Introduction
Aneuploidy refers to the presence of an abnormal
number of chromosomes in a cell and an extra or missing chromosome
is a common genetic disorder (1).
Aneuploidy frequently results in miscarriage, but is also the cause
of a large number of birth defects. Among those defects, trisomy
21, 18 and 13 are most common (1).
The prevalence of trisomy 21 (Down syndrome) is 10.3–13.6 per
10,000 live births in the USA and Europe (2), while the prevalence of trisomy 18 and
trisomy 13 is 4.0 and 1.6 cases per 10,000 pregnancies,
respectively (3). These syndromes
are characterized by multiple malformations, concomitant medical
conditions and cognitive impairment (4–6). These
conditions cannot be cured and their impacts on the parents and
society are important. Therefore, pre-natal diagnosis has a
critical role in the management of aneuploidies.
Even if certain ultrasound features may hint toward
the possibility of aneuploidy in a fetus, the only definitive and
gold-standard diagnostic method remains karyotype analysis.
Nevertheless, this method requires cell culture, time and viable
cells, and has low sensitivity (7).
Novel methods, including quantitative fluorescence polymerase chain
reaction (QF-PCR), fluorescence in situ hybridization and
comparative genomic hybridization (CGH) have certain advantages,
including saving time and cost, the requirement of small numbers of
cells and high accuracy. In general, the results for common
aneuploidies may be obtained within 24–48 h (8). QF-PCR has high sensitivity for
aneuploidies involving chromosomes 21, 18, 13, X and Y, but not for
rare aneuploidies. QF-PCR is increasingly considered as a
complementary investigation (9–13) or as
an alternative to conventional cytogenetic analysis.
In recent years, non-invasive pre-natal testing
(NIPT) technologies have developed rapidly. As the associated
conditions cannot be cured and their impact on the parents' and
child's life and society are non-negligible, the parents desire
result confirmation as soon as possible when the screening results
indicate a high risk of aneuploidy. The value of QF-PCR as a
screening method is controversial (11–14), but
it may be used as a mid-pregnancy confirmation test if common fetal
aneuploidies have been identified. Therefore, the aim of the
present study was to examine the value of QF-PCR for diagnostic
confirmation of aneuploidies and the impact of the parental origin
and meiosis stage on the detected aneuploidy.
Materials and methods
Study design and subjects
The study was approved by the Ethics Committee of
Hebei General Hospital (Shijiazhuang, China). All participants
provided written informed consent.
The present study was a prospective cohort study of
428 consecutive high-risk pregnant women (age, 21–45 years;
gestational age, 17–25 weeks) who consulted between May 2015 and
December 2016 at Hebei General Hospital (Shijiazhuang, China).
Patients with at least one of the following indications were
included: i) High-risk NIPT (Z score >3); ii) mid-pregnancy
screening high-risk value (>1/100); and iii) >2 fetal
abnormalities on ultrasound (15).
No other genetic testing was performed. All indicators prior to
amniotic fluid examination were required to be normal. All female
patients provided written informed consent for the pre-natal
diagnosis.
The indications for NITP were: i) Serological
screening or imaging examinations suggesting borderline risk of
common chromosomal aneuploidy; ii) contraindications to
interventional pre-natal diagnosis (including threatening abortion,
fever, coagulation problems, communicable diseases to the fetus,
including hepatitis B and C viruses, syphilis and human
immunodeficiency virus, ongoing infection and incompatibility of
maternal and fetal RH blood group); and iii) gestational age
>20+6 weeks, i.e., women who missed the optimal
timing for serological screening. The indications for serological
screening in the second trimester were: i) Singleton pregnancy of
16–20+6 weeks; ii) for female patients with irregular
menstruation, the biparietal diameter of the fetus was required to
be ≤48 mm; iii) the age of the mother at the expected due time was
<35 years; iv) without history of abnormal pregnancy; and v) the
ultrasound examinations of the fetus exhibited no
abnormalities.
In the context of routine pre-natal examinations,
color fetal ultrasound is first performed to search for
morphological and developmental abnormalities. Subsequently, blood
routine examinations were performed and blood coagulation indexes,
blood type and viral indexes [including hepatitis B surface antigen
or antibody, hepatitis B antigen or antibody, hepatitis B core
antibody, hepatitis B virus, syphilis and HIV] were determined, and
vaginal secretions (routinely examined for vaginal cleanliness,
pus, vaginal Lactobacillus, Chlamydia trachomatis, Clue
cells, Candida albicans, and Trichomonas vaginalis,
body temperature and pulse of the pregnant subjects were measured
to ensure that no surgical contraindications were present. The
women with any contraindications or with multiple gestations were
excluded.
Sampling
The amniotic fluid was sampled using a 21-G sterile
needle and syringe under real-time ultrasound guidance (ALOKA5500;
ALOKA). The first 1 ml of amniotic fluid was discarded and 5 ml
were sampled for QF-PCR and 20 ml for karyotype analysis.
Furthermore, 2 ml of venous blood were collected from the
antecubital vein of 74 couples (both parents) with confirmed fetal
aneuploidy.
For NITP, 5 ml peripheral blood were obtained from
the pregnant female subjects and used for fetal free-DNA screening
of the plasma at the Boao Clinical Examination Center (Yizhuang
Biomedical Park, Beijing, China). The free DNA was obtained for
high-resolution sequencing and the actual number of nucleotide
fragments distributed in each chromosome was measured. The number
was compared with the result of the high-performance computing
sequencing fragment count. Combined bioinformatics analysis was
then performed to assess whether the fetus had any chromosomal
aneuploidy. The assessment of the stage of abnormal chromosome
segregation was performed using the genetic map. If the redundant
short tandem repeat (STR) was of the parental double-STR type, the
abnormal chromosome segregation had occurred in the first meiosis.
