Introduction
Chronic urticaria (CU), more commonly referred to as
hives, is a frequently occurring condition that may persist for
>6 weeks. CU is subdivided into chronic autoimmune urticaria
(CAU), chronic spontaneous urticaria (CSU) and physical urticaria
(PU), and CSU accounts for 35% of CU patients (1). The mechanisms of CSU are complex and
may be triggered by drugs, physical stimuli, as part of
inflammatory or inherited diseases, or may be idiopathic in nature.
Over half of all CSU cases are thought to involve autoimmune
mechanisms (2). The autologous serum
skin test (ASST) provides an in vivo assay for diagnosing
autoimmune urticaria. The ASST procedure consists of collecting an
autologous serum sample from the CU patient, followed by injection
of this sample into an area of normal skin. A positive response is
indicated by the appearance of an erythematous papule within 30 min
following injection (3).
The ASST serves as an effective clinical screening
tool and has become the established method for the detection of
functional circulating auto-antibodies in patients with CU. The
negative predictive value (NPV) of the ASST has been reported to be
82.5±14% (4). This means that in CU
patients with a negative response to the ASST, no functional
circulating auto-antibodies were present in their serum. However, a
positive ASST response may occur in patients with allergic diseases
and even in healthy controls. Therefore, to confirm the presence of
an autoimmune disorders, a quantitative analysis is required
(5). It has been reported that in
cases with positive ASST responses, a higher urticaria activity
score (UAS), longer disease durations, lower scores on quality of
life questionnaires and increased potentials for accompanying
angioedema were present (6,7).
CU is a benign disease, has an autoimmune basis in
40% of cases (8) and is more
prevalent in females. Immunoglobulin (IgE) has an indispensable
role in the occurrence of CSU (9,10), with
autoantibodies targeting high-affinity IgE receptors (FcεRI) or IgE
in patients with CSU (11). Thyroid
disease is the most commonly reported autoimmune condition in
patients with CSU. CSU patients with coexisting thyroid
autoimmunity tend to have a more severe and prolonged course of
their urticaria than those without thyroid autoimmunity. A recent
study has indicated that 9.8% of CU patients had hypothyroidism,
compared with 0.6% in the control group (12). CU and thyroid disease may be
interlinked, and the latter may promote the occurrence of CU
(13). Previous studies have
produced controversial results about the associations between
positive ASST responses and the clinical features of CSU.
Therefore, the current meta-analysis was undertaken to clarify the
association between ASST and CSU.
Materials and methods
Literature search
The PubMed, Embase, Medline, Ovid, Cochrane Library,
China National Knowledge Infrastructure, China Biology Medicine and
Wangfang databases, as well as the VIP Database for Chinese
Technical Periodicals were searched to identify relevant studies
involving ASST and clinical features of CSU. This search included
the period from inception of the database until March 2018. The
search strategy combined the following terms: ‘Autologous serum
skin test’ and ‘chronic spontaneous urticaria’. There was no
restriction regarding language or the type of article. An
additional manual search was performed by screening the references
listed in key publications retrieved in this search.
Inclusion and exclusion criteria
An overall literature search was performed and
relevant studies were screened independently by two reviewers.
Eligible studies were selected based on the following criteria: i)
Study design: Prospective observational study. ii) Patients with
clinically defined CU. iii) Information on responses to the ASST.
iv) Information on at least one of the following parameters:
Average age, duration of disease, UAS, angioedema, anti-thyroid
antibodies, total serum IgE, erythrocyte sedimentation rate and
allergic rhinitis. The exclusion criteria were as follows: i)
Experiments on animal models; ii) cases lacking a definitive
diagnosis of CU; iii) intervention trials; iv) reports lacking
relevant/sufficient data; v) duplicate publications.
Data extraction
Relevant data were extracted by two reviewers
independently. Information included in the forms prepared by these
reviewers comprised the following: First author's name, publication
year, number of patients, mean age, duration of disease, results of
ASST, UAS, angioedema, anti-thyroid antibodies, total serum IgE and
allergic rhinitis.
Assessment of study quality
Quality assessment of included studies was
independently performed and crosschecked by two reviewers using the
Newcastle-Ottawa scale (NOS), which assesses bias on a scale of up
to nine stars; >6 stars on the NOS were regarded as indicative
of a high quality.
Statistical analysis
The meta-analysis was performed using Review Manager
5.2 (the Cochrane Institute, London, UK). The inverse-variance test
was applied for continuous variables and the Mantel-Haenszel test
for examination of dichotomous variables. The weighted mean
difference (WMD) was used for continuous variables across studies
that were measured on the same scale. Dichotomous variables were
assessed by calculating the odds ratio (OR). All data were
expressed as the WMD or OR along with their associated 95%
confidence intervals (CI). Heterogeneity between studies was tested
by using I2 tests. P<0.1 or I2>50% was
considered to indicate a high degree of heterogeneity between
studies. If a significant heterogeneity was present (P<0.1), the
random effects model was selected for heterogeneous outcomes
(P<0.05 or I2≥50%), otherwise, the fixed effects
model was performed for homogeneous outcomes (P≥0.05 and
I2<50%). P<0.05 was considered to represent
statistically significant differences.
