Introduction
Levofloxacin belongs to the third generation of
fluoroquinolone antibiotics (1),
demonstrating typical broad-spectrum antibacterial properties. It
possesses antibacterial properties against both Gram-positive and
Gram-negative bacteria. The mechanism of action comprises
inhibition of bacterial type II topoisomerase by interfering with
the processes of DNA replication, transcription, repair and
recombination (2). Therefore, it has
been widely applied in the treatment of various systematic
infections, including respiratory tract and urinary tract
infections (3–5).
Levofloxacin has been selected as a component of the
drug-delivery system in previous studies due to its low molecular
mass (6). Magnetic mesoporous silica
nanoparticles (MSNs) are nanostructured materials that have aroused
widespread interest due to their potential application as a
controlled delivery system for therapeutic molecules (4,7,8). MSNs are characterized by having an
adjustable surface, large pore volume, and controllable pore size.
Different antibiotics and biologically active molecules, including
DNA and RNA, may be loaded into MSNs easily (9). In a previous study by our research
group, it was demonstrated that Levofloxacin could be successfully
loaded into MSNs, and those MSNs were subsequently adsorbed onto
the surface of nano-hydroxyapatite/polyurethane (n-HA/PU), which
was then used as the drug-delivery system. A novel biodegradable,
sustainable antibiotic release composites scaffold was synthesized
(10).
High-performance liquid chromatography (HPLC)
(11,12) and ultraviolet-visible
spectrophotometry (UV-Vis) (13) are
two commonly used methods for detecting Levofloxacin. Furthermore,
several studies have determined the concentration and
pharmacokinetics of Levofloxacin in serum or plasma (14,15).
However, few studies have been published that focused on a
comparison of HPLC with UV-Vis, which is the preferred method for
determination of Levofloxacin. Levofloxacin has been mixed with
novel synthetic composite scaffolds that contained several
components (10). However, where
there is a lot of impurity interference in the samples, this has
the tendency to increase the difficulty of detection (16). The aim of the present study was
therefore to compare HPLC with UV-Vis for determining the level of
Levofloxacin released from mesoporous silica microspheres/n-HA
composite scaffolds in order to verify which of the two methods is
preferable for this drug-delivery system.
Materials and methods
Equipment
Chromatographic analysis was performed with the use
of a Shimadzu liquid chromatograph equipped with a model LC-2010AHT
gradient pump, CBM-20A system controller and Shimadzu CLASS-VP
UV-Visible detector (Shimadzu Corporation, Kyoto, Japan). The
present study also utilized a Sigma D-37520 high-speed centrifuge
(Merck KGaA, Darmstadt, Germany), a Kunshan Shu Mei KQ2200B
ultrasonic cleaner (Kunshan Ultrasonic Instruments Co., Ltd.,
Kunshan, China), a Mettler-Toledo one-hundred-thousandth electronic
balance (Mettler-Toledo GmbH, Greifensee, Switzerland), an Elix10
water purification system (Milli-Q Gradient A10; EMD Millipore,
Billerica, MA, USA), and a UV-2600 UV-Vis spectrophotometer
(Shimadzu Corporation). Sample separation was performed on a Sepax
BR-C18 column (250×4.6 mm; Sepax Technologies, Inc., Newark, DE,
USA), with a particle diameter of 5 µm.
Chemicals
Levofloxacin was purchased from the National
Institutes for Food and Drug Control (cat. no. 130455-201106;
Beijing, China). Ciprofloxacin was purchased from Sigma-Aldrich;
Merck KGaA (cat no. 17850-5G-F) and applied as the internal
standard. Methanol [high-performance liquid chromatography
(HPLC)-grade] and tetrabutylammonium bromide (analytically pure)
were purchased from Merck KGaA.
Chromatography
The chromatographic separations were performed using
a Sepax BR-C18 column (250×4.6 mm; Sepax Technologies, Inc.) with
5-µm particle diameter. The column temperature was set at 40°C, and
the mobile phase was prepared by mixing 0.01 mol/l
KH2PO4, methanol and 0.5 mol/l
tetrabutylammonium hydrogen sulphate (proportions, 75:25:4). This
was delivered at a flow rate of 1 ml/min. The wavelength of the
detector was set at 290 nm, and the injection volume was either 20
µl for associated substances, or 10 µl for assay determination.
Synthesis of novel composite
scaffolds
The specific production method was described in
detail in our previous article (10). Briefly, MSNs were synthesized
according to the protocol reported by Argyo et al (17). A total of 0.2 g
cetyltrimethylammonium bromide (CTAB) was combined with 0.75 ml 2
mol/l sodium hydroxide (NaOH), and the resultant solution was then
added to 100 ml H2O with constant stirring.
Subsequently, 20 ml n-hexane was added into the above solution with
stirring (400 rpm). Iron (II, III) oxide
(Fe3O4) was stabilized with oleic acid. Ethyl
acetate (2 ml) and 0.5 ml tetraethyl orthosilicate were added to
the solution at 70°C for 3 h. CTAB and Fe3O4
separated out as nanocrystals when the pH value of the solution was
decreased. The solid product was obtained by filtering, prior to
drying under vacuum at room temperature, and these were named as
MSNs. Finally, MSNs were resolved in suspension and Levofloxacin
(1,500 µg/ml) was loaded by electrostatic attraction.