If the redundant STR was of the parental single-STR type, the
abnormal chromosome segregation had occurred in the second meiosis.
If the two types were present, it was not possible to determine the
time of abnormal chromosome segregation due to exchanges of
parental chromosomes.
QF-PCR
Loci D21S11 (located at 21q21.1), D21S1435 (at
21q21) and PENTAD (at 21q22.3) were used to assess chromosome 21.
The loci D18S51 (located at 18q21.33), D18S1002 (at 18q11.2),
D18S535 (at 18q12.2) and D18S391 (at 18p11.22) were used to assess
chromosome 18. The loci D13S317 (at 13q31.1) and D13S634 (at
13q14.3) were used to assess chromosome 13. The loci Amelogenin
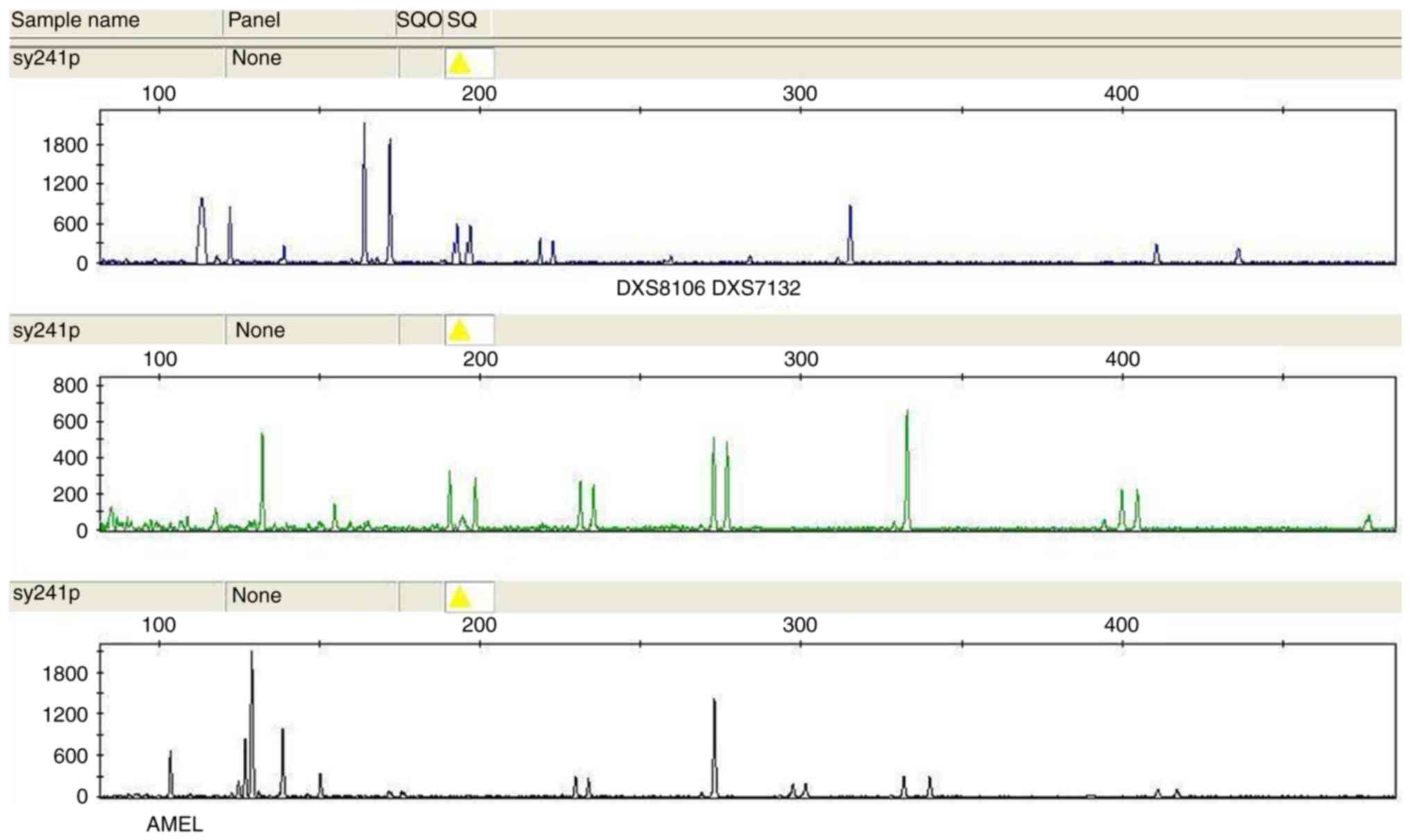
(located at Xp22.3), DXS8106 (at Xq27.3) and DXS7132 (at Xq11.2)
were used to assess the sex chromosomes (16,17).
Primer sequences are presented in Table
I. All primers were synthesized by Shanghai YingweiJieji
Trading Co., Ltd. Fluorescent labeling was performed at the 5′ end
using carboxyfluorescein (blue), carboxytetramethylrhodamine
(yellow) or carboxy-4′,5′-dichloro-2′,7′-dimethylfluorescein
(green).
 | Table I.Primers for chromosomes 21, 18, 13
and X. |
Table I.