A sensitivity analysis was performed by removing
studies one at a time to confirm the robustness of the results.
Finally, publication bias in the analysis was determined using a
funnel plot and Egger's regression test.
Results
Study characteristics
A total of 1,629 relevant studies were retrieved.
After removal of duplicates by title, 851 articles were further
screened. Following a careful review of these studies, the full
text of 39 studies were assessed. Finally, 16 articles met the
inclusion criteria and were included in the systematic review
(6,7,14–27). The
selection process for these studies is summarized in Fig. 1. A total of 2,554 patients, including
1,284 with positive and 1,270 with negative ASST results were
assessed. The characteristics of these patients are presented in
Table I. The publication year ranged
from 2005 to 2017. Of note, the search of all databases for
eligible articles revealed that the region of all studies retrieved
was Asia, including China, Thailand, Korea, India, Taiwan and
Japan.
 | Table I.Characteristic and methodological
quality of included studies. |
Table I.
Characteristic and methodological
quality of included studies.
|
|
|
|
|
| No. of
subjects |
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author | Year | Area | No. of samples | Group | Patients | Controls | Age (years) | Duration
(months) | UAS | IgE (IU/ml) | Anti-thyroid | Angioedema | No. of males | NOS score | (Refs.) |
|---|
| Alpay A | 2013 | Japan | 50 | N1 | 31 | 5 |
| 35.64±67.20 |
|
| 5 (31) | 16 (31) |
| 8 | (19) |
|
|
|
|
| N2 | 19 | 45 |
| 36.24±50.40 |
|
| 2 (19) | 10 (19) |
|
|
|
| Boonpiyathad T | 2016 | Thailand | 128 | N1 | 78 |
| 32.60±8.30 | 6.16±7.26 |
|
|
| 3 (78) | 19 | 7 | (14) |
|
|
|
|
| N2 | 50 |
| 38.10±10.90 | 5.76±6.60 |
|
|
| 4 (50) | 16 |
|
|
| Chen MC | 2008 | China | 100 | N1 | 58 | 0 |
|
| 8.03±1.50 |
|
|
|
| 8 | (25) |
|
|
|
|
| N2 | 42 | 42 |
|
| 7.07±1.50 |
|
|
|
|
|
|
| Kim JH | 2016 | Korea | 138 | N1 | 69 |
| 37.00±11.75 | 6.00±23.63 | 9.00±2.5 | 120.00±296.20 |
|
| 16 | 8 | (17) |
|
|
|
|
| N2 | 69 |
| 41.00±13.25 | 5.50±59.63 | 10.00±2.50 | 113.00±247.50 |
|
| 21 |
|
|
| Krupashankar | 2012 | India | 80 | N1 | 47 |
| 33.81±12.04 | 21.70±30.65 |
|
| 8 (48) | 24 (47) | 19 | 7 | (22) |
| DS |
|
|
| N2 | 33 |
| 37.58±14.36 | 17.42±18.63 |
|
| 5 (62) | 9 (33) | 17 |
|
|
| Kumar YH | 2016 | India | 110 | N1 | 48 |
| 28.54±13.50 |
|
|
| 7 (47) | 13 (48) | 15 | 7 | (20) |
|
|
|
|
| N2 | 62 |
| 31.55±14.43 |
|
|
| 1 (33) | 8 (62) | 22 |
|
