Introduction
Cataract is a disorder of lens metabolism caused by
aging, heredity, malnutrition, abnormal immune function and so on.
The lens protein becomes denatured and cloudy, causing the
diminution of vision and even the lose of vision. Cataract is an
important cause of blindness, especially in the elderly. With the
growth of aging population, incidence of cataract is increasing
(1–3). At present, the most effective means of
treating cataract is cataract extraction combined with intraocular
lens implantation, but ~0.32–1.00% of patients will have
rhegmatogenous retinal detachment after cataract surgery. Although
success rate of treatment for rhegmatogenous retinal detachment has
increased significantly during past several years, some patients
are still incurable, and irreversible visual impairment occurs
(4,5).
At present, mechanism of the occurrence of
postoperative rhegmatogenous retinal detachment in cataract
patients has not been fully clarified. Studies have reported that
most of the lens epithelial cells in the anterior lens capsule of
cataract patients migrate to posterior capsule and undergo
epithelial-mesenchymal transition after surgery, and the secretion
of collagen and extracellular matrix is the main cause of
rhegmatogenous retinal detachment (6,7).
Inhibition of epithelial-mesenchymal transition in lens epithelial
cells may be an effective strategy to prevent rhegmatogenous
retinal detachment. miRNA (21–25 bp) is a kind of small biological
molecule closely related to cell epithelial-mesenchymal transition.
A previous study found that miR-181a can inhibit
epithelial-mesenchymal transition of lens epithelial cells
(8), and has been reported that
TGF-β2 induces epithelial-mesenchymal transition of human lens
epithelial cells through PI3K/Akt signaling pathway (9). The interactions between miR-181a and
TGF-β2 in regulating epithelial-mesenchymal transition of lens
epithelial cells are unknown, while research has found that
miR-181a can promote TGF-β expression and mediate
epithelial-mesenchymal transition in ovarian cancer cells (10).
In this study, we analyzed the expression of
miR-181a and TGF-β2 in lens epithelial cells of patients with
cataract combined with rhegmatogenous retinal detachment, and
explored the effect of miR-181a and TGF-β2 on
epithelial-mesenchymal transition in lens epithelial cells.
Patients and methods
Research subjects
Forty patients with rhegmatogenous retinal
detachment combined with age-related cataract (cast-off group) and
another 40 patients with simple age-related cataract
(non-exfoliated group) in Tongren Hospital (Shanghai, China) were
enrolled between January 2017 and June 2018. Lens epithelial cells
were collected. Inclusion criteria: All patients met the relevant
diagnostic criteria for retinopathy established by the American
Retina Association. Lens was opaque, visual acuity was ≤0.6, and
double lacrimal passage was unobstructed. Patients in the cast-off
group were confirmed with round and horseshoe-shaped holes by
round-eye ultrasonography. Medical records were complete. Exclusion
criteria: Patients with other eye diseases, patients with severe
ocular trauma or history of eye surgery, acute metabolic disorders
such as diabetic ketoacidosis within the previous 1 month, patients
who had used tear film stabilization drug, patients with severe
acute infection, patients with liver and kidney dysfunction or
severe medical problems, and women during pregnancy or lactation
were excluded.
The present study was approved by the Ethics
committee of Tongren Hospital. Patients who participated in this
research had complete clinical data. Signed informed consents were
obtained from the patients or the guardians.
Materials
Primary rabbit anti-human TGF-β2, E-cadherin,
vimentin polyclonal antibodies and secondary goat anti-rabbit IgG
polyclonal antibody (cat. nos. 19999-1-AP, 20874-1-AP, 10366-1-AP
and SA00001-2) were purchased from Wuhan Sanying Biotechnology
(Wuhan, China). Polyacrylamide gel electrophoresis buffer (cat. no.
orb154330) was purchased from Xiamen Huijia Biotechnology Co., Ltd.
(Xiamen, China). Western blot analysis kit (cat. no. UFC04948) was
purchased from Shanghai Junrui Biotechnology Co., Ltd. (Shanghai,
China).
Construction and transfection of
miR-181a expression vector
miR-181a over-expression vector (miR-181a-mimic
group) and empty vector (miR-control group) were designed and
synthesized by Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
The lens epithelial cells of 20 patients in the cast-off group were
selected by random number table method. Lens epithelial cells of
patients were subjected to trypsinization 24 h before transfection.