Primers for chromosomes 21, 18, 13
and X.
| STR | Primers |
|---|
| AMEL | F:
5′-(TAMRA)-CCCTGGGCTCTGTAAAGAA-3′ |
|
| R:
5′-ATCAGAGCTTAAACTGGGAAGCTG-3′ |
| D13S317 | F:
5′-ATTACAGAAGTCTGGGATGTGGAGGA-3′ |
|
| R:
5′-(JOE)-GGCAGCCCAAAAAGACAGA-3′ |
| D13S634 | F:
5′-GGCAGATTCAATAGGATAAATAGA-3′ |
|
| R:
5′-(TAMRA)-GTAACCCCTCAGGTTCTCAAGTCT-3′ |
| D18S1002 | F:
5′-(TAMRA)-CAAAGAGTGAATGCTGTACAAACAGC-3′ |
|
| R:
5′-CAAGATGTGAGTGTGCTTTTCAGGAG-3′ |
| D18S391 | F:
5′-GGACTTACCACAGGCAATGTGACT-3′ |
|
| R:
5′-(JOE)-TAGACTTCACTATTCCCATCTGAG-3′ |
| D18S51 | F:
5′-(FAM)-TTCTTGAGCCCAGAAGGTTA-3′ |
|
| R:
5′-ATTCTACCAGCAACAACACAAATAAAC-3′ |
| D18S535 | F:
5′-CAGCAAACTTCATGTGACAAAAGC-3′ |
|
| R:
5′-(JOE)-CAATGGTAACCTACTATTTACGTC-3′ |
| D21S11 | F:
5′-ATATGTGAGTCAATTCCCCAAG-3′ |
|
| R:
5′-(FAM)-TGTATTAGTCAATGTTCTCCAGAGAC-3′ |
| D21S1435 | F:
5′-CCCTCTCAATTGTTTGTCTACC-3′ |
|
| R:
5′-(TAMRA)-GCAAGAGATTTCAGTGCCAT-3′ |
| DXS7132 | F:
5′-AGCCCATTTTCATAATAAATCC-3′ |
|
| R:
5′-(FAM)-AATCAGTGCTTTCTGTACTATTGG-3′ |
| DXS8106 | F:
5′-(FAM)-CTTGCACTTGCTGTGG-3′ |
|
| R:
5′-AGCTGTAGAGTTGAGGAATG-3′ |
| PENTAD | F:
5′-(JOE)-GAAGGTCGAAGCTGAAGTG-3′ |
|
| R:
5′-ATTAGAATTCTTTAATCTGGACACAAG-3′ |
Amniotic fluid and peripheral blood DNA were
extracted using the Lab-Aid 820 DNA extraction kit (Xiamen Zeesan
Biotech Co., Ltd.), according to the manufacturer's protocols.
Primers were generated with fluorescent tags according to the
sequences in the National Center for Biotechnology Information
database (strbase.nist.gov/str_fact.htm; www.ncbi.nlm.nih.gov). The reaction mixture was
prepared using 100 µl GoTaq hot start colorless master mix (Promega
Corp.), primers (10 µl) and H2O (70 µl). Amplification
was performed using 9 µl of this reaction mixture with 1 µl DNA.
The QF-PCR program was: i) 2 min at 95°C, followed by 20 sec at
95°C and 80 sec at 59°C; ii) 30 cycles of 20 sec at 95°C, 80 sec at
59°C and 40 sec at 73°C; and iii) final extension for 10 min at
73°C. The QF-PCR products (1 µl) were added to 10 ml formamide
(Applied Biosystems; Thermo Fisher Scientific, Inc.), containing
0.25 µl LIS 600 (Promega Corp.) as a standard. After denaturation
at 95°C for 5 min, the mixture was cooled quickly and capillary
electrophoresis was performed on an ABI 3130 Genetic Analyzer
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using a POP4
polymer.
Karyotype analysis
Karyotyping was performed using a routine method
(18). Amniocytes were centrifuged
and precipitated, and cultured in the appropriate medium. G banding
was performed and the chromosome smear was prepared. An automatic
scanning microscope and image analysis system (GSL-120; Leica
Microsystems) were used to perform chromosome karyotype
analysis.
Data analysis
GeneMapper 3.2 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to analyze the results and calculate the
peak areas. Allele ratios (shorter allele/longer allele) between
0.8 and 1.4 were considered as normal, and ratios of >1.8 or
<0.65, or the presence of three alleles of equal peak area were
considered as trisomy. The presence of a single peak was considered
as uninformative and a minimum of two concordant informative
markers for each chromosome (21, 18, 13 and X) were required for a
confident result (19). Parental
origin was obtained from the genotypes of STR markers in the same
locus of the fetus. If parental heterozygosity was retained in the
trisomic offspring, non-disjunction error from meiosis I was
considered. If parental heterozygosity was reduced to homozygosity,
non-disjunction error from meiosis II was considered (20). When the two types existed at the same
time, non-disjunction error from the parental chromosome exchange
was considered.
Results
Patient and specimen
characteristics
The amniotic fluid from the 428 pregnant women was
analyzed by karyotype and QF-PCR. The mean age of the pregnant
women was 30.4 years (range, 21–45 years) and the mean gestational
age was 20.0 weeks (range, 17–25 weeks). The QF-PCR results for
representative cases of aneuploidy by are provided in Figs. 1–7.
Technically, the two methods were 100% successful, a readable
result was obtained from each test and no retesting was
required.
Aneuploidy distribution by
karyotype
The tests identified 105 cases of aneuploid
karyotype, including 71 cases of common trisomy 21 and one case of
ectopic type trisomy 21. Trisomy 21 accounted for 68.6% of all
trisomy cases. There were 20 cases of trisomy 18 (19.0%), 8 cases
of sex chromosome aneuploidy (7.6%; 5 cases of 47,XXX; 2 cases of
47,XXY; and 1 case of 45,XO), 4 cases of trisomy 13 (3.8%;
including one case of ectopic type trisomy 13) and 1 case of
48,XXX,+18 (1.0%) (Table II).
 | Table II.Fetal chromosome aneuploidy
distribution in mid-pregnancy. |
Table II.