The n-HA/PU composite porous scaffolds were
successfully synthesized using the in situ foaming method
(13,18). Initially, 30 g castor oil was
combined with 40 g n-HA particles in a nitrogen atmosphere and
thorough stirring. Subsequently, 30 g isophorone diisocyanate was
added to the suspension at 70°C for 3 h in order to obtain the
prepolymer, and 1 ml 1,4-butanediol was used to extend the
prepolymer. During this step, the n-HA/PU composite scaffolds were
actually obtained. The n-HA/PU was cut into small cuboids (10×6×6
mm) and immersed into the Levofloxacin-MSN suspension at 25°C for
30 min, prior to drying in a vacuum oven at 40°C. Following
completion of the above steps, 1 mg Levofloxacin-MSN-n-HA/PU (Lev@
MSN/n-HA/PU) was successfully synthesized.
Preparation of the standard
solution
Levofloxacin (30.00 mg) was weighed precisely and
dissolved in simulated body fluid (SBF; Hangzhou Haoxin Biotech,
Co., Ltd., Hangzhou, China). Subsequently, the Levofloxacin
solution was transferred to a 10 ml volumetric flask and used to
obtain a standard solution (3 mg/ml Levofloxacin). The standard
solution was diluted into 14 different concentration gradients:
300, 200, 100, 50, 25, 10, 5, 2.5, 1, 0.5, 0.25, 0.1, 0.05 and 0.01
µg/ml. Ciprofloxacin (25.00 mg) was also weighed accurately and
dissolved in methanol. The concentration of ciprofloxacin was
adjusted to 500 µg/ml, and this was used as the internal
standard.
Wavelength selection of UV-Vis
The standard solution of Levofloxacin was prepared
as described above. High (50 µg/ml), medium (25 µg/ml), and low (5
µg/ml) concentrations were selected. After the instrument was
calibrated to zero, the maximum absorption wavelength was
determined by scanning the standard levofloxacin solutions at
200–400 nm.
Establishment of the standard curve by
HPLC
A total of 14 different concentrations of the
Levofloxacin standard solutions (10 µl) were respectively added
into 100 µl blank SBF at room temperature. Subsequently, 10 µl
ciprofloxacin was added into the working solution as an internal
standard. The working solution was vortex-mixed for 5 min, and 800
µl dichloromethane was subsequently added. The solution was further
vortex-mixed for 5 min, and centrifuged at 7,155 × g for 5 min at
25°C. An aliquot (750 µl) of supernatant was extracted, and dried
with nitrogen in a 50°C water bath. A total of 100 µl of the mobile
phase was added into the tube for redissolution. The supernatant
was mixed in an ultrasonicator, vortex-mixed and centrifuged at
16,099 × g for 10 min at 25°C. An aliquot (10 µl) of the
supernatant was injected into a Sepax BR-C18 column (250×4.6 mm;
Sepax Technologies, Inc., Newark, DE, USA) with a constant flow
rate of 1 ml/min. Each concentration of the standard product was
analyzed 3 times. The ratio of the sum-of-peak areas of
Levofloxacin to ciprofloxacin was set as the vertical axis
(y) in the standard curve, while the concentration of
Levofloxacin was set as the horizontal axis (x). Linear
regression analysis was performed using the weighted least-square
method (W=1/C2).
Establishment of standard curve by
UV-Vis
Blank SBF (3 ml) was scanned using UV-Vis, and the
instrument was calibrated to zero. Different concentrations (0.5,
2.5, 5, 12.5, 25 and 50 µg/ml) of the standard product were scanned
by UV-Vis. The absorbance value was set as the vertical axis
(y) in the standard curve, while the concentration of
Levofloxacin was set as the horizontal axis (x). Linear
regression analysis was performed using the weighted least-square
method (W=1/C2).
Recovery of the two methods
Three different concentration levels of Levofloxacin
(low, 5 µg/ml; middle, 25 µg/ml; high, 50 µg/ml) were prepared,
respectively. As quality control samples, these were processed and
tested using HPLC in accordance with the method described above.
The samples were also investigated by UV-Vis. The ratio between the
measured concentration and the theoretical concentration was
regarded as the method recovery rate.
Real sample determination
All the 1 mg Lev@ MSNs/n-HA/PU composite porous
scaffolds (n=10) were sterilized with γ-cobalt 60 radiation. The
scaffolds were subsequently immersed in EP tubes containing 3 ml
modified SBF and incubated for 24 h at 37°. The scaffolds were
subsequently removed. The leach liquor was placed in a refrigerator
at −20°C prior to testing. Each sample was determined 3 times.
Sample processing for the UV-Vis method was performed as follows:
The leach liquor was diluted 100 times with SBF, and 3 ml of the
resultant solution was placed in a quartz cell, and scanned at a
specific wavelength. The absorbance values were then recorded, and
a standard curve was then used in order to calculate the antibiotic
concentrations.
Statistical analysis
SPSS version 19.0 statistical software (IBM Corp.,
Armonk, NY, USA) was used for statistical analysis. All
quantitative data are presented as the mean ± standard deviation.
Linear regression was used to establish the standard curve of the
HPLC and UV-Vis methods, respectively. Paired Student's t-test was
used to compare the mean concentration obtained from the HPLC and
UV-Vis methods. Pearson's correlation analysis was used to analyze
the concentration detected by HPLC and UV-Vis methods. The relative
standard deviation (RSD) was calculated according to the following
equation: Standard deviation/calculated arithmetic mean ×100%.
P<0.05 was considered to indicate a statistically significant
value.
