Introduction
Choroidal neovascularization (CNV) is characterized
by an abnormal growth of blood vessels between the neurosensory
retina and the retinal pigment epithelium, and is a sight
threatening condition most commonly associated with age-related
macular degeneration (AMD) (1) and
pathologic myopia (2). Despite the
development of novel therapies for CNV, including laser
photocoagulation, photodynamic therapy, pharmacotherapy and
targeted gene therapy, this disorder remains a leading cause of
severe central vision loss in individuals above the age of 50 years
(3,4).
Vascular endothelial growth factor (VEGF) is a
chemotactic and angiogenic factor that is considered to be a major
factor in the proliferation and migration of vascular endothelial
cells (ECs) in AMD (5–7). CNV has been experimentally linked to
the overexpression of VEGF, which promotes choroidal endothelial
cell (CEC) proliferation and migration as well as capillary-like
tube formation (8–10). CECs are located on the vascular layer
of the eye, known as the choroidea or choroid coat. CECs have been
reported in multiple previously published studies (11,12),
which have demonstrated that the inhibition of angiogenic signaling
in CECs is able to ameliorate the CNV process. Current treatments
for CNV primarily target VEGF-mediated processes (13). While VEGF is a potent inducer of
angiogenesis, understanding the roles of additional angiogenic
stimuli would be invaluable for the development of novel CNV
therapies (14).
The Slit guidance ligand family of proteins (Slit1,
Slit2 and Slit3) are secreted extracellular matrix proteins
involved in neural development, and participate in additional
physiological and pathological processes, including angiogenesis,
inflammation and cancer (15–17).
Slit2 guides axon growth and controls neurocyte migration (18). The Slit proteins interact with
roundabout guidance (Robo) receptors (Robo1, Robo2, Robo3 and
Robo4), to mediate chemorepulsion of olfactory bulb explants in
vitro (19). The Robo family of
proteins are primarily expressed in the nervous system; however,
they are also detectable in other tissues, including vascular,
renal and tumor tissues (20). In
addition, Slit2 has been demonstrated to influence tumor
angiogenesis, growth and metastasis (21–23)
while inhibiting retinal neovascularization (24,25).
Previous studies have demonstrated that Slit2 may
positively or negatively regulate VEGF-directed permeability
depending on whether it binds to Robo1 or Robo4 receptors,
respectively (26,27). These and the results of additional
studies suggest that Slit2-mediated responses may be determined by
the tissue-specific expression of Robo1 and Robo4 receptors in ECs
(28,29). For instance, Slit2 inhibits
hantavirus-induced enhancement of pulmonary EC permeability via a
mechanism involving Robo4 (30); a
vascular-specific receptor expressed in ECs (31). In addition, Robo4 mediates
Slit2-mediated alternations in cell migration and tube formation in
ECs. A previous study demonstrated that Robo4 activation by Slit2
inhibits VEGF-induced EC migration, tube formation and permeability
in vitro, as well as VEGF-stimulated vascular leakage in
vivo, by inhibiting the activation of Src family kinases
(24). Two additional reports
revealed that Slit2 interacts with Robo4 to inhibit VEGF- and basic
fibroblast growth factor-induced CE migration (32,33).
Notably, Slit2 has also been implicated in the migration of
vascular smooth muscle cells (34).
Despite considerable evidence supporting the
important role of Slit2 in mediating the migration of various types
of ECs, there is limited information regarding the effects of Slit2
on CEC migration and tube formation. The authors of the present
study hypothesized that Slit2 may modulate CEC migration and tube
formation induced by VEGF. To test this hypothesis, the present
study assessed the effects of exogenous Slit2 on VEGF-induced CEC
migration and angiogenesis.
Materials and methods
Cell culture and grouping
All cell culture reagents were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) unless otherwise
specified. Human CECs (cat. no. CP-H092) were purchased from
Procell Life Science & Technology Co., Ltd., (Wuhan, China) and
cultured in complete medium consisting of M199 medium supplemented
with 10% fetal bovine serum, 100 µg/ml endothelial cell growth
supplement, 1,000 µg/ml heparin sulfate (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin. Cells were grown in a humidified atmosphere
with 5% CO2 at 37°C.
CECs were subjected to reverse
transcription-polymerase chain reaction (RT-PCR) and
immunocytochemistry analyses to detect the expression of specific
genes and proteins, respectively. The cells were cultured in 6-well
plates at a density of 5×104 cells/ml and exposed to 0,
50, 75, 100, 125 and 150 ng/ml recombinant human Slit2-N protein
(PeproTech, Inc., Rocky Hill, NJ, USA) for 8 h before they were
harvested for analysis.
For cell migration and tube formation assays, cells
were divided into the following 4 groups: Non-treatment control,
cells cultured in M199 medium only; Slit2, cells cultured in M199
medium containing 125 ng/ml Slit2-N; VEGF, cells cultured in M199
medium containing 20 ng/ml VEGF (PeproTech, Inc.); and Slit2+VEGF,
cells cultured in M199 medium containing 125 ng/ml Slit2-N plus 20
ng/ml VEGF (35).