Fetal chromosome aneuploidy
distribution in mid-pregnancy.
| Chromosome
aneuploidy type | n (%) |
|---|
| Trisomy 21
(47,XX,+21) (including one ectopic type) | 72 (68.6) |
| Trisomy 18
(47,XX,+18) | 20 (19.0) |
| Sex chromosome
aneuploidy | 8 (7.6) |
| Trisomy 13
(47,XX,+13) | 4 (3.8) |
| 48,XXX,+18
(including one ectopic type) | 1 (1.0) |
| Total | 105 (100.0) |
Concordance between karyotype and
QF-PCR
By using karyotyping and QF-PCR analysis, 105 cases
of aneuploidy were identified. Using karyotype as the gold
standard, QF-PCR indicated no false-positive or false-negative
results, due to a concordance of 100%. Among the 62 cases with a
high risk of trisomy 21 according to NIPT, 58 cases had positive
results in the karyotype and STR analyses, while 4 cases exhibited
no obvious abnormality, leading to a true-positive rate of 93.5%.
Among the 18 cases with a high risk of trisomy 18 according to
NIPT, 15 had positive results in the karyotype and STR analyses,
while 3 cases exhibited no obvious abnormality, leading to a
true-positive rate of 83.3% (Table
III).
 | Table III.Concordance between karyotype and
QF-PCR. |
Table III.
Concordance between karyotype and
QF-PCR.
| Abnormality | n (%) |
|---|
| Chromosome 21
(according to NIPT) | 62 (100) |
| Positive on
karyotype and STR (true positive) | 58 (93.5) |
| No obvious
abnormality | 4 (6.5) |
| Chromosome 18 | 18 (100) |
| Positive on
karyotype and STR (true positive) | 15 (83.3) |
| No obvious
abnormality | 3 (16.7) |
Parental origin of non-disjunction
errors of chromosomal aneuploidy
Blood samples from 81 couples (out of 105 cases of
fetal chromosomal aneuploidy; 77.1%) were collected and tested for
parental origin of non-disjunction error. The results indicated
that 47 cases of trisomy 21 were of maternal origin (85.4%) and
five were of paternal origin (9.1%), while the source was unknown
in three cases (5.5%). Among the 17 cases of trisomy 18, 15 cases
were of maternal (88.2%) and two of paternal origin (11.8%). Among
the 4 cases of trisomy 13, 3 cases were of maternal (75.0%) and 1
of paternal origin (25.0%). Among the 5 cases of sex chromosome
abnormality, 2 cases were of maternal (40.0%) and 3 of paternal
origin (60.0%) (Table IV).
 | Table IV.Parental origin of non-disjunction
errors. |
Table IV.
Parental origin of non-disjunction
errors.
| Parental origin of
non-disjunction error | n (%) |
|---|
| Chromosome 21 |
|
|
Maternal origin | 47 (85.4) |
|
Paternal origin | 5 (9.1) |
|
Parental origin not
determined | 3 (5.5) |
|
Total | 55 (100) |
| Chromosome 18 |
|
|
Maternal origin | 15 (88.2) |
|
Paternal origin | 2 (11.8) |
|
Total | 17 (100) |
| Sex chromosome |
|
|
Maternal origin | 2 (40.0) |
|
Paternal origin | 3 (60.0) |
|
Total | 5 (100) |
Frequency of maternal meiosis stage
errors
The frequency of maternal meiosis stage errors was
significantly different (P=0.041) between trisomy 21 [76.6% meiosis
I (n=36), 12.8% meiosis II (n=6), 8.5% maternal transition (n=4)
and 2.1% maternal reproductive cell chimeras (n=1)] and trisomy 18
[46.7% meiosis I (n=7), 40.0% meiosis II (n=6) and 13.3% maternal
transition (n=2)] (Table V).
 | Table V.Maternal meiosis stages of
non-disjunction errors. |
Table V.
Maternal meiosis stages of
non-disjunction errors.
| Maternal meiosis
stage of non-disjunction errors | n (%) |
|---|
| Chromosome 21 |
|
| Meiosis
I | 36 (76.6) |
| Meiosis
II | 6 (12.8) |
|
Maternal transition | 4 (8.5) |
|
Maternal reproductive cell
chimeras | 1 (2.1) |
|
Total | 47 (100) |
| Chromosome 18 |
|
| Meiosis
I | 7 (46.7) |
| Meiosis
II | 6 (40.0) |
|
Maternal transition | 2 (13.3) |
|
Total | 15 (100) |
Discussion
The value of QF-PCR as a screening test is
controversial, but it may be used as a mid-pregnancy test to
confirm the diagnosis of common fetal aneuploidies (11–14). In
this light, the present study aimed to examine the value of QF-PCR
in diagnostic karyotype confirmation and the impact of the parental
origin and meiosis stage on the aneuploidy. The results suggest
that the combination of NIPT and QF-PCR may become a rapid and
effective method for fetal aneuploidy detection. Testing of the
parental origin and meiosis stage of non-disjunction errors by
QF-PCR provides additional genetic information for the diagnosis
and management of aneuploidies, as opposed to karyotyping
alone.