Results
Structural characterization of novel
composite scaffolds
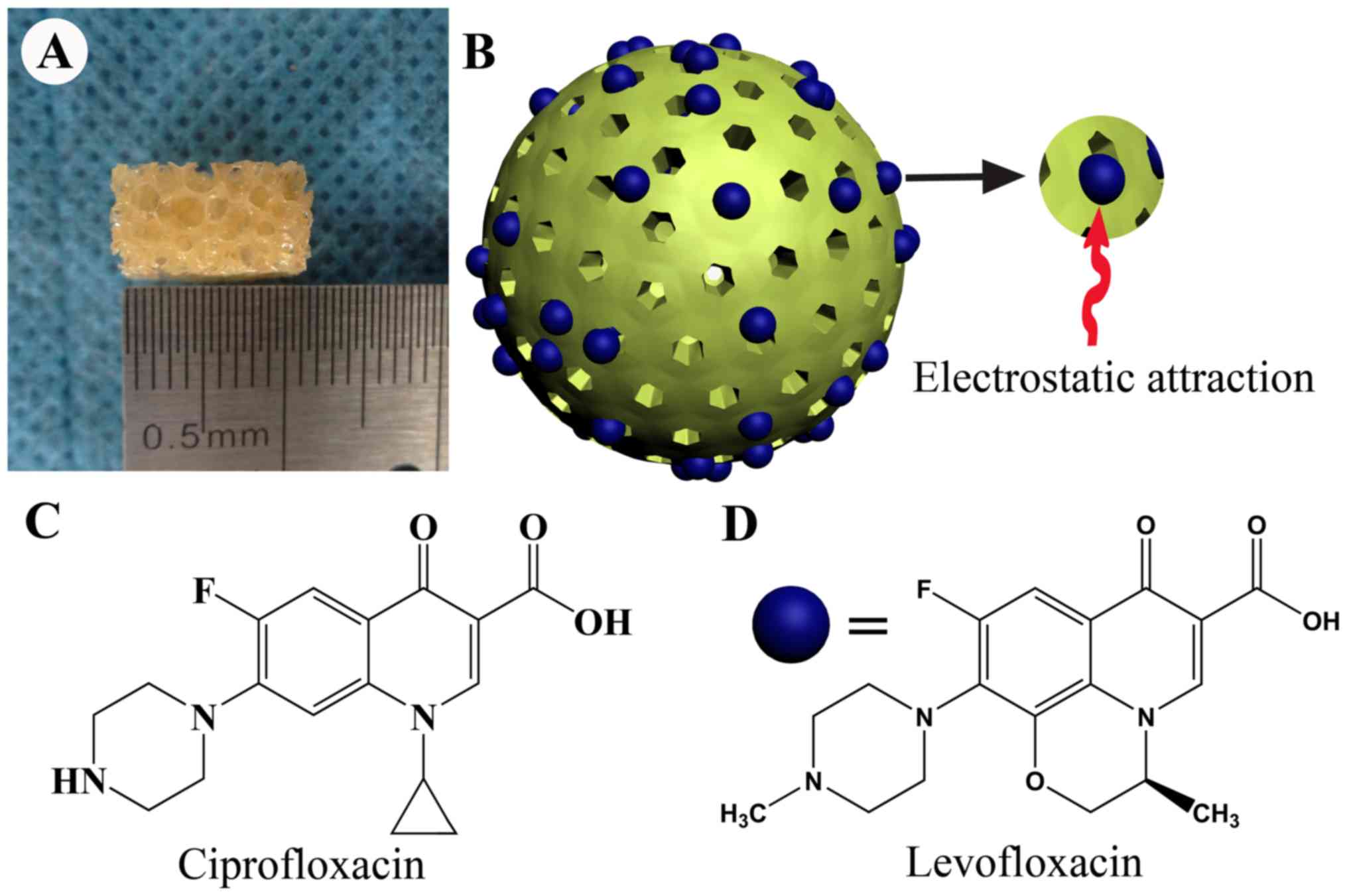
The novel biodegradable composite scaffolds
containing a large quantity of MSNs were successfully constructed,
and the n-HA/PU was cut into small cuboids (size, 10×6×6 mm). A
large number of pores were observed in the material. The appearance
of the n-HA/PU composite porous scaffolds is presented in Fig. 1A, and a three-dimensional structural
diagram of newly fabricated mesoporous silica microspheres is
presented in Fig. 1B. Numerous
perforated channels were observed above the mesoporous silica
microspheres. A large quantity of Levofloxacin was attached to the
surface of the microspheres through electrostatic attraction.
Ciprofloxacin was set as the internal standard in the process of
determination; the molecular structural formula of Ciprofloxacin is
presented in Fig. 1C. Likewise, the
molecular structural formula of Levofloxacin is presented in
Fig. 1D.
Specificity of HPLC
The blank SBF containing no antibiotics was regarded
as the blank control. Following its examination via HPLC, no clear
indication of impurity interference could be identified on the
chromatograms (Fig. 2A). The
standard solution of Levofloxacin and ciprofloxacin was examined by
HPLC, and the results demonstrated that the retention times of
Levofloxacin and ciprofloxacin were ~4.84 and 6.74 min,
respectively (Fig. 2B). These
results demonstrated that the adjusted mobile phase was optimized
for the detection of these composite scaffolds, as it presented the
shortest retention time, an improved shape to the peaks, and good
resolution of Levofloxacin and ciprofloxacin.
Wavelength of UV-Vis
SBF was used as the blank control group, and this
was established as the experimental group following addition of the
standard solution of Levofloxacin. The performance of the blank
control group and the experimental group are presented in Fig. 2C and D. Subsequently, the samples
were detected by UV-Vis. It was revealed that Levofloxacin at
different concentrations had a maximum absorption wavelength of
288.4 nm. Therefore, 288.4 nm was selected as the wavelength for
UV-Vis detection of the samples.