Transfection was performed when 80% confluence was reached. Cells
were incubated at 37°C and 5% CO2 incubator for 12 h,
then cells were washed with normal medium (DMEM + 20% fetal bovine
serum) and cultivated for additional 48 h. Cell culture medium was
changed every 6 h, and transfection was confirmed by RT-qPCR.
Untransfected lens epithelial cells were the blank control cells.
Lipofectamine™ 2000 (cat. no. 11668019) transfection kit was
purchased from Thermo Fisher Scientific, Inc.
RT-qPCR
Single lens epithelial cell suspension
(1×107/ml) was prepared and TRIzol lysate was added in a
ratio of 3:1. TRIzol™ reagent (cat. no. R0016) was purchased from
Beyotime Institute of Biotechnology (Shanghai, China). After
extraction, integrity of the RNA was analyzed by 1.5% agarose gel
electrophoresis, and purity of extracted RNA was detected by a
micro nucleic acid analyzer. Only RNA samples with A260/A280
between 1.8 and 2.1 were used in reverse transcrition, which was
performed using the following system: Oligo (dT) primer (50 µM) 0.5
µl, random 6 mers (100 µM) 0.5 µl, 5X PrimeScript Buffer 2.0 µl,
PrimeScript RT Enzyme Mix 0.5 µl, total RNA 2 µl and 4.5 µl Rnase
Free ddH2O was added to 10 µl in total. Reaction
conditions were: 37°C for 15 min and 85°C for 5 sec. Reverse
transcription kit was purchased from Beyotime Institute of
Biotechnology. PCR amplification was performed after transcription.
PCR amplification system consists of 2 µl of cDNA template, 25 µl
of 2X bare SYBR mixture, and 1 µl of upstream and downstream
primer. Double distilled water was added to 50 µl volume. Reaction
conditions were: 95°C for 5 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. GAPDH was used as endogenous control.
Three replicate wells were set and results were analyzed by
2−ΔCq method (11).
RT-qPCR kit (cat. no. WE0140-UIM) was purchased from BioLab Inc.
(Lawrenceville, USA). Primers were designed and synthesized by
Thermo Fisher Scientific, Inc. (Table
I).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Forward | Reverse |
|---|
| miR-181a |
5′-GCGGTAACATTCAACGCTGTCG-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| TGF-β2 mRNA |
5′-GCTTTGGATGCGGCCTATTG-3′ |
5′-CCAGCACAGAAGTTGGCATTGTA-3′ |
| GAPDH |
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Western blot analysis
Protein in the lens epithelial cells was extracted
with RIPA lysis buffer. A total of 10 μl protein per lane
was separated by 12% polyacrylamide gel electrophoresis. The
initial voltage was 90 V. After the electrophoresis, the
transmembrane to PVDF membrane was performed, with 100 V constant
pressure for 100 min. Membranes were blocked with 5% BSA at 37°C
for 60 min. After incubating with primary antibodies at 4°C
overnight, membranes were washed twice with PBS and incubated with
secondary antibody at 37°C for 2 h. Signal development was
performed using ECL. Gray scale was measured using Quantity One
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
relative expression level = gray value of target gene / gray value
of endogenous control.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analyses. Measurement data were expressed in %, and
ratios were compared using the χ2 test. Count data were
expressed as mean ± standard deviation (SD). t-test was used for
comparison between two groups. Analysis of variance was used for
comparison among groups with Dunnett's post hoc test, and repeated
variance measurement was used for comparison at different time
points within the group. Pearson's correlation was used to analyze
the correlation between the relative expression level of miR-181a
and TGF-β2 in lens epithelial cells in the miR-181a-mimic group.
Differences were statistically significant at P<0.05.
Results
Relative expression levels of miR-181a
in lens epithelial cells
Results of RT-qPCR showed that relative expression
level of miR-181a in lens epithelial cells of the cast-off group
was (1.325±0.094), and relative expression level in lens epithelial
cells of the non-exfoliated group was (2.634±0.121). There was a
statistically significant difference between the two groups.
Relative expression level of miR-181a in the non-exfoliated group
was significantly higher than that in the cast-off group
(P<0.05; Fig. 1).