Cell migration assay
The cell migration assay was performed using 24-well
plates containing Transwell inserts with 8-µm pore polyethylene
terephthalate (PET) membranes separating the inner and outer
chambers. CECs were seeded onto the insert at 1×104
cells/ml and the appropriate medium was added to the wells
according to each group. Following incubation for 8 h, cells on the
PET membrane were fixed with 4% paraformaldehyde for 30 min at room
temperature, while any non-migrating cells on the inner side of the
membrane were removed gently with a cotton swab. Cells that had
migrated through the pores onto the lower surface of the membrane
were stained with 0.01% crystal violet for 20 min at room
temperature, counted and photographed under an inverted microscope
(BX51; Olympus Corporation, Tokyo, Japan).
Tube formation assay
Matrigel was diluted in cold serum-free cell culture
medium at a 1:1 ratio, and used to coat 96-well culture dishes for
2 h at 37°C. CECs were then resuspended in the appropriate culture
medium according to each group and plated at 1×104
cells/ml. Following 8 h, tubular structures were counted and
photographed under an inverted microscope (Olympus Corporation).
For each group, 5 random fields were selected to calculate the
average number and standard deviation of tube formations.
Immunocytochemistry
CECs mounted onto slides were treated and fixed with
4% paraformaldehyde for 15 min at room temperature. The cells were
then washed with PBS and incubated with 0.5% Triton X-100/PBS for
20 min at 4°C. Cells were blocked with 10% goat serum (cat. no.
ab7481; Abcam, Cambridge, UK) for 20 min at room temperature.
Factor VIII-related antigen, Slit2, Robo1 and Robo4 proteins were
then detected. To do this, CECs were incubated with rabbit
polyclonal anti-Factor VIII-related antigen (dilution, 1:50; cat.
no. TA325456; OriGene Technologies, Inc., Rockville, MD, USA), and
anti-human Slit2 (cat. no. sc-514499), Robo1 (cat. no. sc-293444)
and Robo4 (cat. no. sc-166872) (all diluted at 1:1,000 and
purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
primary antibodies at 4°C overnight. Cells were then washed with
PBS and incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG secondary antibodies (dilution, 1:200; cat. no.
ab6721; Abcam) at 37°C for 30 min. A 3,3′-diaminobenzidine
substrate kit (Sangon Biotech, Co., Ltd., Shanghai, China) was used
for chromogenic detection. The nuclei were stained with 0.5%
hematoxylin for 3 min at room temperature. The results were
observed and photographed under an inverted microscope (Olympus
Corporation).
Western blot analysis
Cells were treated with protein lysis solution
(Beyotime Institute of Biotechnology, Shanghai, China) containing
10 mM phenylmethylsulfonyl fluoride. Protein concentrations were
determined using the bicinchoninic acid protein assay (Thermo
Fisher Scientific, Inc.). An equal quantity (30 µg) of protein for
each sample was loaded and resolved by SDS-PAGE using a 6% gel,
followed by transfer onto polyvinylidene difluoride membranes
(Merck KGaA). The membranes were then incubated with primary
antibodies against Robo4 (dilution, 1:1,000; cat. no. sc-166872;
Santa Cruz Biotechnology, Inc.) and GAPDH (dilution, 1:1,000; cat.
no. ab8245; Abcam) at 4°C overnight, followed by incubation with
HRP-conjugated goat anti-rabbit IgG antibodies (dilution, 1:1,000;
cat. no. ab6721; Abcam) for 1 h at room temperature. Protein bands
were visualized using an enhanced chemiluminescence kit (Thermo
Fisher Scientific, Inc.), and protein expression was
semi-quantified using Quantity One software 4.6 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
RT-PCR
Total RNA from CECs was isolated using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Reverse-transcription was performed using
the Takara PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu,
Japan). The mRNA expression levels of Slit2, Robo1, Robo4 and
β-actin were assessed via PCR amplification of target cDNA
sequences using TaKaRa Z-Taq™ DNA Polymerase (Takara Bio, Inc.).
The thermocycling conditions were as follows: 94°C for 5 min
followed by 30 cycles of 94°C for 30 sec, 60°C for 30 sec, 72°C for
60 sec, and maintenance at 72°C for 10 min. PCR products were
resolved by 2% agarose gel electrophoresis, and visualized by
staining with ethidium bromide. The sequences of primers used for
RT-PCR are presented in Table I.
 | Table I.Sequences for RT-PCR and the sizes of
PCR product sizes. |
Table I.
Sequences for RT-PCR and the sizes of
PCR product sizes.