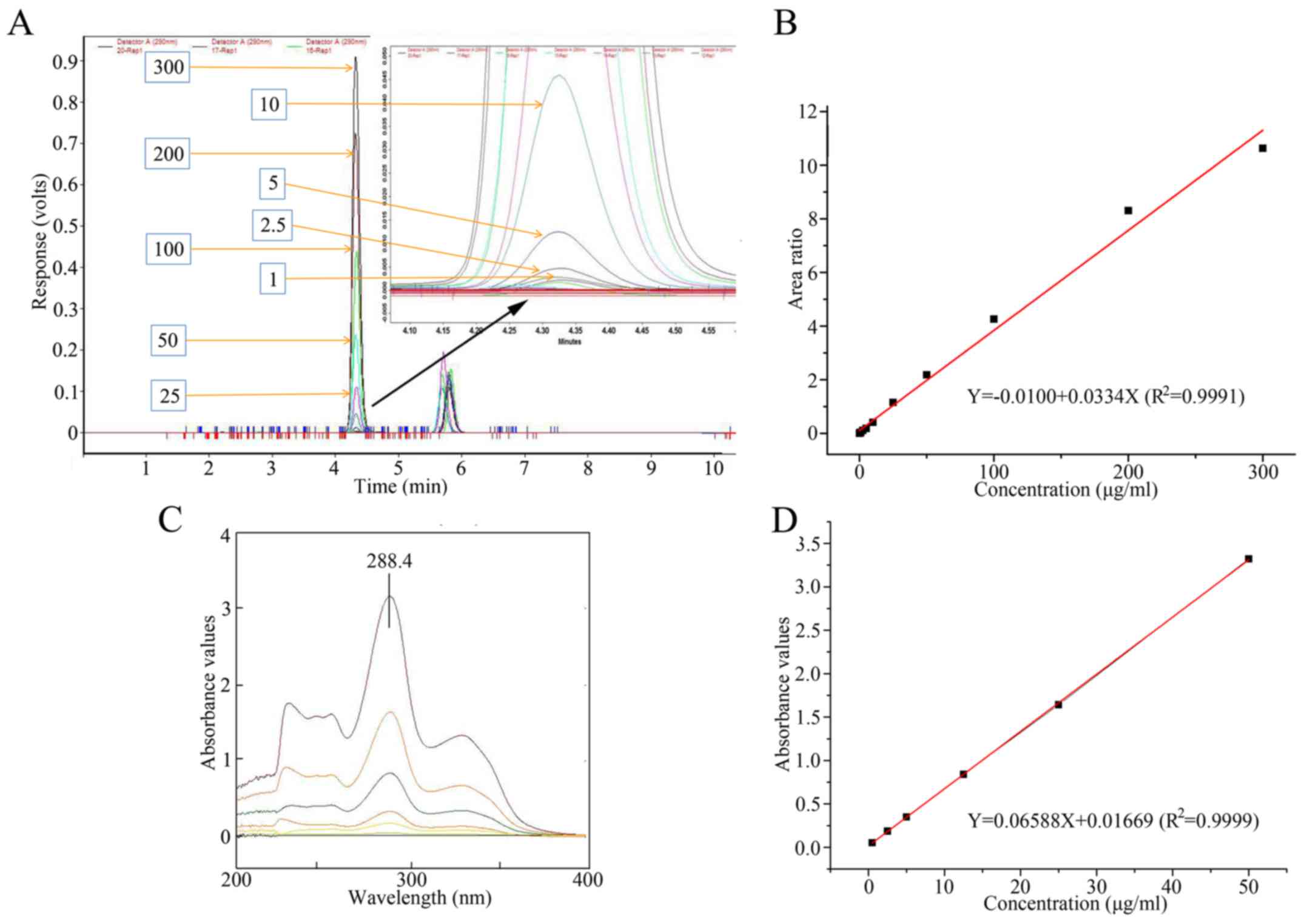
Linearity of the standard curve
The ratio of the sum-of-peak areas of each of
Levofloxacin and ciprofloxacin was set as the vertical axis
(y) in the standard curve according to the HPLC method. The
concentration of Levofloxacin was used as the horizontal axis
(x). Linear regression was calculated using the weighted
least square method (W=1/C2). The linear concentration
range was identified to be 0.05–300 µg/ml for Levofloxacin. The
regression equation of HPLC yielded values of
y=0.033x+0.010, with R2=0.9991. The
chromatograms of Levofloxacin at different concentrations, and
ciprofloxacin are presented in Fig.
3A. The standard curve derived from the HPLC method is
presented in Fig. 3B. The absorbance
value recorded by UV-Vis was set as the vertical axis (y) in
the standard curve according to the UV-Vis method. The
concentration of Levofloxacin was set along the horizontal axis
(x). The regression equation for the UV-Vis method was
identified to be y=0.065x+0.017, with
R2=0.9999. The linear concentration range was
identified to be 2.5–50 µg/ml for Levofloxacin. The wavelength of
Levofloxacin with different concentrations is presented in Fig. 3C, and the representative calibration
curve for UV-Vis is presented in Fig.
3D.
