhoff D, Töx U and Steffen HM:
Insufficient evidence of benefit regarding mortality due to albumin
substitution in HCC-free cirrhotic patients undergoing large volume
paracentesis. J Gastroenterol Hepatol. 32:327–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki K, Firl DJ, Hashimoto K, Fujiki M,
Diago-Uso T, Quintini C, Eghtesad B, Fung JJ, Aucejo FN and Miller
CM: Development and validation of the HALT-HCC score to predict
mortality in liver transplant recipients with hepatocellular
carcinoma: A retrospective cohort analysis. Lancet Gastroenterol
Hepatol. 2:595–603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwarz L, Bubenheim M, Zemour J, Herrero
A, Muscari F, Ayav A, Riboud R, Ducerf C, Regimbeau JM, Tranchart
H, et al: Bleeding recurrence and mortality following
interventional management of spontaneous HCC rupture: Results of a
multicenter European study. World J Surg. 42:225–232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun LY, Zhang H, Li ZL, Li C, Wang MD and
Yang T: How to predict global trends in HCC mortality if neglecting
more than half the world's cases? J Hepatol. 67:887–888. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao W, Li J, Hu C, Shen J, Liu X, Xu Y and
Ye Z: Symptom clusters and symptom interference of HCC patients
undergoing TACE: A cross-sectional study in China. Support Care
Cancer. 21:475–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen M, Therneau T, Orsini LS and Qiao YL:
Design and rationale of the HCC BRIDGE study in China: A
longitudinal, multicenter cohort trial in hepatocellular carcinoma.
BMC Gastroenterol. 11:532011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li GJ, Harrison TJ, Yang JY, Chen QY, Wang
XY and Fang ZL: Combined core promoter mutations and pre-S deletion
of HBV may not increase the risk of HCC: A geographical
epidemiological study in Guangxi, China. Liver Int. 33:936–943.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng YM, Feng CW, Chen SC and Hsu CD:
Unexpected remission of hepatocellular carcinoma (HCC) with lung
metastasis to the combination therapy of thalidomide and
cyproheptadine: Report of two cases and a preliminary HCC cell line
study. BMJ Case Rep. 2012(bcr2012007180)2012.
|
|
12
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou YQ, Yao Y, Bao YL, Song ZB, Yang C,
Gao XL, Zhang WJ, Sun LG, Yu CL, Huang YX, et al: Juglanthraquinone
C induces intracellular ROS increase and apoptosis by activating
the Akt/Foxo signal pathway in HCC cells. Oxid Med Cell Longev.
2016:49416232016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Huang X, Han J, Zheng W and Ma W:
Extract of Perilla frutescens inhibits tumor proliferation of HCC
via PI3K/AKT signal pathway. Afr J Tradit Complement Altern Med.
10:251–257. 2012.PubMed/NCBI
|
|
15
|
Serru V, Dessen P, Boucheix C and
Rubinstein E: Sequence and expression of seven new tetraspans.
Biochim Biophys Acta. 1478:159–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji ZJ, Wang JL and Chen L: Inhibition of
skin squamous cell carcinoma proliferation and promote apoptosis by
dual silencing of NET-1 and survivin. Oncol Rep. 34:811–822. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu B, Liang X, Jing H, Han X, Sun Y, Guo
C, Liu Y and Cheng W: Effect of NET-1 siRNA conjugated sub-micron
bubble complex combined with low-frequency ultrasound exposure in
gene transfection. Oncotarget. 9:4150–4160. 2017.PubMed/NCBI
|
|
18
|
Zuo Y, Ulu A, Chang JT and Frost JA:
Contributions of the RhoA guanine nucleotide exchange factor Net1
to polyoma middle T antigen-mediated mammary gland tumorigenes and
metastasis. Breast Cancer Res. 20:412018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gabitova G and Burke NJ: Improving
healthcare empowerment through breast cancer patient navigation: A
mixed methods evaluation in a safety-net setting. BMC Health Serv
Res. 14:4072014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wheelock AE, Bock MA, Martin EL, Hwang J,
Ernest ML, Rugo HS, Esserman LJ and Melisko ME: SIS. NET: A
randomized controlled trial evaluating a web-based system for
symptom management after treatment of breast cancer. Cancer.