| Primer | Sequence
(5′-3′) | Size (bp) |
|---|
| Slit2 |
|
Forward |
TGGCTATCAGGGAGAAAAGTGTG | 176 |
|
Reverse |
CCGCGATATGGTCTTTGTCAC |
|
| Robo1 |
|
Forward |
CAGCACCAGCCCGACAGGAG | 124 |
|
Reverse |
GCGCATCCGTATCCATATCTGAG |
|
| Robo4 |
|
Forward |
CCACCCATATGCCAGGCTCCTAC | 226 |
|
Reverse |
CCCAGAAGCAGCAGCCAGAGTG |
|
| β-actin |
|
Forward |
GTGATCTCCTTCTGCATCCTGT | 188 |
|
Reverse |
CCACGAAACTACCTTCAACTCC |
|
Statistical analysis
Samples were run in triplicate and all experiments
were repeated three times. The data are presented as the mean ±
standard deviation and analyzed using SPSS 17.0 software (SPSS
Inc., Chicago, IL, USA). Comparisons among groups were analyzed by
one-way analysis of variance followed by the least significant
difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
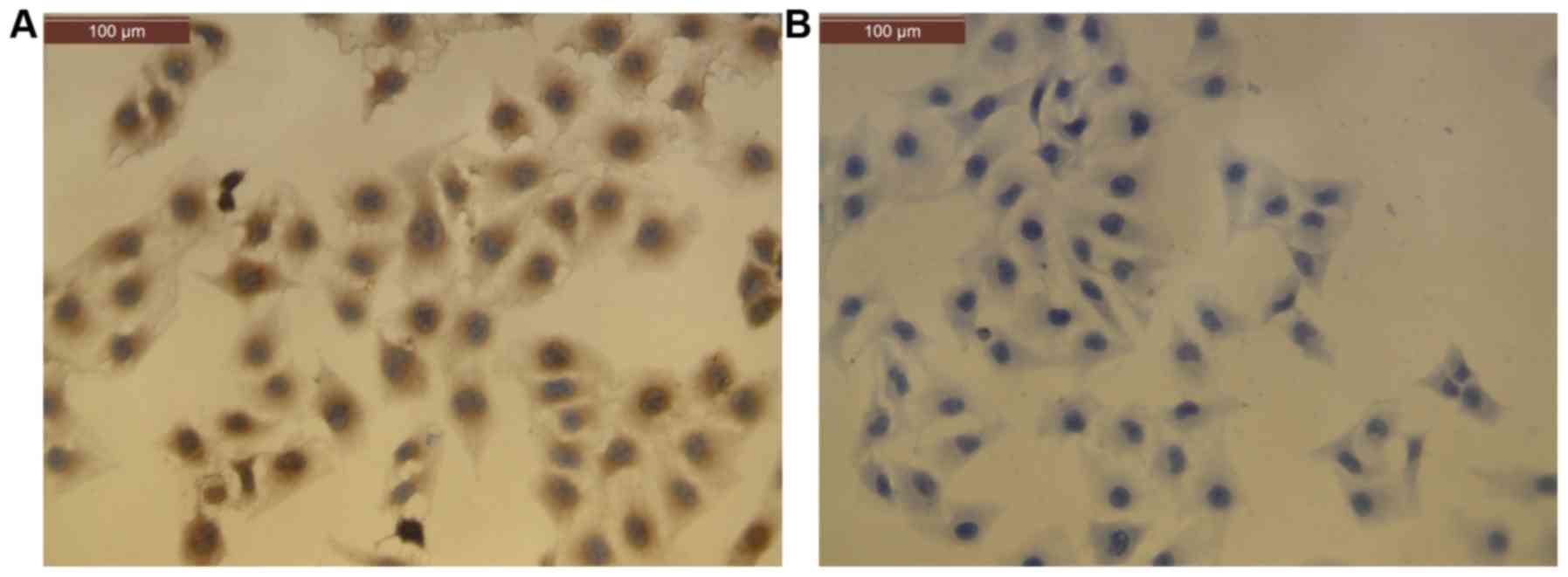
CECs form confluent monolayers and
largely express Factor VIII-related antigen
CECs were observed to form confluent monolayers with
a cobblestone appearance under a microscope. Cells were confirmed
to be vascular ECs by positive immunocytochemistry staining for
Factor VIII-related antigen (36) in
more than 95% of cells (Fig. 1).
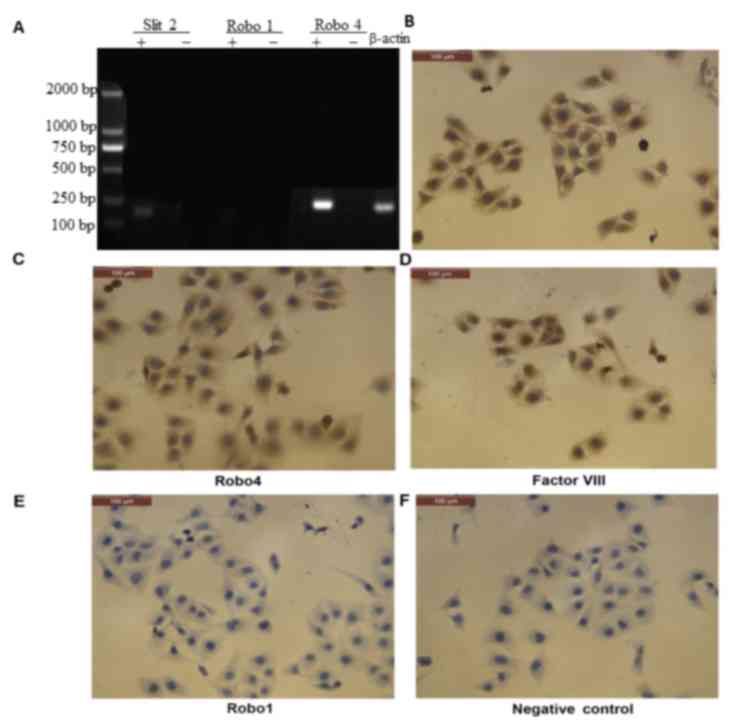
CECs express Slit2 and Robo4, but not
Robo1
Robo1 is expressed in retinal pigment epithelial
cells and vascular endothelial cells (37); however, its expression profile in
CECs is currently unknown. RT-PCR detected Slit2 and Robo4 mRNA
expression, but not Robo1, in CECs (Fig.