Recovery of the two methods
The low, medium, and high (5, 25 and 50 µg/ml)
concentrations of the three standard solutions were determined by
HPLC. The recovery rates were 96.37±0.50, 110.96±0.23 and
104.79±0.06%, respectively. The RSD values were revealed to be
0.52, 0.21 and 0.08%, respectively. The same three concentrations
of low, medium, and high (5, 25 and 50 µg/ml) standard solutions
were also checked by UV-Vis. The recovery rates were demonstrated
to be 96.00±2.00, 99.50±0.00, and 98.67±0.06%, respectively. The
associated RSD values were 2.08, 0.00 and 0.06%, respectively. All
the results of the recovery analysis met the requirement of the
Chinese pharmacopoeia, i.e. 80–120%. The measured concentrations
and recovery of the two methods are presented in Table I.
 | Table I.Measured concentration and recovery
using the two methods. |
Table I.
Measured concentration and recovery
using the two methods.
| Method | Real concentration
(µg/ml) | Measured
concentration (µg/ml) | Recovery (%) | Mean recovery
(%) | RSD (%) |
|---|
| HPLC | 5 | 4.81 | 96.3 | 96.37±0.50 | 0.52 |
|
|
| 4.84 | 96.9 |
|
|
|
|
| 4.79 | 95.9 |
|
|
|
| 25 | 27.67 | 110.69 | 110.96±0.23 | 0.21 |
|
|
| 27.76 | 111.09 |
|
|
|
|
| 27.77 | 111.1 |
|
|
|
| 50 | 52.35 | 104.7 | 104.79±0.06 | 0.08 |
|
|
| 52.42 | 104.8 |
|
|
|
|
| 52.41 | 104.8 |
|
|
| UV-Vis | 5 | 4.83 | 96.0 | 96.00±2.00 | 2.08 |
|
|
| 4.72 | 94.0 |
|
|
|
|
| 4.90 | 98.0 |
|
|
|
| 25 | 24.87 | 99.5 | 99.50±0.00 | 0.00 |
|
|
| 24.87 | 99.5 |
|
|
|
|
| 24.87 | 99.5 |
|
|
|
| 50 | 49.34 | 98.7 | 98.67±0.06 | 0.06 |
|
|
| 49.34 | 98.7 |
|
|
|
|
| 49.31 | 98.6 |
|
|
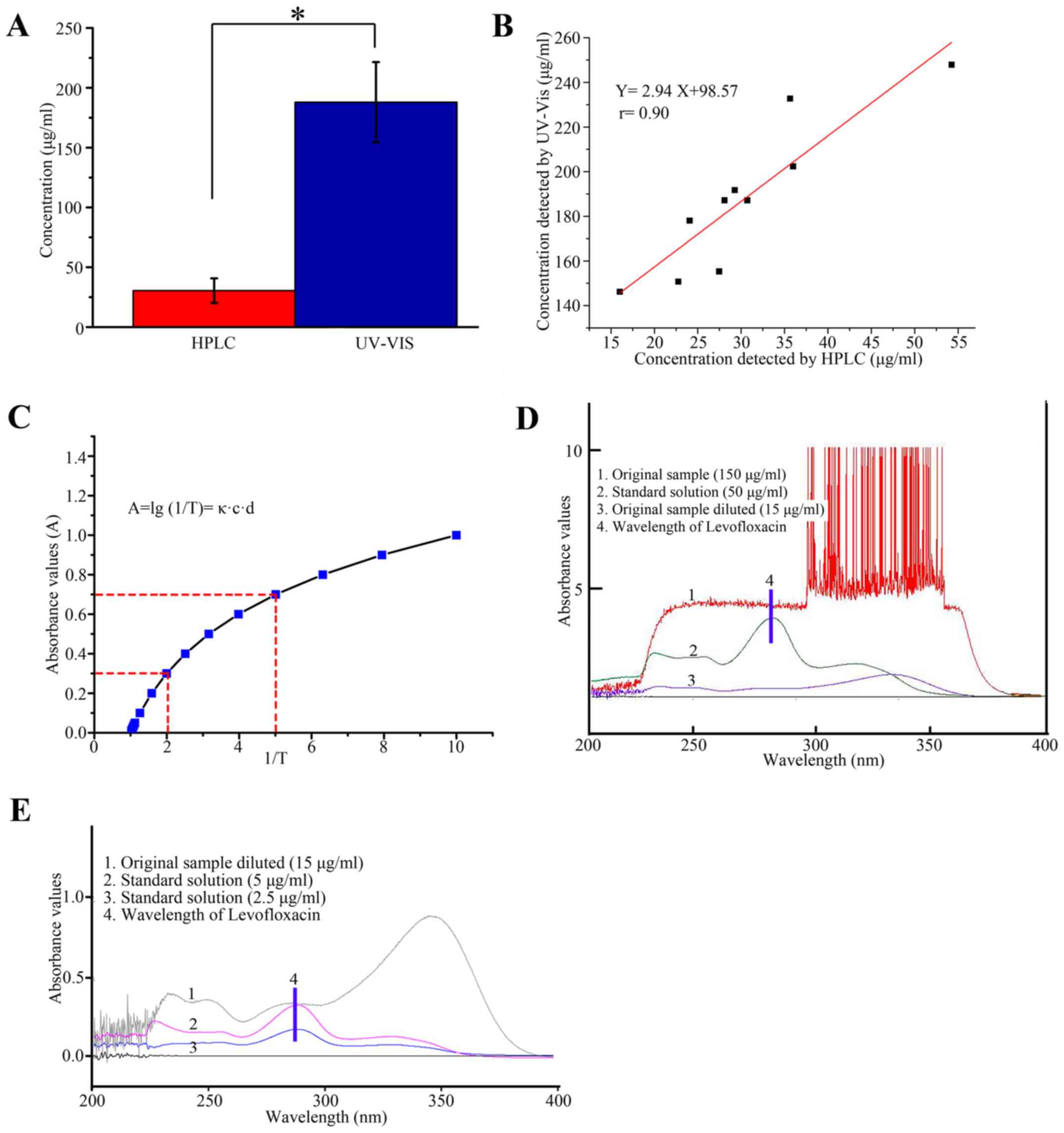
Comparison of HPLC and UV-Vis
The mean concentration of 10 samples measured
according to the UV-Vis method was 187.93±33.52 µg/ml, whereas the
mean concentration of 10 samples measured using the HPLC method was
30.43±10.27 µg/ml. Paired t-tests were used to compare the results
of the two methods. A statistically significant difference was
identified between the two methods (t=20.17, P<0.001;
Fig. 4A). The concentration of
Levofloxacin measured by UV-Vis was set as the vertical axis
(y), while the concentration of Levofloxacin measured by
HPLC was set as the vertical axis (x). These two results
were used for correlation analysis. The linear equation was
determined to be y=2.94x+98.57, and the correlation
coefficient (r) was identified to be 0.90 (P<0.001; Fig. 4B). Characteristic parameters
according to Lambert-Beer's law were examined. The absorbance value
(A) has a logarithmic function with the reciprocal of transmittance
(1/T) number (Fig. 4C). The peak
wave of Levofloxacin could not be seen when the original sample was
detected (Fig. 4D), however, after
the sample was diluted the peak wave of Levofloxacin was observed
(Fig. 4E).