|
| Lee MF | 2014 | Taiwan | 40 | N1 | 20 |
| 37.00±7.25 |
|
| 174.80±49.50 | 2 (18) |
| 5 | 8 | (18) |
|
|
|
|
| N2- | 20 |
| 46.60±15.00 |
|
| 129.10±16.60 | 1 (19) |
| 10 |
|
|
| Li MM | 2016 | China | 136 | N1 | 54 |
| 38.52±12.53 | 45.27±65.67 | 7.60±4.30 | 171.20±331.10 | 9 (54) |
| 10 | 7 | (26) |
|
|
|
|
| N2 | 82 |
| 36.60±11.83 | 35.24±42.54 | 7.60±3.90 | 143.90±294.10 | 16 (82) |
| 24 |
|
|
| Aktar S | 2015 | Japan | 50 | N1 | 23 | 3 | 35.65±9.12 |
| 3.09±2.02 |
|
|
| 5 | 7 | (15) |
|
|
|
|
| N2 | 27 | 20 | 29.59±9.66 |
| 3.15±1.74 |
|
|
| 13 |
|
|
| Song ZQ | 2013 | China | 862 | N1 | 399 |
| 35.50±8.80 | 21.23±10.62 | 4.20±0.70 |
|
|
|
| 8 | (6) |
|
|
|
|
| N2 | 463 |
| 23.50±7.60 | 27.58±18.63 | 3.80±0.50 |
|
|
|
|
|
|
| Sun WL | 2005 | China | 82 | N1 | 29 |
| 38.30±12.30 | 41.80±51.40 | 14.07±2.25 |
| 8 (29) | 16 (29) | 6 | 7 | (27) |
|
|
|
|
| N2 | 53 |
| 33.70±14.40 | 32.60±40.00 | 13.13±3.00 |
| 3 (53) | 13 (53) | 15 |
|
|
| Yadav S | 2013 | India | 80 | N1 | 40 | 4 | 32.50±8.75 |
| 6.80±1.20 |
| 8 (40) |
|
| 7 | (21) |
|
|
|
|
| N2 | 40 | 40 | 33.00±8.75 |
| 6.20±1.40 |
| 6 (40) |
|
|
|
|
| Yang SL | 2016 | China | 79 | N1 | 43 | 0 | 39.23±12.24 | 55.66±38.10 | 14.64±2.11 |
|
|
| 14 | 7 | (24) |
|
|
|
|
| N2 | 36 | 20 | 38.72±12.65 | 23.68±20.00 | 13.16±1.12 |
|
|
| 16 |
|
|
| Ye YM | 2016 | Korea | 75 | N1 | 17 |
| 38.20±11.80 | 30.50±26.50 | 9.50±4.10 | 238.30±306.60 | 6 (17) | 6 (17) | 5 | 7 | (16) |
|
|
|
|
| N2 | 50 |
| 41.10±12.40 | 13.00±89.50 | 10.10±3.60 | 250.20±390.70 | 9 (47) | 21 (50) | 19 |
|
|
| Zhong H | 2014 | China | 390 | N1 | 261 |
| 34.70±13.80 |
| 4.00±1.30 |
|
| 91 (261) | 71 | 7 | (7) |
|
|
|
|
| N2 | 129 |
| 32.30±15.30 |
| 3.40±1.60 |
|
| 16 (129) | 51 |
|
|
| Zhou PM | 2017 | China | 154 | N1 | 67 | 0 | 36.23±15.14 | 34.40±73.07 | 7.30±2.00 |
|
|
| 25 | 8 | (23) |
|
|
|
|
| N2 | 87 | 30 | 34.94±13.27 | 24.17±13.17 | 6.70±1.70 |
|
|
| 37 |
|
|
Quality assessment of included
studies
Detailed results regarding the quality assessment
are summarized in Table I. All
included studies were prospective observational studies. The
present meta-analysis was restricted to studies with a low risk of
bias (NOS ≥7), and the entire analysis was replicated following
removal of the most influential study on the basis of its
weight.
Comparison of the influence of ASST
responses on CSU cases and heathy controls
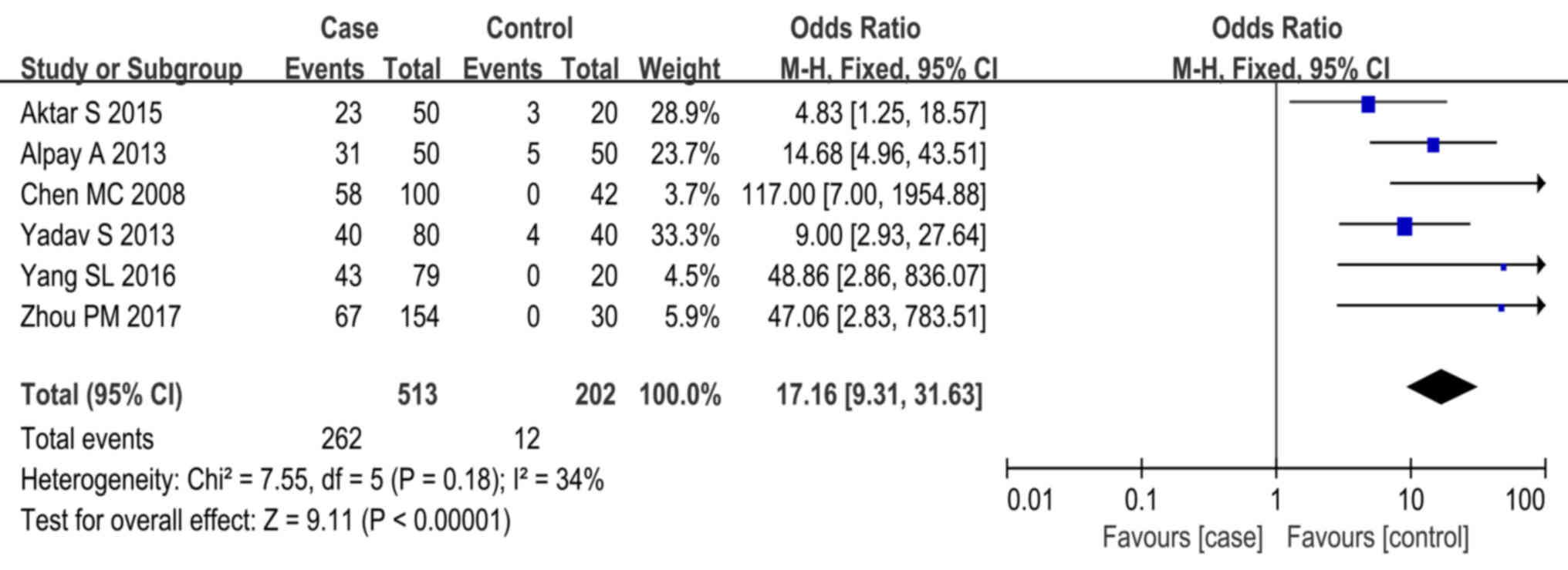
Of all studies reviewed, 6 provided data on ASST
responses in heathy controls (15,19,21,23,25).