Relative expression levels of TGF-β2 in lens
epithelial cells. Results of qRT-PCR showed that relative
expression level of TGF-β2 mRNA in lens epithelial cells of the
non-exfoliated group was (0.351±0.066), and relative expression
level in lens epithelial cells of the cast-off group was
(0.712±0.073). There was a statistically significant difference
between the two groups. Relative expression level of TGF-β2 mRNA in
the cast-off group was significantly higher than that in the
non-exfoliated group (P<0.05; Fig.
2A). Western blot analysis showed that relative expression
level of TGF-β2 protein in lens epithelial cells of the
non-exfoliated group was (4.621±1.247), and relative expression
level in lens epithelial cells of the cast-off group was
(21.522±3.364). There was a statistically significant difference
between the two groups. Relative expression level of TGF-β2 protein
in the cast-off group was significantly higher than that in the
non-exfoliated group (P<0.05; Fig.
2B and 3).
Results of lens epithelial cell
transfection
Results of RT-qPCR showed that relative expression
level of miR-181a in the miR-181a-mimic group was (2.183±0.134)
after transfection, and relative expression level of miR-181a in
the miR-control group was (1.342±0.074). There was a statistically
significant difference between the two groups. After transfection
of miR-181a overexpression vector, relative expression level of
miR-181a in lens epithelial cells was significantly increased
(P<0.05), suggesting successful transfection (P<0.05;
Fig. 4).
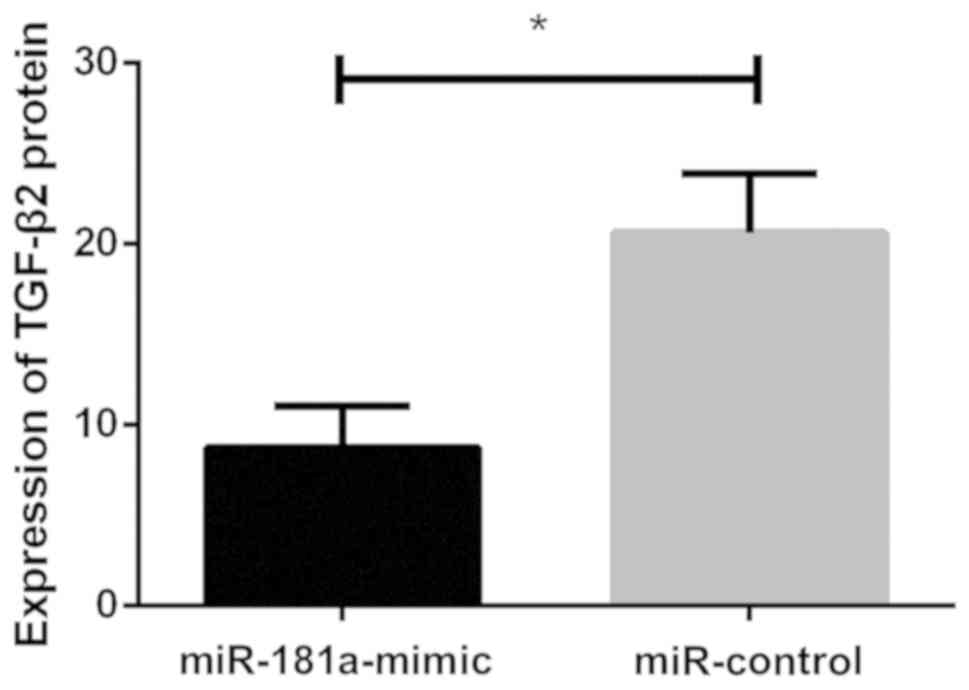
Effects of miR-181a overexpression on
TGF-β2 protein
Western blot analysis showed that relative
expression level of TGF-β2 protein in lens epithelial cells of the
miR-control group was (20.631±3.245), and relative expression level
in lens epithelial cells of the miR-181a-mimic group was
(8.752±2.283). There was a statistically significant difference
between the two groups. Relative expression level of TGF-β2 protein
in the miR-control group was significantly higher than that in the
miR-181a-mimic group (P<0.05). Upregulation of miR-181a
expression decreased the expression of TGF-β2 protein in lens
epithelial cells (P<0.05; Fig. 5
and Fig. 6).