Discussion
In our previous study, scaffolds based on novel
composites were successfully synthesized, consisting of nano-HA, PU
and MSNs. Levofloxacin was encapsulated in MSNs via electrostatic
attraction (10). That publication
demonstrated that these novel composite scaffolds presented an
improved ability for inhibition of the inflammation reaction, and
for treating chronic osteomyelitis with bone defects, compared with
other groups. These novel composite scaffolds may have
applicability in terms of their use as a local antibiotic delivery
system for the treatment of chronic osteomyelitis and bone
regeneration.
For the treatment of infectious diseases, the
concentration of local drugs is required to reach the effective
antibacterial concentration. In order to evaluate sustained release
characteristics of these novel composite scaffolds as a local
antibiotic delivery system in vitro and in vivo, it
is necessary to optimize the detection method for accurate
determination of the Levofloxacin concentration in local infectious
soft tissue. Levofloxacin belongs to the third generation of
fluoroquinolone antibiotics, demonstrating good antibacterial
ability against Gram-positive and Gram-negative bacteria (19). The molecular mass of Levofloxacin is
low; this therefore enables the compound to combine with MSNs
through electrostatic attraction (20).
When the standard solutions of Levofloxacin were
assessed, the two methods of HPLC and UV-Vis presented higher
recovery rates and met the requirements of the Chinese
pharmacopoeia. However, when the sample of novel composite
scaffolds was measured by UV-Vis, the spectrum changed
significantly compared with the standard products. The mean
concentration (30.43±10.27 µg/ml) of the samples determined by HPLC
was significantly lower compared with the mean concentrations
(187.93±33.52 µg/ml) of the samples determined by UV-Vis. It is
considered that, as far as impurity interference absorbance of
Levofloxacin is concerned, impurities can interfere with the
accuracy of the UV-Vis measurements. The working principle of
UV-Vis follows the Lambert-Beer law (21). The absorbance value (A) has a
logarithmic function with the reciprocal of transmittance (1/T)
number, so the absorbance value and concentration are linear only
within a relatively small range: 0.3–0.7 (22). This principle may explain why the
UV-Vis method has a narrower linear range than HPLC. The range of
linearity for UV-Vis was identified to be 2.5–50 µg/ml for
Levofloxacin. The range of linearity for HPLC was identified to be
0.05–300 µg/ml for Levofloxacin. If the concentration of samples
was too high (>50 µg/ml in the present study), it is imprecise
for measuring the concentration of levofloxacin by UV-Vis.
Furthermore, it is considered that the multiple impurities of
samples interference absorbance of Levofloxacin. The multiple
impurities increase the absorbance value. The recovery rate of the
UV-Vis method was determined to be between 96 and 98%. When
determining the recovery rate of the two methods, three different
concentration levels of standard Levofloxacin were prepared
respectively. These samples didn't contain impunities. However,
when determining the concentration of levofloxacin released from
the composite scaffolds, the composite scaffolds were initially
immersed in SBF, the samples contained the multiple impurities
which interference absorbance of Levofloxacin. Although UV-Vis
revealed a satisfactory recovery rate, the concentration of
Levofloxacin calculated was therefore not accurate. The
concentration of the identical sample detected by the UV-Vis method
was significantly higher compared with that of the HPLC method. The
results of the two methods exhibited a positive correlation.
Impurities in the sample may increase the absorbance of UV-Vis and
the linear range of UV-Vis is narrow. With a difference in drug
loading, a large difference ensues in terms of the amount of
drug-sustained release, and this would fall outside the linear
range of UV-Vis. These factors thereby result in an inability of
the UV-Vis method to accurately measure the drug concentration.