QF-PCR has numerous advantages. Without a doubt, the
major advantages of QF-PCR are that the results are obtained
rapidly and only require a small amount of amniotic fluid and no
cell culture. Hence, QF-PCR is more cost-effective than karyotyping
(21). Furthermore, QF-PCR is able
to detect >90% of clinically significant chromosomal
abnormalities (11,22–25), but
this is controversial and certain studies suggest that QF-PCR may
fail to detect 15–30% of the abnormalities identified by
karyotyping (26,27). On the other hand, one limitation of
QF-PCR is that it fails to detect structural abnormalities and
mosaicism of <30% (28). It has
been suggested that restricting the use of QF-PCR for low-risk
pregnancies or combining QF-PCR with other modalities, e.g. nuchal
translucency, may be sufficient for screening, but there is a
concern regarding the lack of high-quality data from karyotyping,
and this issue remains controversial (12).
In the present study, QF-PCR had a concordance rate
of 100% with the karyotype, without any false-positive or -negative
results. This observation is particularly good and is supported by
previous studies that also obtained accuracies of almost 100%.
Indeed, Badenas et al (11)
reported a concordance rate of 98.8% among 7,679 pre-natal samples.
In a study by De la Paz-Gallardo et al (12), the results of 99% of the 928 samples
included were concordant; in addition, if QF-PCR had been used as
the major diagnostic method, with confirmation by karyotyping only
in high-risk individuals identified based on imaging, only 12.5% of
the samples would have required karyotype confirmation. Rostami
et al (13) reported that
among 4,058 pre-natal samples, 98.6% were successfully diagnosed by
QF-PCR, but karyotyping detected additional cases. On the other
hand, Papoulidis et al (14)
reported that karyotyping detected 320 aneuploidies among 13,500
cases, but QF-PCR did not detect 70 (21.9%) of these 320 cases. In
a Chinese study, only two cases out of 210 were discordant between
karyotype and QF-PCR (16). However,
there are certain variations among studies, populations and methods
(10). A recent meta-analysis
indicated that the sensitivity/specificity of QF-PCR compared with
karyotyping vary depending on the tested chromosome, with
respective values of 99.4/99.9% for trisomy 21, 97.7/99.9% for
trisomy 18, 92.9/99.9% for monosomy X and 90.6/100% for trisomy 13
(29).
Early studies suggested that the detection of sex
chromosome aneuploidies by QF-PCR was poor (30,31), but
more recent studies indicated a good performance (32–34). The
difficulties are due to the low polymorphic level of sex chromosome
STRs, but the discovery of appropriate markers improved the
detection of sex chromosome aneuploidies (33,34).
Hence, the choice of the primers for QF-PCR influences the results
and diagnostic performance. Additional studies are required to
address these issues.
NIPT for aneuploidy using cell-free DNA in maternal
plasma is a novel direction for pre-natal screening and diagnosis.
Clinical trials have demonstrated the efficacy of NIPT for trisomy
21, 18 and 13 in high-risk females, but positive NIPT results must
be confirmed using invasive techniques (35). Of note, the NIPT data for the three
abovementioned aneuploidies had 100% (or close to) diagnostic
sensitivity and specificity; the test also correctly identified the
fetal sex in all cases (36–38). However, it must be emphasized that
for aneuploidies, the diagnostic performance of cell-free fetal DNA
methods, including QF-PCR, may be affected by disease prevalence
and placental mosaicism, and QF-PCR should be considered, for now,
as a screening test (29). In the
present study, chromosome analysis and QF-PCR detection indicated
that the true-positive rates of NIPT for trisomy 21 and 18 were
93.5 and 83.3%, respectively. These discrepancies among previously
published studies may be due to differences in populations
regarding factors including genetics or polymorphisms. Additional
studies are required to examine this issue.
Determining from which parent the aneuploidy
originates may be useful in certain situations, e.g. in egg or
sperm donation, or to determine which parent is at higher risk of
yielding aneuploid gametes (39).
Aneuploid embryos mostly occur due to maternal aneuploid gametes,
but 1–2% of aneuploid embryos are due to aneuploid gametes from the
sperm. The aneuploidy incidence of oocytes is higher than that of
sperm due to more effective checkpoints in the processes of
spermatogenesis compared to oogenesis (40). This strong maternal bias occurs
mainly in autosomal chromosomes, and sex chromosome abnormalities
(e.g. those associated with Klinefelter's syndrome) are usually
from the father (41). The present
study indicated that 85.4% of the cases of trisomy 21 had a
maternal and 9.1% a paternal source, while the source was unknown
for 5.5% (the selected markers were present in the mother and
father, and the exact source remained undetermined), as supported
by previous studies (20,42). Studies suggested that 5–9% of trisomy
21 cases result from paternal meiosis errors (43,44). The
development of complete human gametes involves two meiotic
divisions. The first meiotic division is the separation of
homologous chromosomes and the second separates sister chromatids.
Previous studies suggested that in trisomy 21, more errors occur in
meiosis I than in meiosis II (45).
In the present study, for trisomy 21, 76.6% meiosis I and 12.8%
meiosis II errors, 8.5% maternal transition and 2.1% maternal
reproductive cell chimeras were detected. This result was in
disagreement with a previous study from Europe (42). Differences in ethnicity and genetics
may explain, at least in part, the discrepancies.
Trisomy 18 is the second most common trisomy
syndrome after trisomy 21 (5). It is
also important in pre-natal diagnosis due to being associated with
a high risk of fetal loss and stillbirth (46–48). The
present study indicated that in the context of mid-pregnancy
diagnosis, trisomy 18 accounted for 19.0% of the cases, second to
trisomy 21. The extra chromosome of trisomy 18 cases was usually of
maternal origin. Indeed, 88.2% of cases of trisomy 18 were maternal
and 11.8% were paternal. This is different from other autosomal
abnormalities, which more frequently arise in meiosis I. About half
of the non-disjunction errors occur in meiosis II of oocytes
(49,50). The results of the present study were
consistent with these results and indicated that the stages of
meiotic separation affected in trisomy 18 were 46.7% for meiosis I,
40.0% for meiosis II and 13.3% for maternal transition.