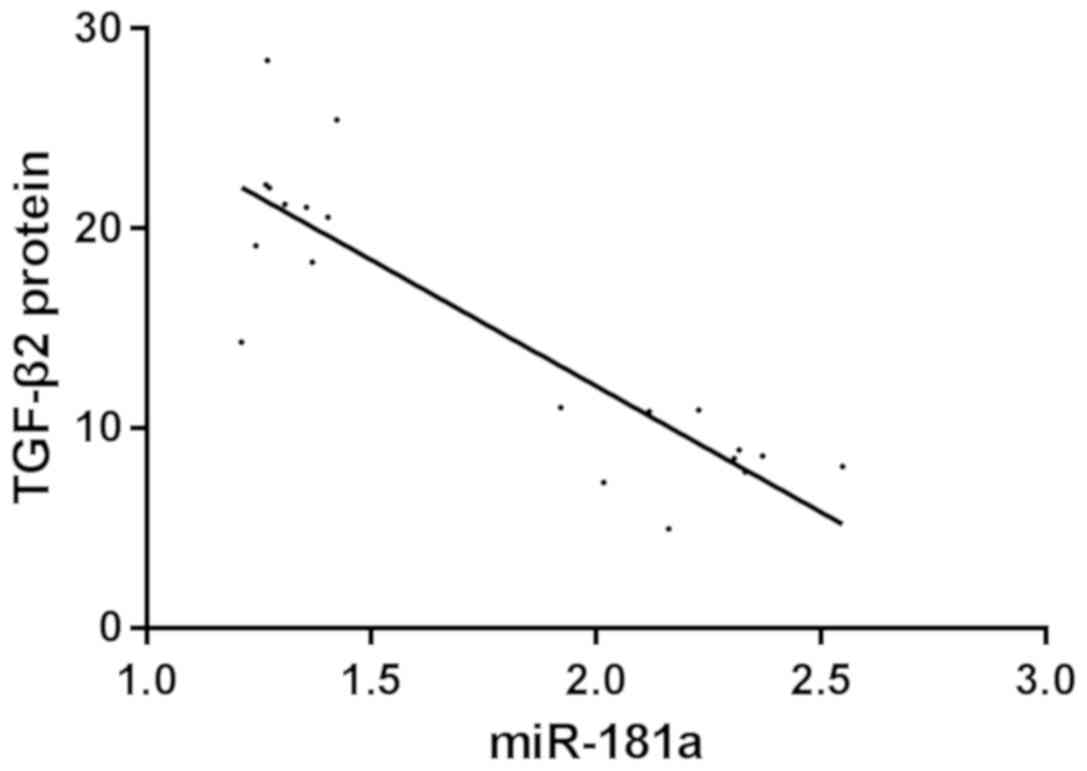
Correlation analysis between miR-181a
and TGF-β2 protein expression
Pearson's correlation analysis showed that the
expression level of miR-181a was negatively correlated with the
expression of TGF-β2 protein in miR-181a-mimic group. The higher
the expression level of miR-181a, the lower the expression level of
TGF-β2 protein (r=−0.875, P<0.001) (Fig. 7).
Effect of transfection of miR-181a
overexpression vector on epithelial-mesenchymal transition in lens
epithelial cells
Western blot analysis showed that relative
expression level of E-cadherin in lens epithelial cells of the
miR-control group was (0.983±0.023), and relative expression level
of E-cadherin in lens epithelial cells of the miR-181a-mimic group
was (1.345±0.042). There was a statistically significant difference
between the two groups. Relative expression level of E-cadherin in
the miR-control group was significantly lower than that in the
miR-181a-mimic group (P<0.05; Fig.
8A). Relative expression level of vimentin in the miR-control
group was (1.465±0.038), and relative expression level of vimentin
in the miR-181a-mimic group was (1.121±0.013). There was
significant statistical difference between the two groups. Relative
expression level of vimentin in the non-exfoliated group was
significantly higher than that in the miR-181a-mimic group
(P<0.05; Fig. 8B and 9).
Discussion
Rhegmatogenous retinal detachment is the most common
type of clinical retinal detachment. The prevention of
rhegmatogenous retinal detachment is important to improve the
postoperative prognosis of cataract patients. Many biological
factors have been shown to be involved in the development of
rhegmatogenous retinal detachment, and TGF-β, especially TGF-β2,
plays a key role in epithelial-mesenchymal transition of lens
epithelial cells (12,13). miRNAs also play an important role in
epithelial-mesenchymal transition, and many miRNAs are expressed in
the eye. miR-181a has also been shown to be expressed in lens
epithelial cells (14,15). miR-181a was found to inhibit
epithelial-mesenchymal transition in lens epithelial cells
(8), while the specific mechanism
remains unknown. The role of miR-181a in patients with
rhegmatogenous retinal detachment after cataract surgery has not
been reported.