121:893–899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen SQ, Li K, Zhu N and Nakao A:
Expression and clinical significance of NET-1 and PCNA in
hepatocellular carcinoma. Med Oncol. 25:341–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang L, Zhu J, Ma Y, Hong C, Xiao S and
Jin L: Neuroepithelial transforming gene 1 functions as a potential
prognostic marker for patients with non-small cell lung cancer. Mol
Med Rep. 12:7439–7446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun CK, Chua MS, He J and So SK:
Suppression of glypican 3 inhibits growth of hepatocellular
carcinoma cells through up-regulation of TGF-β2. Neoplasia.
13:735–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J, Sherman M, Llovet JM, Beaugrand
M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M
and Rodés J; EASL Panel of Experts on HCC: Clinical management of
hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL
conference. European association for the study of the liver. J
Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu L, Lau SH, Tzang CH, Wen JM, Wang W,
Xie D, Huang M, Wang Y, Wu MC, Huang JF, et al: Association of
Vimentin overexpression and hepatocellular carcinoma metastasis.
Oncogene. 23:298–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong
XY, Gu Q, Yao Z and Sun BC: Promotion of hepatocellular carcinoma
metastasis through matrix metalloproteinase activation by
epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med.
15:691–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lauridsen HM, Pellowe AS, Ramanathan A,
Liu R, Miller-Jensen K, McNiff JM, Pober JS and Gonzalez AL: Tumor
necrosis factor-α and IL-17A activation induces pericyte-mediated
basement membrane remodeling in human neutrophilic dermatoses. Am J
Pathol. 187:1893–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma YF, Chen C, Li D, Liu M, Lv ZW, Ji Y
and Xu J: Targeting of interleukin (IL)-17A inhibits PDL1
expression in tumor cells and induces anticancer immunity in an
estrogen receptor-negative murine model of breast cancer.
Oncotarget. 8:7614–7624. 2017.PubMed/NCBI
|
|
29
|
Xu LL, Li ZJ, Niu XL and Deng WM: The
mechanisms of IL-17A on promoting tumor metastasis. Int Rev
Immunol. 36:360–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Lau GK, Chen L, Dong SS, Lan HY,
Huang XR, Li Y, Luk JM, Yuan YF and Guan XY: Interleukin 17A
promotes hepatocellular carcinoma metastasis via NF-kB induced
matrix metalloproteinases 2 and 9 expression. PLoS One.
6:e218162011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW,
Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al: MicroRNA-122, a
tumor suppressor microRNA that regulates intrahepatic metastasis of
hepatocellular carcinoma. Hepatology. 49:1571–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding SJ, Li Y, Shao XX, Zhou H, Zeng R,
Tang ZY and Xia QC: Proteome analysis of hepatocellular carcinoma
cell strains, MHCC97-H and MHCC97-L, with different metastasis
potentials. Proteomics. 4:982–994. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song P, Bao H, Yu Y, Xue Y, Yun D, Zhang
Y, He Y, Liu Y, Liu Q, Lu H, et al: Comprehensive profiling of
metastasis-related proteins in paired hepatocellular carcinoma
cells with different metastasis potentials. Proteomics Clin Appl.
3:841–852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen L, Wang Z, Zhan X, Li DC, Zhu YY and
Zhu J: Association of NET-1 gene expression with human
hepatocellular carcinoma. Int J Surg Pathol. 15:346–353. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bagci EZ, Vodovotz Y, Billiar TR,
Ermentrout GB and Bahar I: Bistability in apoptosis: Roles of bax,
bcl-2, and mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prokop A, Wieder T, Sturm I, Essmann F,
Seeger K, Wuchter C, Ludwig WD, Henze G, Dörken B and Daniel PT:
Relapse in childhood acute lymphoblastic leukemia is associated
with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous
caspase-3 processing in vivo. Leukemia. 14:1606–1613. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manero F, Gautier F, Gallenne T, Cauquil
N, Grée D, Cartron PF, Geneste O, Grée R, Vallette FM and Juin P:
The small organic compound HA14-1 prevents Bcl-2 interaction with
Bax to sensitize malignant glioma cells to induction of cell death.