Statistical analysis revealed a high degree of homogeneity across
studies (P=0.18, I2=34%). Meta-analysis using the
fixed-effects model indicated that CSU cases were more frequently
associated with positive ASST responses than the healthy controls
(P<0.01, OR=17.16, 95% CI, 9.31–31.63; Fig. 2).
Comparison of age, duration of disease
and UAS in positive and negative ASST cases
Of all 16 studies, 14 (6,7,14,18,20,24,26,27) and
10 (6,14,16,17,19,22,24,26,27)
provided data on age and duration of disease, respectively, in the
two groups. A significant heterogeneity was present across the
studies (age: P<0.001, I2=95%; duration: P<0.001,
I2=83%), necessitating use of the random-effects model.
The meta-analysis indicated that the two groups displayed no
statistically significant differences in age (P=0.96, WMD=0.11, 95%
CI, −4.31 to 4.52; Fig. 3) and
duration (P=0.08, WMD=5.48, 95% CI, −0.71 to 11.68; Fig. 4). A subgroup analysis regarding
patient age was then performed, in which patients were stratified
by region. Results from China and India indicated that the two
groups exhibited no difference in age (China: P=0.15, WMD=3.95, 95%
CI, −1.49 to 9.39; India: P=0.18, WMD=−1.88, 95% CI, −4.63 to
0.87). Of note, the results from Korea, Thailand and Taiwan
indicated that a positive ASST response was associated with younger
age (Korea: P=0.04, WMD=−3.68, 95% CI, −7.21 to −0.16; Taiwan:
P=0.01, WMD=−9.6, 95% CI, −16.9 to −2.30; Thailand: P=0.002,
WMD=−5.50, 95% CI, −9.04 to −1.96), while the opposite result was
obtained in Japan (P=0.02, WMD=6.06, 95% CI, 0.85–11.27; Fig. 3). Another subgroup analysis was
performed with stratification by duration of disease (≤30 vs.
>30 months). It was revealed that in patients with a duration of
disease of ≤30 months, the disease duration was not significantly
associated with the result of the ASST (P=0.59, WMD=−1.52, 95% CI,
−7.03 to 3.99), while in those with a duration of disease of >30
months, a positive ASST response was associated with a
significantly increased duration of disease as compared with that
in patients with a negative ASST response (P=0.002, WMD=15.93, 95%
CI, 56.64–26.22; Fig. 4).
A total of 11 studies provided data on UAS (6,7,15,17,21,23,27).
Statistically significant heterogeneity was present across these
studies (P=0.01, I2=59%), again resulting in the use of
the random-effects model. The meta-analysis revealed that the
positive ASST response group had a higher UAS than that of the
negative ASST response group (P<0.001, WMD=0.42, 95% CI,
0.35–0.50; Fig. 5). Therefore, a
subgroup analysis was performed based on the range of UAS as
estimated by employing different guidelines, including European
(28) and Korean guidelines
(29). The results of this subgroup
analysis also indicated that the positive ASST response group had a
higher UAS than the negative ASST response group [UAS (0–6):
P<0.001, WMD=0.41, 95% CI, 0.33–0.49; UAS (0–9): P<0.001,
WMD=0.77, 95% CI, 0.36–1.18; UAS (0–18): P=0.00, WMD=1.18, 95% CI,
0.57–1.80]. However, the results of UAS of 0–12 was at the verge of
statistical significance, however it was not statistically
significant (P=0.05, WMD=0.60, 95% CI, 0.00–1.20, and an inverse
result was observed for UAS of 0–15 (P=0.04, WMD=−0.73, 95% CI,
−1.41 to −0.05). In addition, two studies provided the median and
range of UAS but no standard deviation (SD) (25,27). The
SD was then calculated using an established formula (30).
