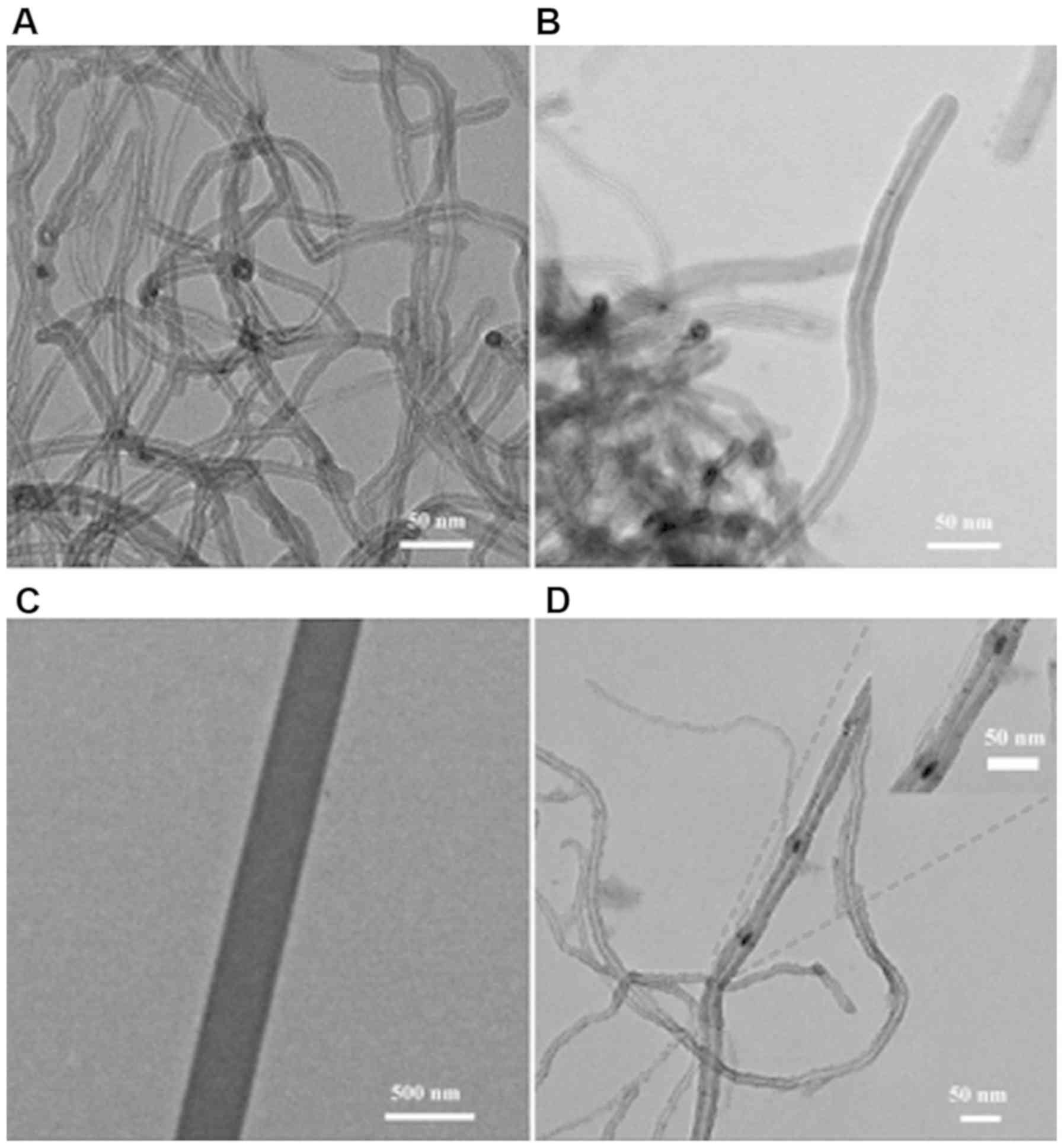
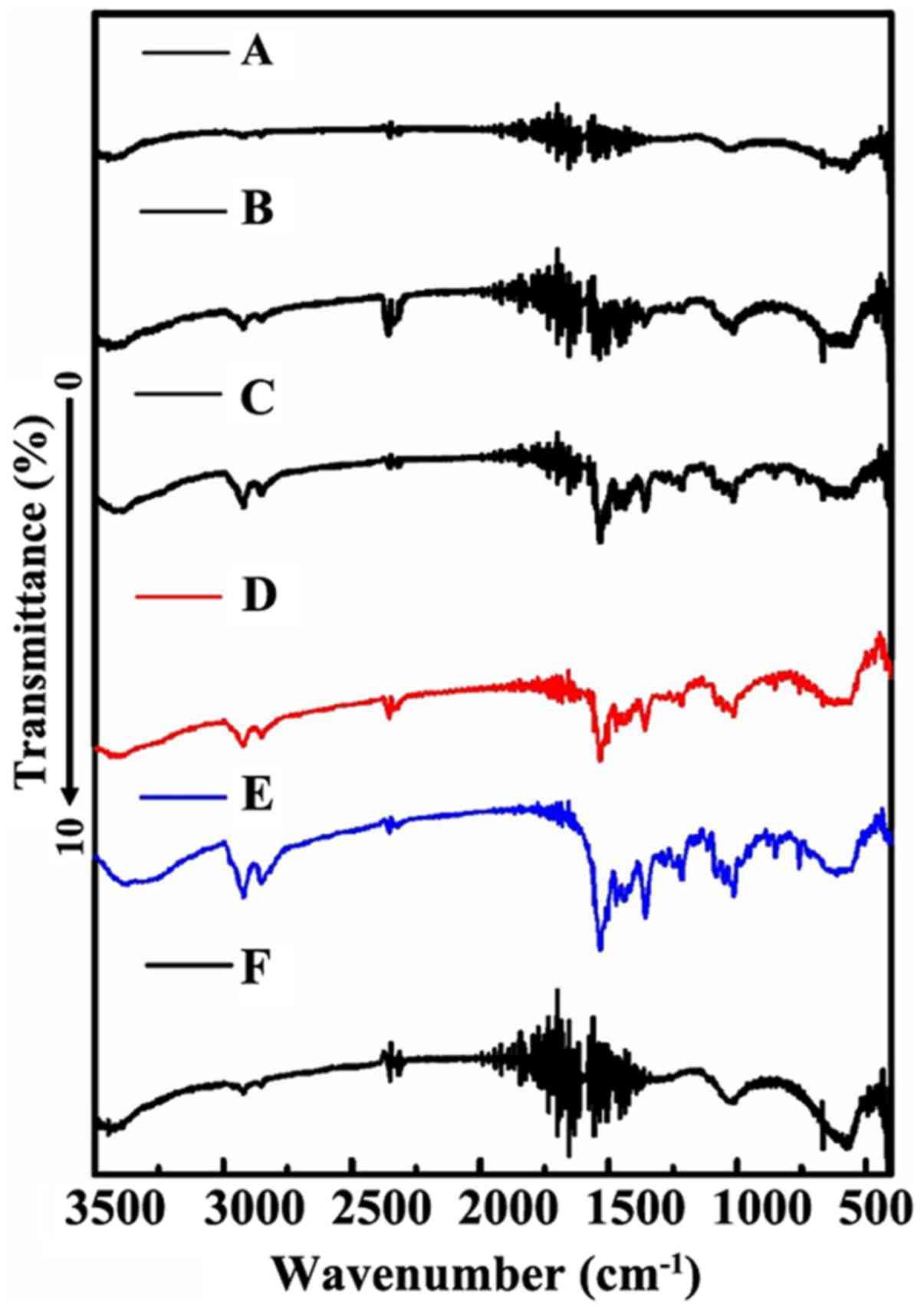
|
1
|
D'Souza SL, Deshmukh B, Bhamore JR, Rawat
KA, Lenka N and Kailasa SK: Synthesis of fluorescent nitrogen-doped
carbon dots from dried shrimps for cell imaging and boldine drug
delivery system. Rsc Adv. 6:12169–12179. 2016. View Article : Google Scholar
|
|
2
|
Zhang H, Hou L, Jiao X, Ji Y, Zhu X and
Zhang Z: Transferrin-mediated fullerenes nanoparticles as
Fe(2+)-dependent drug vehicles for synergistic anti-tumor efficacy.