2A). Consistent with these results, immunocytochemistry
analysis detected Slit2 and Robo4, but not Robo1, protein
expression in CECs (Fig. 2B-F).
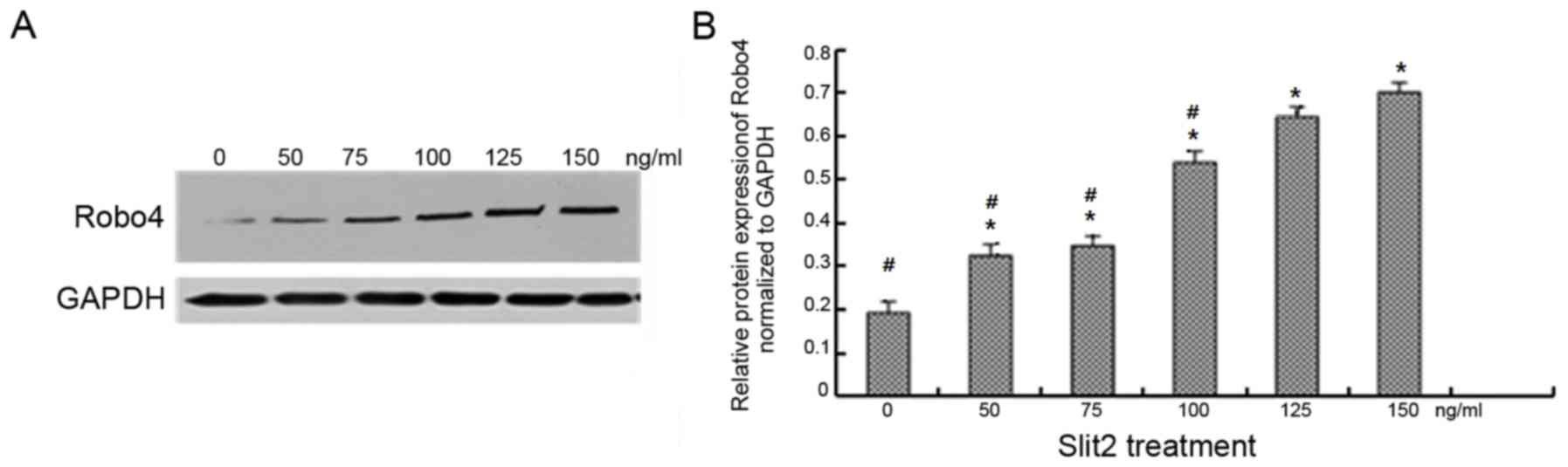
Exogenous Slit2 upregulates Robo4
protein expression in a concentration-dependent manner
Treatment with 0, 50, 75, 100, 125 and 150 ng/ml
exogenous Slit2 was associated with a concentration-dependent
increase in Robo4 protein levels in CECs (Fig. 3). Robo4 protein expression levels
were significantly higher in CECs treated with 125 ng/ml Slit2 when
compared with cells exposed to lower concentrations (P<0.05;
Fig. 3B). However, no significant
difference in Robo4 protein expression was observed between the 125
and 150 ng/ml Slit2-treated groups. For this reason, a
concentration of 125 ng/ml Slit2 was selected for subsequent cell
migration and tube formation assays.