Comparison of total serum IgE,
angioedema and anti-thyroid autoantibodies in positive and negative
ASST groups
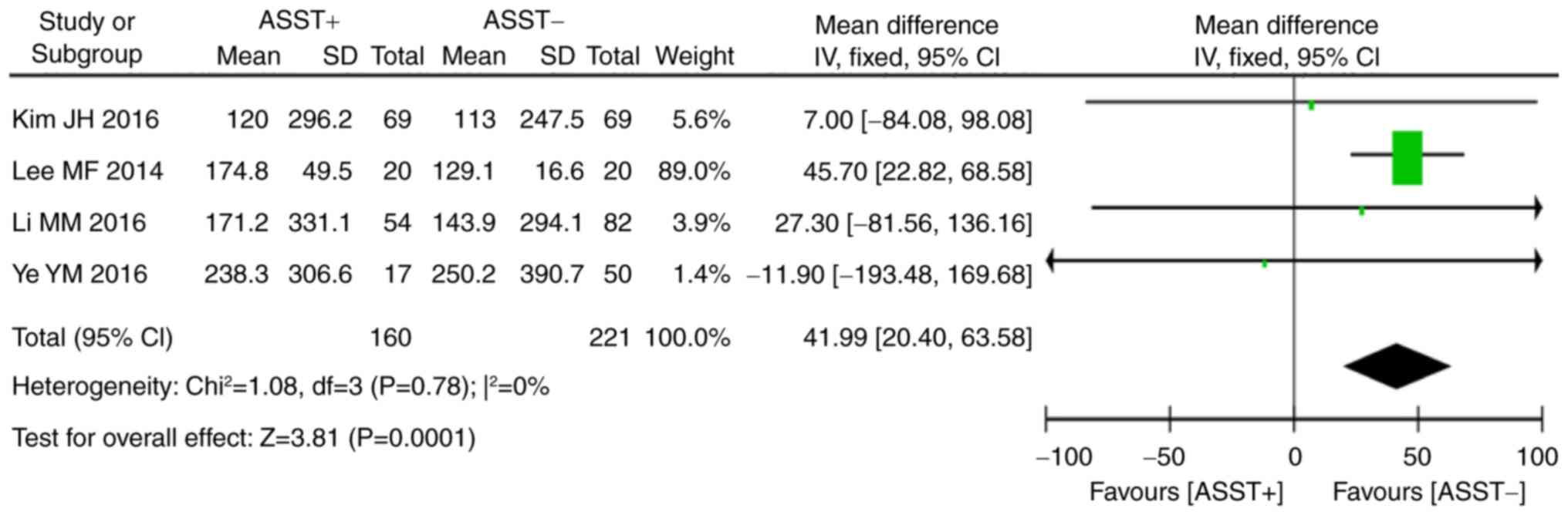
Of all studies included in the present review, 4
(16–18,26)
provided data on total serum IgE and 8 (16,18–22,26,27) on
the presence of thyroid autoantibodies in the two groups.
Statistical analysis indicated that a significant homogeneity was
present across these studies (serum total IgE: P=0.78,
I2=0%; anti-thyroid autoantibodies: P=0.38,
I2=7%). Meta-analysis using the fixed-effects model
indicated that a positive ASST result was associated with higher
levels of total serum IgE (P<0.001, WMD=41.99, 95% CI, 20.40 to
63.58; Fig. 6) and thyroid
autoantibodies (P<0.01, OR=1.87, 95% CI, 1.19–2.94; Fig. 7) than a negative ASST result.
Data from 7 studies provided information on
angioedema in the two groups. As the results on angioedema
displayed a statistically significant heterogeneity across studies
(P=0.03, I2=57%), it was necessary to use the
random-effects model. Meta-analysis revealed that a positive ASST
result was associated with a higher risk of angioedema than that of
a negative ASST result (P=0.03, OR=1.92, 95% CI, 1.08–3.40;
Fig. 8). Angioedema is a subjective
index of urticaria and manifests in a relatively short-lived edema
in the skin. The occurrence of angioedema is affected by inherited
and environmental factors; furthermore, physical stimulators,
including shock and pressure, may also induce angioedema (31). As a result, a substantial
heterogeneity in angioedema data may be present.
Sex differences among subjects with
positive ASST responses
An included study demonstrated that females are more
likely to have a positive ASST response as rates of ASST were 78%
and 22% in females and males, respectively (15). Of all studies included in the present
review, 12 (7,14,18,20,22,24,27),
which accounted for 1462 cases, contained 756 cases with positive
responses to ASST. Of these 756 cases, 210 were males and 546 were
females. Statistical analysis of positive ASST responses in CSU
cases indicated that a statistically significant homogeneity was
present across studies (P=0.97, I2=0%), and the
fixed-effects model was therefore used. The meta-analysis revealed
that females had a higher prevalence of positive ASST responses
(P<0.001, OR=0.63, 95% CI, 0.50–0.79; Fig. 9). The detailed results of the
meta-analysis for the above indexes are presented in Table I.
Sensitivity analysis
Studies that failed to satisfy the criterion of high
quality were excluded from the present review. In addition, a
sensitivity analysis was applied for each index involving ASST
comparisons. The sensitivity analysis demonstrated that the results
obtained using the random- and fixed-effects models were in
accordance with each study included in the review. These results
suggested that no individual studies significantly affected the
pooled results. This indicated that the meta-analysis performed
provided reliable results.