We first detected the difference of miR-181a and
TGF-β2 in lens epithelial cells of cataract patients with
rhegmatogenous retinal detachment and simple cataract patients by
RT-qPCR. Results showed that the expression level of miR-181a was
decreased, while expression levels of TGF-β2 mRNA and TGF-β2
protein were increased in lens epithelial cells of cataract
patients with rhegmatogenous retinal detachment. One of the
important causes of rhegmatogenous retinal detachment is
epithelial-mesenchymal transition in residual lens epithelial cells
in cataract patients (16). Previous
studies have found that miR-181a inhibits epithelial-mesenchymal
transition of lens epithelial cells (8), while TGF-β2 can promote
epithelial-mesenchymal transition of lens epithelial cells
(9), indicating the potential
interactions between miR-181a and TGF-β2. This should be an
important reason for the decreased or increased expression of
miR-181a and TGF-β2 in lens epithelial cells of cataract patients
with rhegmatogenous retinal detachment.
To further investigate the role of miR-181a in
epithelial-mesenchymal transition of lens epithelial cells in
cataract patients with rhegmatogenous retinal detachment and the
mechanism, we constructed a miR-181a overexpression vector. RT-qPCR
confirmed the significant increase in the expression level of
miR-181a in lens epithelial cells and successful transfection. We
also measured the expression levels of two factors closely related
to epithelial-mesenchymal transition-intracellular epithelial
marker E-cadherin and interstitial marker Vimentin. Studies have
shown that upregulation of Vimentin expression and inhibition of
E-cadherin expression promote epithelial-mesenchymal transition
(17,18). In our study it was found that after
over-expression of miR-181a, expression level of E-cadherin was
significantly increased, while expression level of Vimentin was
significantly decreased in lens epithelial cells, suggesting
inhibition of epithelial-mesenchymal transition. This is consistent
with the findings of Dong et al (8), which showed that miR-181a inhibits
epithelial-mesenchymal transition of lens epithelial cells.
miR-181a has been reported to promote epithelial-mesenchymal
transition in tumor cells (19,20),
which is completely contrary to our findings and may be due to the
existence of multiple downstream targets of miR-181a. After
over-expression of miR-181a, we found that the expression level of
TGF-β2 protein in lens epithelial cells decreased, and the results
of Pearson's correlation analysis showed that the expression level
of miR-181a was negatively correlated with the expression level of
TGF-β2 protein. Therefore, we speculate that miR-181a and TGF-β2
may interact with each other to participate in the
epithelial-mesenchymal transition of lens epithelial cells.
miR-181a may play a role in inhibiting epithelial-mesenchymal
transition of lens epithelial cells by downregulating TGF-β2.
Previous studies have reported that miR-181a is involved in cell
epithelial-mesenchymal transition by regulating TGF-β (10,19–21),
which is consistent with our study.
In conclusion, expression level of miR-181a was
decreased, while expression level of TGF-β2 protein was increased
in lens epithelial cells of cataract patients with rhegmatogenous
retinal detachment. Overexpression of miR-181a may inhibit
epithelial-mesenchymal transition in lens epithelial cells by
inhibiting TGF-β2 expression. Our findings provide new insights
into the treatment of cataract patients with rhegmatogenous retinal
detachment.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific
Research Project of Changning District Health and Family Planning
Commission (grant no. 20164Y008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ wrote the manuscript. YJ and YG performed PCR and
western blot analysis. NL and WQ were responsible for construction
and transfection of miR-181a expression vector. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee of Tongren Hospital (Shanghai, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Patinet consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Waltz KL, Featherstone K, Tsai L and
Trentacost D: Clinical outcomes of TECNIS toric intraocular lens
implantation after cataract removal in patients with corneal
astigmatism. Ophthalmology. 122:39–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamiya K, Hayashi K, Shimizu K, Negishi K,
Sato M and Bissen-Miyajima H: Survey Working Group of the Japanese
Society of Cataract and Refractive Surgery: Multifocal intraocular
lens explantation: A case series of 50 eyes. Am J Ophthalmol.