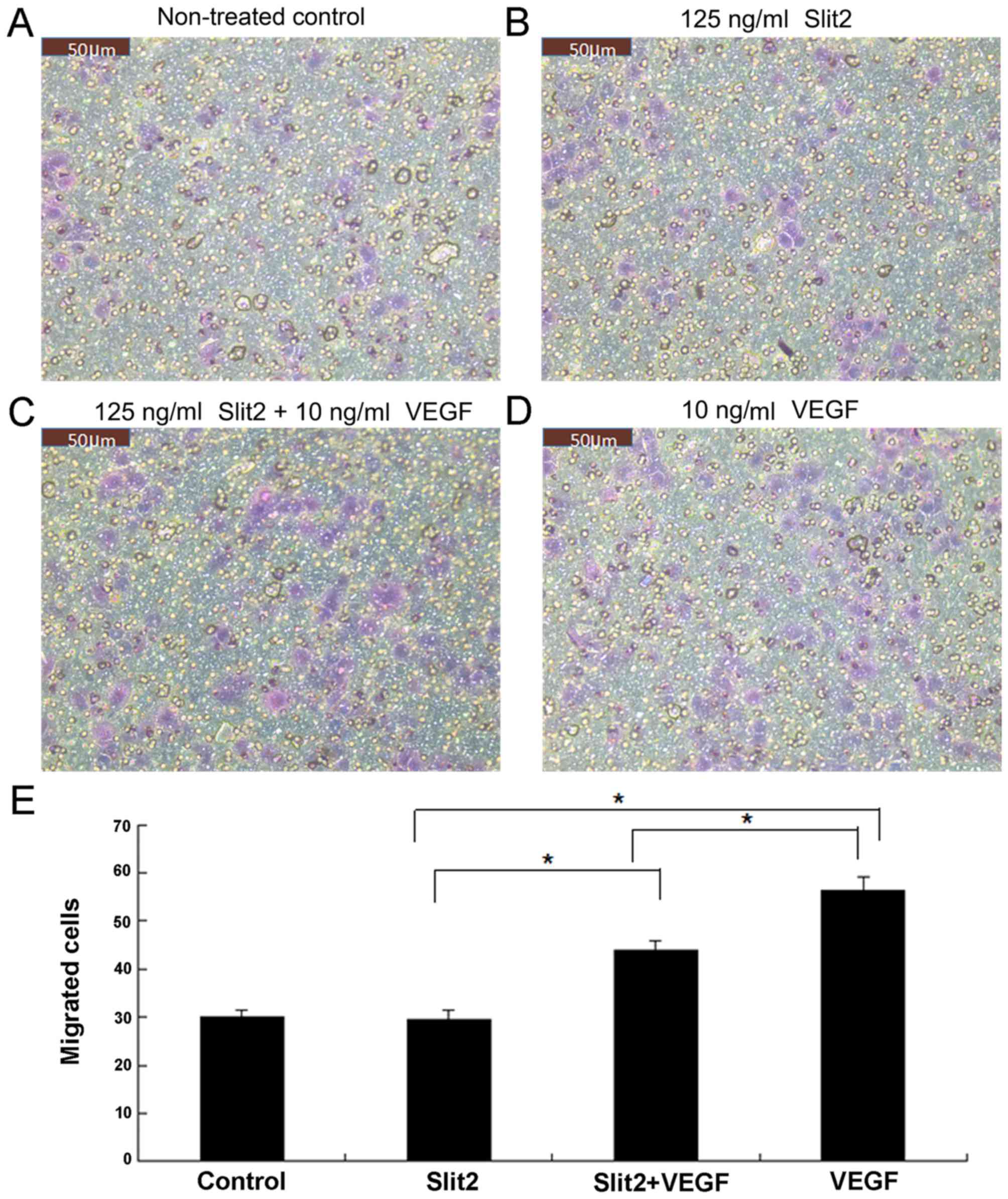
Slit2 inhibits VEGF-induced CEC
migration
Using Transwell migration assays, exogenous VEGF (10
ng/ml) was observed to enhance the migration of CECs when compared
with untreated controls (Fig. 4).
Despite the observation that Slit2 treatment (125 ng/ml) alone
demonstrated no significant effect on CEC migration when compared
with the control group, it significantly inhibited VEGF-induced CEC
migration (P<0.05; Fig. 4).
Slit2 inhibits VEGF-induced CEC tube
formation
As presented in Fig.
5, CECs migrated gradually and formed connections to produce
simple tubular structures of differing shapes and sizes. Treatment
with VEGF (10 ng/ml) was associated with an increase in tube
formation when compared with controls (Fig. 5). Treatment with 125 ng/ml Slit2
alone demonstrated no significant effect on tube formation when
compared with controls (Fig. 5);
however, Slit2 (125 ng/ml) significantly attenuated VEGF-induced
tube formation (P<0.05; Fig.
5).
Discussion
A noteworthy observation of the current study was
that Slit2 and Robo4 but not Robo1, were expressed in human CECs.
In addition, Robo4 was upregulated by exogenous Slit2 treatment
(0–125 ng/ml) in a concentration-dependent manner. Importantly,
VEGF-induced CEC migration and tube formation was inhibited by
exogenous Slit2 treatment. Combined with the results of previously
published studies (28–34), these results support the hypothesis
that Slit2 may interact with Robo4 to inhibit VEGF-induced CEC
migration and tube formation, and subsequent angiogenesis. However,
further studies are necessary to confirm this hypothesis and
determine whether the Slit2/Robo4 signaling pathway may present a
therapeutic target for the development of novel CNV therapies.
The present study utilized RT-PCR and
immunocytochemistry analyses and determined that Slit2 and Robo4
mRNA and protein were expressed in human CECs, whereas Robo1 was
not. These findings corroborate previous studies demonstrating that
Robo4, but not Robo1, is expressed in microvascular ECs (38) and pulmonary microvascular ECs
(30). EC-specific expression of
Robo4 is well established (39).
However, in contrast to the results of previous studies, Robo1
expression was reportedly expressed in vascular ECs from rabbits
with experimental proliferative vitreoretinopathy (37), in retinal and choroidal tissue
samples from mice with experimental laser-induced CNV (40), in retinal tissue specimens from mice
with experimental retinal neovascularization (41) and in human umbilical vein ECs
(HUVECs) (30,40). It is therefore possible that Robo1
expression in ECs is species-specific, as the majority of studies
that have demonstrated positive expression of Robo1 in ECs used
mouse or rabbit models. In addition, it is also possible that Robo1
expression may be enhanced under pathological conditions such as
CNV and proliferative vitreoretinopathy. Therefore, although Robo4
may be the predominant isoform expressed in CECs, additional
studies are required to establish whether Robo1 may also be
upregulated and whether this may contribute to the pathogenesis of
CNV.