The wavelength of Levofloxacin reached a maximum at
288.4 nm. First, the undiluted solution was assessed directly by
UV-Vis, and due to impurity interference and the high drug
concentration employed, which exceeded the response absorbance of
the UV-Vis method, the wavelength of Levofloxacin disappeared. The
wavelength of Levofloxacin reappeared following dilution of the
sample. Although the absorbance value lay within the linear range,
the impurity resulted in an increase in the absorbance value, and
the finally calculated concentration of Levofloxacin was higher
than the real concentration. Therefore, the UV-Vis method could not
meet all the requirements for the measurement.
In the preliminary experiments for the present
study, the mobile phase of the HPLC method comprised a 0.01 mol/l
potassium dihydrogen phosphate-methanol solution. The HPLC
chromatograms revealed that the chromatographic peak of
Levofloxacin was not completely separated from the chromatographic
peak associated with impurity, and furthermore, the chromatographic
peak was irregular. The chromatographic performance was
significantly improved following the addition of 0.5 mol/l
tetrabutylammonium bromide into the mobile phase (23,24).
Finally, the mobile phase consisting of 0.01 mol/l potassium
dihydrogen phosphate, methanol and 0.5 mol/l tetrabutylammonium
bromide (75:25:4) met with the requirements, and the
chromatographic peaks of Levofloxacin and ciprofloxacin were
separated from impurities. The chemical properties of ciprofloxacin
are similar to those of Levofloxacin, and so the former was
regarded as the internal standard (25). The two compounds are highly soluble
in organic solvents. Therefore, dichloromethane was chosen as the
extractant. In order to ensure sufficient extraction of the
antibiotic in the sample, the ratio of sample to extractant was set
as 1:8 (26). The recoveries of the
HPLC method ranged from 96–105%, reaching the requirements of the
Chinese pharmacopoeia, i.e. 80–120%.
HPLC and UV-Vis are two commonly used methods for
detecting Levofloxacin. The advantage of the UV-Vis method is that
it is simple to operate, and is relatively inexpensive. A number of
previous studies used UV-Vis to investigate the sustained release
properties of drugs loaded on the composite composites (27,28).
However, a number of disadvantages also exist: Impurities in the
sample could not be ruled out, and these may increase the
calculated concentration; furthermore, the linear range is narrow.
Therefore, it is not clear whether the studies using UV-Vis to
measure drug concentrations are reliable. An aim of the present
study was to investigate which method is preferable for this drug
delivery system. Another purpose of the present study was to
explain why the concentration of Levofloxacin detected by these two
methods was different.
In conclusion, in the present study, the
concentration of Levofloxacin was measured by HPLC and UV-Vis. The
aim of the present study was to compare the techniques of HPLC and
UV-Vis. The findings demonstrated that although UV-Vis is simple to
operate, and the expenses are low, it is not accurate to measure
the concentration of drugs loaded on the biodegradable composite
composites. The present study provides guidance on which methods
should be selected for investigating the sustained release
properties of drugs in tissue engineering. The HPLC method could be
used to separate Levofloxacin from various impurities in the
chromatographic column, which eliminated the interference of
impurities with Levofloxacin. As a method, HPLC exhibited the
advantages of high precision and high recovery. Therefore, HPLC is
the preferred method to evaluate sustained release characteristics
of Levofloxacin released from mesoporous silica microspheres/n-HA
composite scaffolds.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
Key Research and Development Program of Shandong Province (grant
no. 2017GSF218065) and Natural Science Foundation of Shandong
Province (grant no. ZR201702140118).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and XZ designed the experiments. QW, GW and SX
performed the experiments. YZ analyzed and interpreted the data. QW
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HPLC
|
high-performance liquid
chromatography
|
|
UV-Vis
|
ultraviolet-visible
spectrophotometry
|
|
MSN
|
magnetic mesoporous silica
nanoparticle
|
|
n-HA
|
nano-hydroxyapatite
|
|
PU
|
polyurethane
|
References
|
1
|
Das A, Mukherjee J, Dey G, Sarkar AK,
Sahoo BK, Chakrabarty US, Nandi U and Pal TK: Bioequivalence study
of levofloxacin tablets in healthy Indian volunteers using HPLC.
Arzneimittelforschung. 61:61–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wimer SM, Schoonover L and Garrison MW:
Levofloxacin: A therapeutic review. Clin Ther. 20:1049–1070. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carral N, Lukas JC, Oteo I and Suarez E:
Impact of poor compliance with levofloxacin and moxifloxacin on
respiratory tract infection antimicrobial efficacy: A
pharmacokinetic/pharmacodynamic simulation study. Int J Antimicrob
Agents. 45:79–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun H, Wang H and Ge X: Simultaneous
determination of the combined drugs of ceftriaxone sodium,
metronidazole, and levofloxacin in human urine by high-performance
liquid chromatography. J Clin Lab Anal. 26:486–492. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SS, Ratliff PD and Judd WR:
Retrospective review of ceftriaxone versus levofloxacin for
treatment of E.coli urinary tract infections. Int J Clin Pharm.
40:143–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cicuéndsez M, Izquierdo-Barba I, Portolés
MT and Vallet-Regí M: Biocompatibility and levofloxacin delivery of
mesoporous materials. Eur J Pharm Biopharm. 84:115–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croissant JG, Fatieiev Y, Almalik A and
Khashab NM: Mesoporous silica and organosilica nanoparticles:
Physical chemistry, biosafety, delivery strategies, and biomedical
applications. Adv Healthc Mater. 7:2018.doi:
10.1002/adhm.201700831. View Article : Google Scholar
|
|
8
|
Shi S, Chen F and Cai W: Biomedical
applications of functionalized hollow mesoporous silica
nanoparticles: Focusing on molecular imaging. Nanomedicine (Lond).