Cancer Res. 66:2757–2764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Del Bufalo D, Biroccio A, Leonetti C and
Zupi G: Bcl-2 overexpression enhances the metastatic potential of a
human breast cancer line. FASEB J. 11:947–953. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mukhopadhyay A, Banerjee S, Stafford LJ,
Xia C, Liu M and Aggarwal BB: Curcumin-induced suppression of cell
proliferation correlates with down-regulation of cyclin D1
expression and CDK4-mediated retinoblastoma protein
phosphorylation. Oncogene. 21:8852–8861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: Normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aouacheria A, Cunningham KW, Hardwick JM,
Palková Z, Powers T, Severin FF and Váchová L: Comment on
‘Sterilizing immunity in the lung relies on targeting fungal
apoptosis-like programmed cell death’. Science.
360(eaar6910)2018.PubMed/NCBI
|
|
43
|
Shlezinger N, Irmer H, Dhingra S, Beattie
SR, Cramer RA, Braus GH, Sharon A and Hohl TM: Response to comment
on ‘Sterilizing immunity in the lung relies on targeting fungal
apoptosis-like programmed cell death’. Science.
360(eaas9457)2018.PubMed/NCBI
|
|
44
|
Le DA, Wu Y, Huang Z, Matsushita K,
Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA
and Moskowitz MA: Caspase activation and neuroprotection in
caspase-3- deficient mice after in vivo cerebral ischemia and in
vitro oxygen glucose deprivation. Proc Natl Acad Sci USA.
99:15188–15193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Richardson-Burns SM, Kominsky DJ and Tyler
KL: Reovirus-induced neuronal apoptosis is mediated by caspase 3
and is associated with the activation of death receptors. J
Neurovirol. 8:365–380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fu Y, Ye X, Lee M, Rankin G and Chen YC:
Prodelphinidins isolated from Chinese bayberry leaves induces
apoptosis via the p53-dependent signaling pathways in OVCAR-3 human
ovarian cancer cells. Oncol Lett. 13:3210–3218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pidugu VR, Yarla NS, Bishayee A, Kalle AM
and Satya AK: Novel histone deacetylase 8-selective inhibitor
1,3,4-oxadiazole-alanine hybrid induces apoptosis in breast cancer
cells. Apoptosis. 22:1394–1403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou M, Liu X, Li Z, Huang Q, Li F and Li
CY: Caspase-3 regulates the migration, invasion and metastasis of
colon cancer cells. Int J Cancer. 143:921–930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Persad R, Liu C, Wu TT, Houlihan PS,
Hamilton SR, Diehl AM and Rashid A: Overexpression of caspase-3 in
hepatocellular carcinomas. Mod Pathol. 17:861–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and -independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Garcia-Echeverria C and Sellers WR: Drug
discovery approaches targeting the PI3K/Akt pathway in cancer.
Oncogene. 27:5511–5526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cao J, Lv W, Wang L, Xu J, Yuan P, Huang
S, He Z and Hu J: Ricolinostat (ACY-1215) suppresses proliferation
and promotes apoptosis in esophageal squamous cell carcinoma via
miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis. 9:8172018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Leng J, Wang Z, Fu CL, Zhang J, Ren S, Hu
JN, Jiang S, Wang YP, Chen C and Li W: NF-κB and AMPK/PI3K/Akt
signaling pathways are involved in the protective effects of
Platycodon grandiflorum saponins against acetaminophen-induced
acute hepatotoxicity in mice. Phytother Res. 32:2235–2246. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang HW, Hu JJ, Fu RQ, Liu X, Zhang YH,
Li J, Liu L, Li YN, Deng Q, Luo QS, et al: Flavonoids inhibit cell
proliferation and induce apoptosis and autophagy through
downregulation of PI3Kgamma mediated PI3K/AKT/mTOR/p70S6K/ULK
signaling pathway in human breast cancer cells. Sci Rep.
8:112552018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|