The results of the present study demonstrated that
Robo4 was upregulated by Slit2 treatment in a
concentration-dependent manner. To the best of the authors'
knowledge, this is the first study to demonstrate that Robo4
protein levels in human CECs may be altered by Slit2. However,
these results are consistent with a previously published similar
study demonstrating that Slit2 overexpression in HUVECs was
associated with upregulation of Robo1 expression (40). As Robo4 may serve a role in mediating
the effects of Slit2 in attenuating VEGF-induced angiogenesis by
CECs, Slit2-mediated upregulation of Robo4 would be predicted to
further enhance the potentially beneficial effects of Slit2 against
CNV.
In the current study, VEGF-induced CEC migration and
tube formation were significantly attenuated by co-treatment with
Slit2. These results are consistent with the study from Park et
al (38), which reported that
Slit2 inhibits VEGF-induced migration in primary human ECs.
Multiple additional studies have demonstrated that Slit2 and/or
Robo4 inhibit EC migration and/or tube formation induced by VEGF
(31,32,42,43).
Previous reports have also revealed that Robo1 affects EC migration
(29,44–46).
However, the lack of Robo1 expression observed in human CECs in the
present study suggests that Robo4 may be involved in the mechanism
by which Slit2 attenuates VEGF-induced CEC migration.
The current study did not investigate the potential
mechanisms by which the Slit2/Robo4 signaling pathway may attenuate
VEGF-induced CEC migration and tube formation. However, previous
investigations have yielded some insight into these potential
mechanisms. EC migration in response to VEGF requires activation of
the protein kinase B (Akt)/endothelial nitric oxide synthase
signaling pathway, as well as the extracellular signal-regulated
protein kinase 1/2 (Erk1/2) signaling pathway (35). VEGF receptor (VEGFR)-2 has also been
hypothesized to activate the small guanosine 5′-triphosphatase
(GTPase), Rac1, via Src-dependent phosphorylation of Vav2; a
guanine nucleotide-exchange factor (47,48).
Regarding how Slit2 may interact with these signaling pathways,
Slit2-N reportedly led to VEGFR-3 internalization, thereby
inhibiting PI3K/Akt signaling pathway activation by VEGF (43). Meanwhile, Cai et al (31) provided evidence to suggest that Robo4
inhibits VEGFR-mediated activation of PI3K/Akt and FAK signaling
pathways. In addition, Slit2-N attenuates platelet-derived growth
factor-mediated activation of Rac1 (34). Notably, Robo4-induced inhibition of
EC migration is partly mediated by the Ras/Raf/Mek/Erk signaling
pathway (32). Moreover, Robo4
mediates the effects of Slit2 by forming a complex with paxillin,
which inhibits the activation of the small GTPase, ADP ribosylation
factor 6, and consequently inhibits Rac (25). Therefore, it is possible that
inhibition of VEGF-induced CEC migration by Slit2/Robo4 signaling
may involve Rac1, although further studies are required to confirm
this.
In conclusion, the results of the current study
demonstrate that Slit2 inhibits VEGF-induced CEC migration and tube
formation. Further studies are required to determine whether Robo4
is involved in these Slit2-mediated effects and to identify the
underlying mechanisms. Our exploring on Slit2/Robo4 signaling
related mechanisms in reducing human CEC angiogenesis would
facilitate the development of novel therapies for the treatment of
CNV.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Natural Science
Foundation of China (grant no. 81170858).
Availability of data and materials
Data are available on request.
Authors' contributions
YT performed the experiments, participated in data
collection and drafted the manuscript. XZ designed the experiments,
revised the manuscript and supervised the current study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
CEC
|
choroidal endothelial cell
|
|
CNV
|
choroidal neovascularization
|
|
AMD
|
age-related macular degeneration
|
References
|
1
|
Shao J, Choudhary MM and Schachat AP:
Neovascular age-related macular degeneration. Dev Ophthalmol.
55:125–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong TY, Ferreira A, Hughes R, Carter G
and Mitchell P: Epidemiology and disease burden of pathologic
myopia and myopic choroidal neovascularization: An evidence-based
systematic review. Am J Ophthalmol. 157:9–25.e12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blinder KJ, Bradley S, Bressler NM,
Bressler SB, Donati G, Hao Y, Ma C, Menchini U, Miller J, Potter
MJ, et al: Effect of lesion size, visual acuity, and lesion
composition on visual acuity change with and without verteporfin
therapy for choroidal neovascularization secondary to age-related
macular degeneration: TAP and VIP report no.1. Am J Ophthalmol.