8:2027–2039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guisasola E, Asín L, Beola L, de la Fuente
JM, Baeza A and Vallet-Regí M: Beyond traditional hyperthermia: In
vivo cancer treatment with magnetic-responsive mesoporous silica
nanocarriers. ACS App Mater Interfaces. 10:12518–12525. 2018.
View Article : Google Scholar
|
|
10
|
Wang Q, Chen C, Liu W, He X, Zhou N, Zhang
D, Gu H, Li J, Jiang J and Huang W: Levofloxacin loaded mesoporous
silica microspheres/nano-hydroxyapatite/polyurethane composite
scaffold for the treatment of chronic osteomyelitis with bone
defects. Sci Rep. 7:418082017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szerkus O, Jacyna J, Gibas A, Sieczkowski
M, Siluk D, Matuszewski M, Kaliszan R and Markuszewski MJ: Robust
HPLC-MS/MS method for levofloxacin and ciprofloxacin determination
in human prostate tissue. J Pharm Biomed Anal. 132:173–183. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao XX, Yao GC, Guo N, An F and Guo XJ: A
simple and rapid high performance liquid chromatography method to
determine levofloxacin in human plasma and its use in a
bioequivalence study. Drug Discov Ther. 1:136–140. 2007.PubMed/NCBI
|
|
13
|
Netz DJ, Sepulveda P, Pandolfelli VC,
Spadaro AC, Alencastre JB, Bentley MV and Marchetti JM: Potential
use of gelcasting hydroxyapatite porous ceramic as an implantable
drug delivery system. Int J Pharm. 213:117–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siewert S: Validation of a levofloxacin
HPLC assay in plasma and dialysate for pharmacokinetic studies. J
Pharm Biomed Anal. 41:1360–1362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arayne MS, Sultana N and Siddiqui FA:
Optimization of levofloxacin analysis by RP-HPLC using multivariate
calibration technique. Pak J Pharm Sci. 20:100–106. 2007.PubMed/NCBI
|
|
16
|
Lalitha Devi M and Chandrasekhar KB: A
validated stability-indicating RP-HPLC method for levofloxacin in
the presence of degradation products, its process related
impurities and identification of oxidative degradant. J Pharm
Biomed Anal. 50:710–717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Argyo C, Weiss V, Bräuchle C and Bein T:
Multifunctional mesoporous silica nanoparticles as a universal
platform for drug delivery. Chemistry Materials. 26:435–451. 2014.
View Article : Google Scholar
|
|
18
|
Sepulveda P, Binner JG, Rogero SO, Higa OZ
and Bressiani JC: Production of porous hydroxyapatite by the
gel-casting of foams and cytotoxic evaluation. J Biomed Mater Res.
50:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghaffar KA, Hussein WM, Khalil ZG, Capon
RJ, Skwarczynski M and Toth I: Levofloxacin and indolicidin for
combination antimicrobial therapy. Curr Drug Deliv. 12:108–114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guenter SG, Iven H, Boos C, Bruch HP and
Muhl E: Pharmacokinetics of levofloxacin during continuous
venovenous hemodiafiltration and continuous venovenous
hemofiltration in critically ill patients. Pharmacotherapy.
22:175–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Langergraber G, Fleischmann N and
Hofstädter F: A multivariate calibration procedure for UV/VIS
spectrometric quantification of organic matter and nitrate in
wastewater. Water Sci Technol. 47:63–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagaraja P, Al-Tayar NG, Shivakumar A,
Shrestha AK and Gowda AK: A simple and sensitive spectrophotometric
method for the determination of trace amounts of nitrite in
environmental and biological samples using
4-amino-5-hydroxynaphthalene-2,7-disulphonic acid monosodium salt.
Spectrochim Acta A Mol Biomol Spectrosc. 75:1411–1416. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chamseddin C and Jira TH: Comparison of
the chromatographic behavior of levofloxacin, ciprofloxacin and
moxifloxacin on various HPLC phases. Pharmazie. 66:244–248.
2011.PubMed/NCBI
|
|
24
|
Nguyen HA, Grellet J, Ba BB, Quentin C and
Saux MC: Simultaneous determination of levofloxacin, gatifloxacin
and moxifloxacin in serum by liquid chromatography with column
switching. J Chromatogr B Analyt Technol Biomed Life Sci.
810:77–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watabe S, Yokoyama Y, Nakazawa K,
Shinozaki K, Hiraoka R, Takeshita K and Suzuki Y: Simultaneous
measurement of pazufloxacin, ciprofloxacin, and levofloxacin in
human serum by high-performance liquid chromatography with
fluorescence detection. J Chromatogr B Analyt Technol Biomed Life
Sci. 878:1555–1561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji HY, Jeong DW, Kim YH, Kim HH, Sohn DR
and Lee HS: Hydrophilic interaction liquid chromatography-tandem
mass spectrometry for the determination of levofloxacin in human
plasma. J Pharm Biomed Anal. 41:622–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borcan LC, Dudas Z, Len A, Fuzi J, Borcan
F and Tomescu MC: Synthesis and characterization of a polyurethane
carrier used for a prolonged transmembrane transfer of a chili
pepper extract. Int J Nanomedicine. 6:7155–7166. 2018. View Article : Google Scholar
|
|
28
|
Rahimi S, Khoee S and Ghandi M:
Development of photo and pH dual crosslinked coumarin-containing
chitosan nanoparticles for controlled drug release. Carbohydr
Polym. 201:236–245. 2018. View Article : Google Scholar : PubMed/NCBI
|