The present study included certain cases of trisomy
13 and sex chromosome aneuploidy. In the two cases in which the
parents' blood was provided, the aneuploidies were of paternal
origin. Paternal sex chromosome non-disjunction is associated with
reduced recombination between X and Y (51,52). It
has been indicated that G-group and sex chromosomes are more likely
to exhibit aneuploidy than other chromosomes (53). Most individual autosomes have a
disomic frequency of about 0.1%, but sex chromosomes have a disomic
frequency of about 0.43% (54),
likely due to these chromosomes normally having only one crossover;
if recombination fails and this single chiasma is not present, the
homologous chromosomes do not properly move to opposite poles
(55).
QF-PCR is not a perfect technique and the results
may be negative even in the presence of fetal abnormalities on
ultrasound. Array comparative genomic hybridization (aCGH) is able
to detect copy number variations with high resolution (56). In cases of fetal abnormalities
identified on ultrasound but with negative QF-PCR results, aCGH may
indeed detect a gain or deletion in a portion of a chromosome. In
the present study, no cases of negative QF-PCR were encountered due
to the strict selection criteria applied to the population
(pregnancies at high risk of aneuploidy). aCGH will be evaluated in
a future large-scale screening study performed by our group. In
addition, QF-PCR cannot fully replace the traditional karyotype
analysis, but it may be used to screen for common chromosomal
aberrations, including trisomy 21, 18 and 13, and sex chromosome
aneuploidy (9–13,29). The
advantage of the technique is that the results may be quickly
obtained. In the presence of normal QF-PCR screening test results
but fetal abnormalities on ultrasound, more invasive,
time-consuming and costly karyotyping may be performed.
In the present study, 62 subjects had ≥2 fetal
ultrasound abnormalities; among them, karyotyping indicated that 18
subjects had aneuploidies. QF-PCR confirmed the karyotyping
results. In addition, karyotyping also identified three cases of
chromosomal translocation, which were not detected by QF-PCR.
However, the concordance between QF-PCR and karyotyping in
examining aneuploidies in chromosomes 21, 18 and 13, and sex
chromosomes in the second trimester was 100%. The frequency of
aneuploidies was 29.0% (18/62) for the cases with ≥2 fetal
ultrasound abnormalities in the second trimester. Therefore, for
those cases with fetal ultrasound abnormalities in the second
trimester, particularly those with ≥2 abnormalities, pre-natal
diagnosis should be performed, even if pre-natal screening was not
performed or suggested a low risk, in order to rule out any
chromosomal abnormalities (57).
Of note, the present study had certain limitations.
The patients were from a single center and their number was
relatively small. However, as the study population, all females
with a high risk of aneuploidies were selected from all consecutive
and consenting females encountered during the recruitment period
according to the criteria. In addition, due to limited funding, the
recruitment period was restricted to 18 months. Of note, increasing
the sample size would increase the likelihood of observing
false-negative and false-positive results. A multi-center study may
further address this issue. In the present study, only the most
common aneuploidies were examined, which is a limitation of QF-PCR
itself. Additional studies are required to improve the
generalizability of these results. It is important to highlight
that only a limited number of chromosomes were tested using QF-PCR
in the present study. Testing for additional chromosomes should be
developed, examined for cost-benefits and implemented if required
(11,58).
In conclusion, the combination of NIPT and QF-PCR
may become a rapid and effective method for the detection of fetal
aneuploidy. Assessment of parental origin and meiosis stage of
non-disjunction errors by QF-PCR may provide additional genetic
information for the diagnosis and management of aneuploidy compared
to karyotyping alone.
Acknowledgements
Not applicable.
Funding
The present study was funded using Department of
Reproductive Genetics, Hebei General Hospital (Shijiazhuang, China)
research funds.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW conceived and supervised the study; PH, BJ and LR
performed the experiments; BJ and JZ analysed the data; PH and JL
wrote the manuscript; XW and QL revised the manuscript. All authors
reviewed the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hebei General Hospital (Shijiazhuang, China). All participants
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
QF-PCR
|
quantitative fluorescence-polymerase
chain reaction
|
|
CGH
|
comparative genomic hybridization
|
|
NIPT
|
non-invasive pre-natal testing
|
|
aCGH
|
array comparative genomic
hybridization
|
References
|
1
|
MacLennan M, Crichton JH, Playfoot CJ and
Adams IR: Oocyte development, meiosis and aneuploidy. Semin Cell
Dev Biol. 45:68–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin M, Besser LM, Kucik JE, Lu C, Siffel
C and Correa A; Congenital Anomaly Multistate Prevalence and
Survival Collaborative, : Prevalence of down syndrome among
children and adolescents in 10 regions of the United States.