Biomaterials. 37:353–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Huang X, Long Y, Wang X, Zhang H,
Zhu R, Liang L, Teng P and Zheng H: Hollow luminescent carbon dots
for drug delivery. Carbon. 59:192–199. 2013. View Article : Google Scholar
|
|
4
|
Potter C, Tian Y, Walker G, McCoy C,
Hornsby P, Donnelly C, Jones DS and Andrews GP: Novel supercritical
carbon dioxide impregnation technique for the production of
amorphous solid drug dispersions: A comparison to hot melt
extrusion. Mol Pharm. 12:1377–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muzi L, Ménard-Moyon C, Russier J, Li J,
Chin CF, Ang WH, Pastorin G, Risuleo G and Bianco A:
Diameter-dependent release of a cisplatin pro-drug from small and
large functionalized carbon nanotubes. Nanoscale. 7:5383–5394.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao MZ, Huang-Fu MY, Liu HN, Wang XR,
Sheng X and Gao JQ: Fabrication and characterization of drug-loaded
nano-hydroxyapatite/polyamide 66 scaffolds modified with carbon
nanotubes and silk fibroin. Int J Nanomedicine. 11:6181–6194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sciortino N, Fedeli S, Paoli P, Brandi A,
Chiarugi P, Severi M and Cicchi S: Multiwalled carbon nanotubes for
drug delivery: Efficiency related to length and incubation time.
Int J Pharm. 521:69–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhirde AA, Patel V, Gavard J, Zhang G,
Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS and
Rusling JF: Targeted killing of cancer cells in vivo and in vitro
with EGF-directed carbon nanotube-based drug delivery. ACS Nano.
3:307–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ajima K, Yudasaka M, Murakami T, Maigné A,
Shiba K and Iijima S: Carbon nanohorns as anticancer drug carriers.
Mol Pharm. 2:475–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heister E, Neves V, Lamprecht C, Silva
SRP, Coley HM and McFadden J: Drug loading, dispersion stability,
and therapeutic efficacy in targeted drug delivery with carbon
nanotubes. Carbon. 50:622–632. 2012. View Article : Google Scholar
|
|
11
|
Cheng Y, Lu S, Zhang H, Varanasi CV and
Liu J: Synergistic effects from graphene and carbon nanotubes
enable flexible and robust electrodes for high-performance
supercapacitors. Nano Lett. 12:4206–4211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan L, Lu XH, Xiao X, Zhai T, Dai J,
Zhang F, Hu B, Wang X, Gong L, Chen J, et al: Flexible solid-state
supercapacitors based on carbon nanoparticles/MnO2
nanorods hybrid structure. ACS Nano. 6:656–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park H, Afzali A, Han SJ, Tulevski GS,
Franklin AD, Tersoff J, Hannon JB and Haensch W: High-density
integration of carbon nanotubes via chemical self-assembly. Nat
Nanotechnol. 7:787–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ncibi MC and Sillanpää M: Optimizing the
removal of pharmaceutical drugs Carbamazepine and Dorzolamide from
aqueous solutions using mesoporous activated carbons and
multi-walled carbon nanotubes. J Mol Liq. 238:379–388. 2017.
View Article : Google Scholar
|
|
15
|
Lee G, Choong H, Chiang G and Woo K:
Three-year randomized controlled trial of dipyridamole and low-dose
warfarin in patients with IgA nephropathy and renal impairment.
Nephrology (Carlton). 3:117–121. 2010. View Article : Google Scholar
|
|
16
|
Tang Y, Liu SY, Armes SP and Billingham
NC: Solubilization and controlled release of a hydrophobic drug
using novel micelle- forming ABC triblock copolymers.
Biomacromolecules. 4:1636–1645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Waard H, Hessels MJT, Boon M, Sjollema
KA, Hinrichs WLJ, Eissens AC and Frijlink HW: CLSM as quantitative
method to determine the size of drug crystals in a solid
dispersion. Pharm Res. 28:2567–2574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zecevic DE, Meier R, Daniels R and Wagner
KG: Site specific solubility improvement using solid dispersions of
HPMC-AS/HPC SSL-mixtures. Eur J Pharm Biopharm. 87:264–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakai T and Thommes M: Investigation into
mixing capability and solid dispersion preparation using the DSM
Xplore Pharma Micro Extruder. J Pharm Pharmacol. 66:218–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Gray L, Leonardi-Bee J, Weaver CS,
Heptinstall S and Bath PM: Effect of aspirin, clopidogrel and
dipyridamole on soluble markers of vascular function in normal
volunteers and patients with prior ischaemic stroke. Platelets.