158:215–220.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lundström M, Dickman M, Henry Y, Manning
S, Rosen P, Tassignon MJ, Young D and Stenevi U: European Society
of Cataract and Refractive Surgeons Femtosecond laser-assisted
cataract surgery study collaborators Femtosecond laser-assisted
cataract surgeries reported to the European Registry of Quality
Outcomes for Cataract and Refractive Surgery: Baseline
characteristics, surgical procedure, and outcomes. J Cataract
Refract Surg. 43:1549–1556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antoun J, Azar G, Jabbour E, Kourie HR,
Slim E, Schakal A and Jalkh A: Vitreoretinal surgery with silicone
oil tamponade in primary uncomplicated rhegmatogenous retinal
detachment: Clinical outcomes and complications. Retina.
36:1906–1912. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen SN, Lian IeB and Wei YJ: Epidemiology
and clinical characteristics of rhegmatogenous retinal detachment
in Taiwan. Br J Ophthalmol. 100:1216–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wormstone IM: Posterior capsule
opacification: A cell biological perspective. Exp Eye Res.
74:337–347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wormstone IM, Wang L and Liu CS: Posterior
capsule opacification. Exp Eye Res. 88:257–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong N, Tang X and Xu B: miRNA-181a
inhibits the proliferation, migration, and epithelial-mesenchymal
transition of lens epithelial cells. Invest Ophthalmol Vis Sci.
56:993–1001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao K, Ye PP, Tan J, Tang XJ and Shen Tu
XC: Involvement of PI3K/Akt pathway in TGF-beta2-mediated
epithelial mesenchymal transition in human lens epithelial cells.
Ophthalmic Res. 40:69–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parikh A, Lee C, Joseph P, Marchini S,
Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, et al:
microRNA-181a has a critical role in ovarian cancer progression
through the regulation of the epithelial-mesenchymal transition.
Nat Commun. 5:29772014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative geneexpression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Tang X and Chen X: Comparative
effects of TGF-β2/Smad2 and TGF-β2/Smad3 signaling pathways on
proliferation, migration, and extracellular matrix production in a
human lens cell line. Exp Eye Res. 92:173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zode GS, Sethi A, Brun-Zinkernagel AM,
Chang IF, Clark AF and Wordinger RJ: Transforming growth factor-β2
increases extracellular matrix proteins in optic nerve head cells
via activation of the Smad signaling pathway. Mol Vis.
17:1745–1758. 2011.PubMed/NCBI
|
|
14
|
Ryan DG, Oliveira-Fernandes M and Lavker
RM: MicroRNAs of the mammalian eye display distinct and overlapping
tissue specificity. Mol Vis. 12:1175–1184. 2006.PubMed/NCBI
|
|
15
|
Makarev E, Spence JR, Del Rio-Tsonis K and
Tsonis PA: Identification of microRNAs and other small RNAs from
the adult newt eye. Mol Vis. 12:1386–1391. 2006.PubMed/NCBI
|
|
16
|
Caiado RR, Magalhães O Jr, Badaró E, Maia
A, Novais EA, Stefanini FR, Navarro RM, Arevalo JF, Wu L, Moraes N,
et al: Effect of lens status in the surgical success of 23-gauge
primary vitrectomy for the management of rhegmatogenous retinal
detachment: The Pan American Collaborative Retina Study (PACORES)
group results. Retina. 35:326–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Zhang ZX, Lian D, Haig A,
Bhattacharjee RN and Jevnikar AM: IL-37 inhibits IL-18-induced
tubular epithelial cell expression of pro-inflammatory cytokines
and renal ischemia-reperfusion injury. Kidney Int. 87:396–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Zhang P, Sun X, Sun Y, Shi C, Liu H
and Liu X: MicroRNA-181a regulates epithelial-mesenchymal
transition by targeting PTEN in drug-resistant lung adenocarcinoma
cells. Int J Oncol. 47:1379–1392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor MA, Sossey-Alaoui K, Thompson CL,
Danielpour D and Schiemann WP: TGF-β upregulates miR-181a
expression to promote breast cancer metastasis. J Clin Invest.
123:150–163. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brockhausen J, Tay SS, Grzelak CA,
Bertolino P, Bowen DG, d'Avigdor WM, Teoh N, Pok S, Shackel N,
Gamble JR, et al: miR-181a mediates TGF-β-induced hepatocyte EMT
and is dysregulated in cirrhosis and hepatocellular cancer. Liver
Int. 35:240–253. 2015. View Article : Google Scholar : PubMed/NCBI
|