Detection of publication bias
An analysis of publication bias was performed by
using Egger's regression test. The results indicated that no
publication bias was present regarding the UAS and sex differences
among patients with positive ASST responses (UAS: Z=−2.18, P=0.06;
sex differences among patients with positive ASST response:
Z=−0.73, P=0.48). However, publication bias was present with regard
to age and duration of CSU (age: Z=−3.93, P=0.002; duration of CSU:
Z=4.23, P=0.003). Although the above results revealed that studies
included in the present review displayed an inconformity in
publication bias, the funnel plots had typical shapes, and the
funnel plot of anti-thyroid autoantibodies is presented in Fig. 10.
Discussion
To the best of our knowledge, the present study is
the first systematic review comparing ASST responses in patients
with CSU. The results obtained by the meta-analysis suggest that
cases with positive ASST responses had higher UAS and higher levels
of serum total IgE than those of cases with negative ASST
responses. In addition, cases with positive responses to ASST were
more likely to have accompanying angioedema and were positive for
thyroid autoantibodies. CSU was more prevalent in females, who were
also more likely to exhibit a positive response to ASST. It was
also confirmed that a greater incidence of ASST was present in CSU
patients as compared with that in heathy controls. No statistically
significant differences were present between cases with positive
vs. negative responses to ASST with regard to patient age and
duration of disease.
Angioedema, which is the clinical manifestation of
urticaria, develops when urticaria is located within the subcutis.
It is a syndrome characterized by a sudden and limited subcutaneous
and/or submucous swelling. Angioedema in CU is caused by a
non-specific histamine release from activated mast cells (32). The occurrence of angioedema in CU,
while not an indication for disease severity, is associated with a
longer duration of urticarial disease. Non-steroidal
anti-inflammatory drugs and/or systemic corticotherapy are classic
triggers of angioedema in CU (33).
Mast cells may be activated primarily by IgE-dependent (allergen,
anti-IgE) as well as by IgE-independent mechanisms (34). Increased levels of IgE are thought to
provoke urticaria. Auto-antibodies for IgE and the α-chain of FcεRI
contribute to the occurrence of CU (35). It has been reported that one third of
patients with CU have significantly elevated levels of total IgE
and levels of serum total IgE are associated with disease severity
and duration (36). The severity of
CSU was also identified to be associated with a positive response
in the ASST (37), which is in
accordance with the results of the present meta-analysis.
CSU is linked with thyroid diseases, which are the
most commonly reported autoimmune condition in patients with CSU.
Patients with thyroid dysfunction and CSU have a more severe and
prolonged course of urticaria than those without thyroid
dysfunction. A significantly greater number of anti-thyroid
antibodies are present in CSU patients. Even in clinically
euthyroid CSU patients, anti-thyroid antibodies remain present and
are considered to be associated with CSU. Thyroid disease may
worsen urticaria through activation of the complement system
(2).
CU is characterized by mast/basophil cell
activation, which initiates an inflammatory response. Sex hormones
modulate immune and inflammatory cell functions, including mast
cell secretion. Of note, urticaria may be associated with certain
diseases and conditions associated with hormonal changes, including
endocrinopathy, the menstrual cycle, pregnancy, menopause and
hormonal contraceptives or hormone replacement therapy.
Dehydroepiandrosterone (DHEA) is a modulator of endocrine and
immune functions and depletion of DHEA may lead to adverse events
(38). Serum concentrations of DHEA
sulfate in CSU patients are significantly lower than those in
healthy subjects and are associated with positive responses to ASST
(39). This is in accordance with
the results of the present meta-analysis, which indicate a female
predominance for CSU and a positive response in the ASST (6–8,15).
Although the average NPV of the ASST was 92.8%, a
negative ASST was identified as a significant determinant of
urticaria remission and a negative ASST serves as a good predictor
for achieving urticaria remission within 2 years (8,14,40). A
positive ASST response is a significant predictor of CSU in
controls (14).
While the studies included in the present
meta-analyses were selected based on strict inclusion and exclusion
criteria, certain unavoidable limitations and bias remain. As
compared with a randomized controlled trial, the quality of
observational studies is low, which represents a limitation of the
present meta-analysis. However, all subjects enrolled in the
present meta-analysis were patients with CU who voluntarily
underwent an ASST. The major observational endpoints were UAS,
serum total IgE and anti-thyroid autoantibodies, all of which are
objective parameters. Furthermore, all of the studies included were
on Asian populations, which is a cause of publication bias.
Although ASST has a high specificity to test for functional
autoantibodies, their absence has a high specificity for CU.
According to the European expert consensus from 2009 (8), the value and meaning of the ASST
remains to be fully established. Furthermore, skin testing requires
the collection of venous blood and separation of serum prior to
hypodermic injection. It is essential that failsafe precautions are
taken to ensure that the patient's own serum is used for skin
testing and aseptic procedures are required. Commercial ELISA is
now sufficiently mature for testing for functional autoantibodies,
including IgE. The dermatologists of developed countries may be
more likely to identify anti-IgE antibodies using ELISA compared
with certain Asian dermatologists. An additional source of
potential bias may be differences within health care providers and
hospitals regarding the techniques applied for detecting ASST and
the conditions of the patients. Although all studies included in
the present review involved comparisons of/within case groups with
positive and negative ASST responses, inherent differences in
researchers' approaches to, and interpretations of, CSU may exist.