Pediatrics. 124:1565–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Centers for Disease Control, Prevention
(CDC), . Improved national prevalence estimates for 18 selected
major birth defects-United States, 1999–2001. MMWR Morb Mortal Wkly
Rep. 54:1301–1305. 2006.PubMed/NCBI
|
|
4
|
Roizen NJ and Patterson D: Down's
syndrome. Lancet. 361:1281–1289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cereda A and Carey JC: The trisomy 18
syndrome. Orphanet J Rare Dis. 7:812012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crider KS, Olney RS and Cragan JD:
Trisomies 13 and 18: Population prevalences, characteristics, and
prenatal diagnosis, metropolitan Atlanta, 1994–2003. Am J Med Genet
A 146A. 820–826. 2008. View Article : Google Scholar
|
|
7
|
Bridge JA: Advantages and limitations of
cytogenetic, molecular cytogenetic, and molecular diagnostic
testing in mesenchymal neoplasms. J Orthop Sci. 13:273–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Eggeling F, Freytag M, Fahsold R,
Horsthemke B and Claussen U: Rapid detection of trisomy 21 by
quantitative PCR. Hum Genet. 91:567–570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogilvie CM: Prenatal diagnosis for
chromosome abnormalities: Past, present and future. Pathol Biol
(Paris). 51:156–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leung WC, Lau ET, Lao TT and Tang MH:
Rapid aneuploidy screening (FISH or QF-PCR): The changing scene in
prenatal diagnosis? Expert Rev Mol Diagn. 4:333–337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Badenas C, Rodriguez-Revenga L, Morales C,
Mediano C, Plaja A, Pérez-Iribarne MM, Soler A, Clusellas N,
Borrell A, Sánchez MÁ, et al: Assessment of QF-PCR as the first
approach in prenatal diagnosis. J Mol Diagn. 12:828–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Paz-Gallardo MJ, Garcia FS, de
Haro-Munoz T, Padilla-Vinuesa MC, Zafra-Ceres M, Gomez-Capilla JA
and Gomez-Llorente C: Quantitative-fluorescent-PCR versus full
karyotyping in prenatal diagnosis of common chromosome aneuploidies
in Southern Spain. Clin Chem Lab Med. 53:1333–1338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rostami P, Valizadegan S, Ghalandary M,
Mehrjouy MM, Esmail-Nia G, Khalili S, Shahmoradi SS, Imanian H,
Hadavi V, Ghaderi-Sohi S, et al: Prenatal screening for
aneuploidies using QF-PCR and karyotyping: A comprehensive study in
Iranian population. Arch Iran Med. 18:296–303. 2015.PubMed/NCBI
|
|
14
|
Papoulidis I, Siomou E, Sotiriadis A,
Efstathiou G, Psara A, Sevastopoulou E, Anastasakis E, Sifakis S,
Tsiligianni T, Kontodiou M, et al: Dual testing with QF-PCR and
karyotype analysis for prenatal diagnosis of chromosomal
abnormalities. Evaluation of 13,500 cases with consideration of
using QF-PCR as a stand-alone test according to referral
indications. Prenat Diagn. 32:680–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ermito S, Dinatale A, Carrara S, Cavaliere
A, Imbruglia L and Recupero S: Prenatal diagnosis of limb
abnormalities: Role of fetal ultrasonography. J Prenat Med.
3:18–22. 2009.PubMed/NCBI
|
|
16
|
Xu AQ, Xia M, Liu JT, Yao FX, Zhang WM,
Hao N, Zhou J and Bian XM: Validation of quantitative
fluorescent-PCR for rapid prenatal diagnosis of common aneuploidies
in the Chinese population. Genet Mol Res. 12:6379–6388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emad A, Lamoureux J, Ouellet A and Drouin
R: Rapid aneuploidy detection of chromosomes 13, 18, 21, X and Y
using quantitative fluorescent polymerase chain reaction with few
microdissected fetal cells. Fetal Diagn Ther. 38:65–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Howe B, Umrigar A and Tsien F: Chromosome
preparation from cultured cells. J Vis Exp. 28:e502032014.
|
|
19
|
Guzel A, Yilmaz M, Demirhan O, Pazarbasi
A, Kocaturk-Sel S, Erkoc M, Inandiklioglu N, Ozgunen F and Sariturk
C: Rapid detection of fetal aneuploidies by quantitative
fluorescent-polymerase chain reaction for prenatal diagnosis in the
Turkish population. Balkan J Med Genet. 15:11–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramírez NJ, Belalcázar HM, Yunis JJ,
Quintero LN, Arboleda GH and Arboleda H: Parental origin,
nondisjunction, and recombination of the extra chromosome 21 in
Down syndrome: A study in a sample of the Colombian population.
Biomedica. 27:141–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gekas J, van den Berg DG, Durand A, Vallée
M, Wildschut HI, Bujold E, Forest JC, Rousseau F and Reinharz D:
Rapid testing versus karyotyping in down's syndrome screening:
Cost-effectiveness and detection of clinically significant
chromosome abnormalities. Eur J Hum Genet. 19:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allingham-Hawkins DJ, Chitayat D,
Cirigliano V, Summers A, Tokunaga J, Winsor E and Chun K:
Prospective validation of quantitative fluorescent polymerase chain
reaction for rapid detection of common aneuploidies. Genet Med.
13:140–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Comas C, Echevarria M, Carrera M and Serra
B: Rapid aneuploidy testing versus traditional karyotyping in
amniocentesis for certain referral indications. J Matern Fetal
Neonatal Med. 23:949–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hills A, Donaghue C, Waters J, Waters K,
Sullivan C, Kulkarni A, Docherty Z, Mann K and Ogilvie CM: QF-PCR
as a stand-alone test for prenatal samples: The first 2 years'
experience in the London region. Prenat Diagn. 30:509–517.