136:407–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D'Amico DJ, Goldberg MF, Hudson H, Jerdan
JA, Krueger S, Luna S, Robertson SM, Russell S, Singerman L,
Slakter JS, et al: Anecortave acetate as monotherapy for the
treatment of subfoveal lesions in patients with exudative
age-related macular degeneration (AMD): Interim (month 6) analysis
of clinical safety and efficacy. Retina. 23:14–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakamoto T, Ishibashi T, Kimura H,
Yoshikawa H, Spee C, Harris MS, Hinton DR and Ryan SJ: Effect of
tecogalan sodium on angiogenesis in vitro by choroidal endothelial
cells. Invest Ophthalmol Vis Sci. 36:1076–1083. 1995.PubMed/NCBI
|
|
6
|
Ohno-Matsui K, Morita I, Tombran-Tink J,
Mrazek D, Onodera M, Uetama T, Hayano M, Murota SI and Mochizuki M:
Novel mechanism for age-related macular degeneration: An
equilibrium shift between the angiogenesis factors VEGF and PEDF. J
Cell Physiol. 189:323–333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klagsbrun M and D'Amore PA: Regulators of
angiogenesis. Annu Rev Physiol. 53:217–239. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frank RN: Growth factors in age-related
macular degeneration: Pathogenic and therapeutic implications.
Ophthalmic Res. 29:341–353. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwesinger C, Yee C, Rohan RM, Joussen
AM, Fernandez A, Meyer TN, Poulaki V, Ma JJ, Redmond TM, Liu S, et
al: Intrachoroidal neovascularization in transgenic mice
overexpressing vascular endothelial growth factor in the retinal
pigment epithelium. Am J Pathol. 158:1161–1172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrara N: Vascular endothelial growth
factor and age-related macular degeneration: From basic science to
therapy. Nat Med. 16:1107–1111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan Z, Shi H, Zhu R, Li L, Qin B, Kang L,
Chen H and Guan H: Inhibition of YAP ameliorates choroidal
neovascularization via inhibiting endothelial cell proliferation.
Mol Vis. 24:83–93. 2018.PubMed/NCBI
|
|
12
|
Gunda V, Verma RK and Sudhakar YA:
Inhibition of elastin peptide-mediated angiogenic signaling
mechanism(s) in choroidal endothelial cells by the α6(IV)NC1
collagen fragment. Invest Ophthalmol Vis Sci. 54:7828–35. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stone EM: A very effective treatment for
neovascular macular degeneration. N Engl J Med. 355:1493–1495.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Wijngaarden P, Coster DJ and Williams
KA: Inhibitors of ocular neovascularization: Promises and potential
problems. JAMA. 293:1509–1513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Howitt JA, Clout NJ and Hohenester E:
Binding site for Robo receptors revealed by dissection of the
leucine-rich repeat region of Slit. EMBO J. 23:4406–4412. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morlot C, Thielens NM, Ravelli RB, Hemrika
W, Romijn RA, Gros P, Cusack S and McCarthy AA: Structural insights
into the Slit-Robo complex. Proc Natl Acad Sci USA.
104:14923–14928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujiwara M, Ghazizadeh M and Kawanami O:
Potential role of the Slit/Robo signal pathway in angiogenesis.
Vasc Med. 11:115–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li HS, Chen JH, Wu W, Fagaly T, Zhou L,
Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, et al: Vertebrate slit,
a secreted ligand for the transmembrane protein roundabout, is a
repellent for olfactory bulb axons. Cell. 96:807–818. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel K, Nash JA, Itoh A, Liu Z,
Sundaresan V and Pini A: Slit proteins are not dominant
chemorepellents for olfactory tract and spinal motor axons.
Development. 128:5031–5037. 2001.PubMed/NCBI
|
|
20
|
Hohenester E: Structural insight into
Slit-Robo signalling. Biochem Soc Trans. 36:251–256. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Xiao Y, Ding BB, Zhang N, Yuan X,
Gui L, Qian KX, Duan S, Chen Z, Rao Y and Geng JG: Induction of
tumor angiogenesis by Slit-Robo signaling and inhibition of cancer
growth by blocking Robo activity. Cancer Cell. 4:19–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang LJ, Zhao Y, Han B, Ma YG, Zhang J,
Yang DM, Mao JW, Tang FT, Li WD, Yang Y, et al: Targeting
Slit-Roundabout signaling inhibits tumor angiogenesis in
chemical-induced squamous cell carcinogenesis. Cancer Sci.
99:510–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang XM, Han HX, Sui F, Dai YM, Chen M and
Geng JG: Slit-Robo signaling mediates lymphangiogenesis and
promotes tumor lymphatic metastasis. Biochem Biophys Res Commun.
396:571–577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones CA, London NR, Chen H, Park KW,
Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F,
Mukouyama YS, et al: Robo4 stabilizes the vascular network by
inhibiting pathologic angiogenesis and endothelial
hyperpermeability. Nat Med. 14:448–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones CA, Nishiya N, London NR, Zhu W,
Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, et al:
Slit2-Robo4 signalling promotes vascular stability by blocking Arf6
activity. Nat Cell Biol. 11:1325–1331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Acevedo LM, Weis SM and Cheresh DA: Robo4
counteracts VEGF signaling. Nat Med. 14:372–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koch AW, Mathivet T, Larrivée B, Tong RK,
Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K,
Rathore N, et al: Robo4 maintains vessel integrity and inhibits
angiogenesis by interacting with UNC5B. Dev Cell. 20:33–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dickinson RE and Duncan WC: The SLIT-ROBO
pathway: A regulator of cell function with implications for the
reproductive system. Reproduction. 139:697–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheldon H, Andre M, Legg JA, Heal P,
Herbert JM, Sainson R, Sharma AS, Kitajewski JK, Heath VL and
Bicknell R: Active involvement of Robo1 and Robo4 in filopodia
formation and endothelial cell motility mediated via WASP and other
actin nucleation-promoting factors. FASEB J. 23:513–522. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gorbunova EE, Gavrilovskaya IN and Mackow
ER: Slit2-Robo4 receptor responses inhibit ANDV directed
permeability of human lung microvascular endothelial cells.