Finally, the limited sample size and resultant data available for
the present meta-analyses may have affected the final results
obtained. The conclusions require verification by subsequent
studies and the resolution of remaining issues.
In conclusion, the results of the present analyses
of pooled data indicate that CSU patients with a positive ASST
response have more severe clinical features and are more likely to
have an accompanying autoimmunity condition as compared with those
with a negative response to the ASST.
Acknowledgements
Not applicable.
Funding
The current study was supported by National Natural
Science Foundation of China (grant nos. 81673070 and 81872538).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XLN analyzed autologous serum skin test data of all
involved studies and drafted the manuscript. LLZ searched the
literature and collected the data associated with chronic
urticaria. MHS and YJZ performed the statistical analysis. XHG and
RQQ critically revised the manuscript for important intellectual
content. All authors have read and approved the final version of
the manuscript prior to submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
References
|
1
|
Irinyi B, Széles G, Gyimesi E, Tumpek J,
Herédi E, Dimitrios G, Adány R, Hunyadi J and Szegedi A: Clinical
and laboratory examinations in the subgroups of chronic urticaria.
Int Arch Allergy Immunol. 144:217–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fraser K and Robertson L: Chronic
urticaria and autoimmunity. Skin Therapy Lett. 18:5–9.
2013.PubMed/NCBI
|
|
3
|
Al-Hamamy HR, Hameed AF and Abdulhadi AS:
Autologous serum skin test as a diagnostic aid in chronic
idiopathic urticaria. ISRN Dermatol. 2013:2915242013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sabroe RA, Grattan CE, Francis DM, Barr
RM, Kobza Black A and Greaves MW: The autologous serum skin test: A
screening test for autoantibodies in chronic idiopathic urticaria.
Br J Dermatol. 140:446–452. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng L, Song ZQ and Hao F: Application of
autologous serum skin test in chronic urticaria: Current advances.
J Clin Dermatol. 08:508–510. 2011.
|
|
6
|
Song Z, Zhai Z, Zhong H, Zhou Z, Chen W
and Hao F: Evaluation of autologous serum skin test and skin prick
test reactivity to house dust mite in patients with chronic
spontaneous urticaria. PLoS One. 8:e641422013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong H, Song Z, Chen W, Li H, He L, Gao
T, Fang H, Guo Z, Xu J, Yu B, et al: Chronic urticaria in Chinese
population: A hospital-based multicenter epidemiological study.
Allergy. 69:359–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Konstantinou GN, Asero R, Maurer M, Sabroe
RA, Schmid-Grendelmeier P and Grattan CE: EAACI/GA(2)LEN task force
consensus report: The autologous serum skin test in urticaria.
Allergy. 64:1256–1268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Auyeung P, Mittag D, Hodgkin PD and
Harrison LC: Autoreactive T cells in chronic spontaneous urticaria
target the IgE Fc receptor Iα subunit. J Allergy Clin Immunol.
138:761–768.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kulthanan K, Nuchkull P, Ungaksornpairote
C, Chularojanamontri L and Tuchinda P: Prevalence and clinical
correlation of serum immunoglobulin E in patients with chronic
spontaneous urticaria. Ann Allergy Asthma Immunol. 116:258–259. e2.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Navines-Ferrer A, Serrano-Candelas E,
Molina-Molina GJ and Martin M: IgE-related chronic diseases and
Anti-IgE-based treatments. J Immunol Res. 2016:81638032016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Confino-Cohen R, Chodick G, Shalev V,
Leshno M, Kimhi O and Goldberg A: Chronic urticaria and
autoimmunity: Associations found in a large population study. J
Allergy Clin Immunol. 129:1307–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugiyama A, Nishie H, Takeuchi S,
Yoshinari M and Furue M: Hashimoto's disease is a frequent
comorbidity and an exacerbating factor of chronic spontaneous
urticaria. Allergol Immunopathol (Madr). 43:249–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boonpiyathad T and Sangasapaviliya A:
Autologous serum and plasma skin test to predict 2-year outcome in
chronic spontaneous urticaria. Asia Pac Allergy. 6:226–235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aktar S, Akdeniz N, Ozkol HU, Calka O and
Karadag AS: The relation of autologous serum and plasma skin test
results with urticarial activity score, sex and age in patients
with chronic urticaria. Postepy Dermatol Alergol. 32:173–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye YM, Park JW, Kim SH, Ban GY, Kim JH,
Shin YS, Lee HY and Park HS;: PRANA Group: Prognostic factors for
chronic spontaneous urticaria: A 6-month prospective observational
study. Allergy Asthma Immunol Res. 8:115–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH, Lee HY, Ban GY, Shin YS, Park HS
and Ye YM: Serum clusterin as a prognostic marker of chronic
spontaneous urticaria. Medicine (Baltimore). 95:e36882016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee MF, Lin TM, Liu SW and Chen YH: A
rapid method of detecting autoantibody against FcεRIα for chronic
spontaneous urticaria. PLoS One. 9:e1095652014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alpay A, Solak Tekin N, Tekin IÖ,
Altinyazar HC, Koca R and Cinar S: Autologous serum skin test
versus autologous plasma skin test in patients with chronic
spontaneous urticaria. Dermatol Res Pract. 2013:2672782013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar YH, Bhaskar S and Shankar K:
Comparative study of positive versus negative autologous serum skin
test in chronic spontaneous urticaria and its treatment outcome. N
Am J Med Sci. 8:25–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav S, Kanwar A, Parsad D and Minz R:
Chronic idiopathic urticaria and thyroid autoimmunity: Perplexing
association. Indian J Dermatol. 58:3252013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krupashankar DS, Shashikala K and Madala
R: Clinical and investigative assessment of patients with positive
versus negative autologous serum skin test: A study of 80 South
Indian patients. Indian J Dermatol. 57:434–438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou PM, Lu YH, Chen T, Huang J, Fang J,
Liu P and Liu H: Analysis of autologous serum skin test results in
154 cases of patients with chronic spontaneous urticaria. Chin J
Dermatov Integr Tradit and West Med. 02:135–137. 2017.