17:100–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan YD, Sung JH, Kim KK, Kim DW, Kim JO,
Lee BJ, Yong CS and Choi HG: Novel valsartan-loaded solid
dispersion with enhanced bioavailability and no crystalline
changes. Int J Pharm. 422:202–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmoodi M, Arjmand M, Sundararaj U and
Park S: The electrical conductivity and electromagnetic
interference shielding of injection molded multi-walled carbon
nanotube/polystyrene composites. Carbon. 50:1455–1464. 2012.
View Article : Google Scholar
|
|
23
|
Gupta TK, Singh BP, Mathur RB and Dhakate
SR: Multi-walled carbon nanotube-graphene-polyaniline multiphase
nanocomposite with superior electromagnetic shielding
effectiveness. Nanoscale. 6:842–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pippa N, Chronopoulos DD, Stellas D,
Fernández-Pacheco R, Arenal R, Demetzos C and Tagmatarchis N:
Design and development of multi-walled carbon nanotube-liposome
drug delivery platforms. Int J Pharm. 528:429–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bottini M, Bruckner S, Nika K, Bottini N,
Bellucci S, Magrini A, Bergamaschi A and Mustelin T: Multi-walled
carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett.
160:121–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gabizon AA, Barenholz Y and Bialer M:
Prolongation of the circulation time of doxorubicin encapsulated in
liposomes containing a polyethylene glycol-derivatized
phospholipid: Pharmacokinetic studies in rodents and dogs. Pharm
Res. 10:703–708. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu W, Zhao Q, Sun C, Zhang Z, Jiang T,
Sun J, Li Y and Wang S: Mesoporous carbon with spherical pores as a
carrier for celecoxib with needle-like crystallinity: Improve
dissolution rate and bioavailability. Mater Sci Eng C. 39:13–20.
2014. View Article : Google Scholar
|
|
28
|
Zhu WQ, Wan L, Zhang C, Gao YK, Zheng X,
Jiang TY and Wang SL: Exploitation of 3D face-centered cubic
mesoporous silica as a carrier for a poorly water soluble drug:
Influence of pore size on release rate. Mater Sci Eng C. 34:78–85.
2014. View Article : Google Scholar
|
|
29
|
Zhang X, Hui Z, Wan D, Huang H, Huang J,
Yuan H and Yu J: Alginate microsphere filled with carbon nanotube
as drug carrier. Int J Biol Macromol. 47:389–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao W, Paul A, Zhao B, Lee C, Rodes L and
Prakash S: Carbon nanotube lipid drug approach for targeted
delivery of a chemotherapy drug in a human breast cancer xenograft
animal model. Biomaterials. 34:10109–10119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong BS, Yoong SL, Jagusiak A, Panczyk T,
Ho HK, Ang WH and Pastorin G: Carbon nanotubes for delivery of
small molecule drugs. Adv Drug Deliv Rev. 65:1964–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan J, Zeng F, Xu J and Wu S: Targeted
anti-cancer prodrug based on carbon nanotube with photodynamic
therapeutic effect and pH-triggered drug release. J Nanopart Res.
15:19112013. View Article : Google Scholar
|
|
33
|
Chen J, Sarma B, Evans JMB and Myerson AS:
Pharmaceutical crystallization. Cryst Growth Des. 11:887–895. 2011.
View Article : Google Scholar
|
|
34
|
Weimann PA, Hajduk DA, Chu C, Chaffin KA,
Brodil JC and Bates FS: Crystallization of tethered polyethylene in
confined geometries. J Polym Sci Pol Phys37. 2053–2068. 2015.
|
|
35
|
Leon RAL, Wan WY, Badruddoza AZM, Hatton
TA and Khan SA: Simultaneous spherical crystallization and
co-formulation of drug(s) and excipient from microfluidic double
emulsions. Cryst Growth Des. 14:140–146. 2014. View Article : Google Scholar
|
|
36
|
Löbmann K, Laitinen R, Grohganz H, Gordon
KC, Strachan C and Rades T: Coamorphous drug systems: Enhanced
physical stability and dissolution rate of indomethacin and
naproxen. Mol Pharm. 8:1919–1928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shegokar R and Müller RH: Nanocrystals:
Industrially feasible multifunctional formulation technology for
poorly soluble actives. Int J Pharm. 399:129–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ezzati Nazhad Dolatabadi J, Omidi Y and
Losic D: Carbon nanotubes as an advanced drug and gene delivery
nanosystem. Curr Nanosci. 7:297–314. 2011. View Article : Google Scholar
|