Antiviral Res. 99:108–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai H, Xue Y, Li Z, Hu Y, Wang Z, Liu W,
Li Z and Liu Y: Roundabout4 suppresses glioma-induced endothelial
cell proliferation, migration and tube formation in vitro by
inhibiting VEGR2-mediated PI3K/AKT and FAK signaling pathways. Cell
Physiol Biochem. 35:1689–1705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seth P, Lin Y, Hanai J, Shivalingappa V,
Duyao MP and Sukhatme VP: Magic roundabout, a tumor endothelial
marker: Expression and signaling. Biochem Biophys Res Commun.
332:533–541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suchting S, Heal P, Tahtis K, Stewart LM
and Bicknell R: Soluble Robo4 receptor inhibits in vivo
angiogenesis and endothelial cell migration. FASEB J. 19:121–123.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu D, Hou J, Hu X, Wang X, Xiao Y, Mou Y
and De Leon H: Neuronal chemorepellent Slit2 inhibits vascular
smooth muscle cell migration by suppressing small GTPase Rac1
activation. Circ Res. 98:480–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YS, Eichler W, Friedrichs U, Yafai Y,
Hoffmann S, Yasukawa T, Hui YN and Wiedemann P: Impact of
endostatin on bFGF-induced proliferation, migration, and matrix
metalloproteinase-2 expression/secretion of bovine choroidal
endothelial cells. Curr Eye Res. 30:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schneeweis C, Gräfe M, Bungenstock A,
Spencer-Hänsch C, Fleck E and Goetze S: Chronic CRP-exposure
inhibits VEGF-induced endothelial cell migration. J Atheroscler
Thromb. 17:203–212. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang L, Xu Y, Yu W, Li Y, Chu L, Dong J
and Li X: Effect of Robo1 on retinal pigment epithelial cells and
experimental proliferative vitreoretinopathy. Invest Ophthalmol Vis
Sci. 51:3193–3204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park KW, Morrison CM, Sorensen LK, Jones
CA, Rao Y, Chien CB, Wu JY, Urness LD and Li DY: Robo4 is a
vascular-specific receptor that inhibits endothelial migration. Dev
Biol. 261:251–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huminiecki L, Gorn M, Suchting S, Poulsom
R and Bicknell R: Magic roundabout is a new member of the
roundabout receptor family that is endothelial specific and
expressed at sites of active angiogenesis. Genomics. 79:547–552.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Huang L, Sun Y, Bai Y, Yang F, Yu W,
Li F, Zhang Q, Wang B, Geng JG and Li X: Slit2 promotes angiogenic
activity via the Robo1-VEGFR2-ERK1/2 pathway in both in vivo and in
vitro studies. Invest Ophthalmol Vis Sci. 56:5210–5217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han S, Kong YC, Sun B, Han QH, Chen Y and
Wang YC: microRNA-218 inhibits oxygen-induced retinal
neovascularization via reducing the expression of roundabout 1.
Chin Med J (Engl). 129:709–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen GX, Wang HY, Liu T, Yang MT, Zhou ZY
and Feng G: Myocardial Slit2/Robo4 expression and impact of
exogenous Slit2 on proliferation and migration of cardiac
microvascular endothelial cells. Zhonghua Xin Xue Guan Bing Za Zhi.
41:1034–1039. 2013.(In Chinese). PubMed/NCBI
|
|
43
|
Yu J, Zhang X, Kuzontkoski PM, Jiang S,
Zhu W, Li DY and Groopman JE: Slit2N and Robo4 regulate
lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun
Signal. 12:252014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han X and Zhang MC: Potential
anti-angiogenic role of Slit2 in corneal neovascularization. Exp
Eye Res. 90:742–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Enomoto S, Mitsui K, Kawamura T, Iwanari
H, Daigo K, Horiuchi K, Minami T, Kodama T and Hamakubo T:
Suppression of Slit2/Robo1 mediated HUVEC migration by Robo4.
Biochem Biophys Res Commun. 469:797–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rama N, Dubrac A, Mathivet T, Ní
Chárthaigh RA, Genet G, Cristofaro B, Pibouin-Fragner L, Ma L,
Eichmann A and Chédotal A: Slit2 signaling through Robo1 and Robo2
is required for retinal neovascularization. Nat Med. 21:483–491.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garrett TA, Van Buul JD and Burridge K:
VEGF-induced Rac1 activation in endothelial cells is regulated by
the guanine nucleotide exchange factor Vav2. Exp Cell Res.
313:3285–3297. 2007. View Article : Google Scholar : PubMed/NCBI
|