|
|
24
|
Yang SL, Xie SX, Yin SC, Ou FX, Zhang YQ
and Lai W: Analysis of the clinical value of autologous serum skin
test in chronic urticaria. Chin J Health Lab Technol. 21:3158–3160.
2016.
|
|
25
|
Chen MC, Li D, Guo Q and Zhai FQ:
Investigation into the correlation of clinical features and
autologous serum skin test of chronic urticaria. China Tropical
Med. 05:736–738. 2008.
|
|
26
|
Li MM, Guo ZP, Li JY, Xie XQ, Song Q, Ye
YY and Chen SY: Autologous serum skin test and some laboratory test
analysis in 136 patients with chronic urticaria. Chin J Leprosy and
Skin Dis. 10:595–597. 2016.
|
|
27
|
Sun WL and Bi ZG: Clinical features of
chronic urticaria in patients with positive and negative autologous
serum skin test. Chin J Dermatol. 06:342–344. 2005.
|
|
28
|
Zuberbier T, Bindslev-Jensen C, Canonica
W, Grattan CE, Greaves MW, Henz BM, Kapp A, Kozel MM, Maurer M,
Merk HF, et al: EAACI/GA2LEN/EDF guideline: Definition,
classification and diagnosis of urticaria. Allergy. 61:316–320.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye YM, Park JW, Kim SH, Choi JH, Hur GY,
Lee HY, Lee EH and Park HS: Clinical evaluation of the computerized
chronic urticaria-specific quality of life questionnaire in Korean
patients with chronic urticaria. Clin Exp Dermatol. 37:722–728.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou XW, Shi JP and Chen X: How to estimate
the mean and standard deviation based on the median, range and
sample size when conducting meta-analysis. Chin J Evid-based Med.
15:484–487. 2015.
|
|
31
|
Bork K: Angioedema. Immunol Allergy Clin
North Am. 34:23–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boccon-Gibod I and Bouillet L: Angioedema
and urticaria. Ann Dermatol Venereol. 141 (Suppl 3):S586–S595.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hacard F, Nosbaum A, Bensaid B, Nicolas
JF, Augey F, Goujon C and Bérard F: Histaminergic angioedema and
chronic urticaria. Presse Med. 44:37–42. 2015.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petra AI, Panagiotidou S, Stewart JM,
Conti P and Theoharides TC: Spectrum of mast cell activation
disorders. Expert Rev Clin Immunol. 10:729–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang KL, Yang YH, Yu HH, Lee JH, Wang LC
and Chiang BL: Analysis of serum total IgE, specific IgE and
eosinophils in children with acute and chronic urticaria. J
Microbiol Immunol Infect. 46:53–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kessel A, Helou W, Bamberger E, Sabo E,
Nusem D, Panassof J and Toubi E: Elevated serum total IgE-a
potential marker for severe chronic urticaria. Int Arch Allergy
Immunol. 153:288–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Toubi E, Kessel A, Avshovich N, Bamberger
E, Sabo E, Nusem D and Panasoff J: Clinical and laboratory
parameters in predicting chronic urticaria duration: A prospective
study of 139 patients. Allergy. 59:869–873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kasperska-Zajac A, Brzoza Z and Rogala B:
Sex hormones and urticaria. J Dermatol Sci. 52:79–86. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kasperska-Zajac A, Brzoza Z and Rogala B:
Lower serum concentration of dehydroepiandrosterone sulphate in
patients suffering from chronic idiopathic urticaria. Allergy.
61:1489–1490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hizal M, Tüzün B, Wolf R and Tüzün Y: The
relationship between Helicobacter pylori IgG antibody and
autologous serum test in chronic urticaria. Int J Dermatol.
39:443–445. 2000. View Article : Google Scholar : PubMed/NCBI
|