2010.PubMed/NCBI
|
|
25
|
Speevak MD, McGowan-Jordan J and Chun K:
The detection of chromosome anomalies by QF-PCR and residual risks
as compared to G-banded analysis. Prenat Diagn. 31:454–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hulten MA, Dhanjal S and Pertl B: Rapid
and simple prenatal diagnosis of common chromosome disorders:
Advantages and disadvantages of the molecular methods FISH and
QF-PCR. Reproduction. 126:279–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caine A, Maltby AE, Parkin CA, Waters JJ
and Crolla JA; UK Association of Clinical Cytogeneticists (ACC), :
Prenatal detection of down's syndrome by rapid aneuploidy testing
for chromosomes 13, 18, and 21 by FISH or PCR without a full
karyotype: A cytogenetic risk assessment. Lancet. 366:123–128.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Donaghue C, Mann K, Docherty Z and Ogilvie
CM: Detection of mosaicism for primary trisomies in prenatal
samples by QF-PCR and karyotype analysis. Prenat Diagn. 25:65–72.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mackie FL, Hemming K, Allen S, Morris RK
and Kilby MD: The accuracy of cell-free fetal DNA-based
non-invasive prenatal testing in singleton pregnancies: A
systematic review and bivariate meta-analysis. BJOG. 124:32–46.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mansfield ES: Diagnosis of down syndrome
and other aneuploidies using quantitative polymerase chain reaction
and small tandem repeat polymorphisms. Hum Mol Genet. 2:43–50.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adinolfi M, Pertl B and Sherlock J: Rapid
detection of aneuploidies by microsatellite and the quantitative
fluorescent polymerase chain reaction. Prenat Diagn. 17:1299–1311.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmidt W, Jenderny J, Hecher K, Hackelöer
BJ, Kerber S, Kochhan L and Held KR: Detection of aneuploidy in
chromosomes X, Y, 13, 18 and 21 by QF-PCR in 662 selected
pregnancies at risk. Mol Hum Reprod. 6:855–860. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cirigliano V, Voglino G, Cañadas MP,
Marongiu A, Ejarque M, Ordoñez E, Plaja A, Massobrio M, Todros T,
Fuster C, et al: Rapid prenatal diagnosis of common chromosome
aneuploidies by QF-PCR. Assessment on 18,000 consecutive clinical
samples. Mol Hum Reprod. 10:839–846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cirigliano V, Sherlock J, Conway G,
Quilter C, Rodeck C and Adinolfi M: Rapid detection of chromosomes
X and Y aneuploidies by quantitative fluorescent PCR. Prenat Diagn.
19:1099–1103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benn P, Cuckle H and Pergament E:
Non-invasive prenatal testing for aneuploidy: Current status and
future prospects. Ultrasound Obstet Gynecol. 42:15–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benachi A, Letourneau A, Kleinfinger P,
Senat MV, Gautier E, Favre R, Bidat L, Houfflin-Debarge V, Querol
V, Bouyer J, et al: Performance and indications of noninvasive
prenatal testing using cell free circulating fetal DNA (cffDNA) for
the detection of fetal trisomy 21, 18 and 13 in France. J Gynecol
Obstet Biol Reprod (Paris). 45:633–640. 2016.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hernández-Gómez M, Ramirez-Arroyo E,
Meléndez-Hernández R, Garduño-Zaraza LM and Mayén-Molina DG: Non
invasive prenatal test (NIPT) in maternal blood by parallel massive
sequencing. Initial experience in Mexican women and literature
review. Ginecol Obstet Mex. 83:277–288. 2015.(In Spanish).
PubMed/NCBI
|
|
38
|
Koumbaris G, Kypri E, Tsangaras K,
Achilleos A, Mina P, Neofytou M, Velissariou V, Christopoulou G,
Kallikas I, González-Liñán A, et al: Cell-free DNA analysis of
targeted genomic regions in maternal plasma for non-invasive
prenatal testing of trisomy 21, trisomy 18, trisomy 13, and fetal
sex. Clin Chem. 62:848–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sills ES, Li X, Frederick JL, Khoury CD
and Potter DA: Determining parental origin of embryo aneuploidy:
Analysis of genetic error observed in 305 embryos derived from
anonymous donor oocyte IVF cycles. Mol Cytogenet. 7:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Templado C, Uroz L and Estop A: New
insights on the origin and relevance of aneuploidy in human
spermatozoa. Mol Hum Reprod. 19:634–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hassold T and Hunt P: To err (meiotically)
is human: The genesis of human aneuploidy. Nat Rev Genet.
2:280–291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vranekovic J, Bozovic IB, Grubic Z, Wagner
J, Pavlinić D, Dahoun S, Bena F, Culić V and Brajenović-Milić B:
Down syndrome: Parental origin, recombination, and maternal age.
Genet Test Mol Biomarkers. 16:70–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoon PW, Freeman SB, Sherman SL, Taft LF,
Gu Y, Pettay D, Flanders WD, Khoury MJ and Hassold TJ: Advanced
maternal age and the risk of down syndrome characterized by the
meiotic stage of chromosomal error: A population-based study. Am J
Hum Genet. 58:628–633. 1996.PubMed/NCBI
|
|
44
|
Petersen MB and Mikkelsen M:
Nondisjunction in trisomy 21: Origin and mechanisms. Cytogenet Cell
Genet. 91:199–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghosh S, Bhaumik P, Ghosh P and Dey SK:
Chromosome 21 non-disjunction and down syndrome birth in an Indian
cohort: Analysis of incidence and aetiology from family linkage
data. Genet Res (Camb). 92:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morris JK and Savva GM: The risk of fetal
loss following a prenatal diagnosis of trisomy 13 or trisomy 18. Am
J Med Genet A 146A. 827–832. 2008. View Article : Google Scholar
|
|
47
|
Won RH, Currier RJ, Lorey F and Towner DR:
The timing of demise in fetuses with trisomy 21 and trisomy 18.
Prenat Diagn. 25:608–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hill LM: The sonographic detection of
trisomies 13, 18, and 21. Clin Obstet Gynecol. 39:831–850. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eggermann T, Nothen MM, Eiben B, Hofmann
D, Hinkel K, Fimmers R and Schwanitz G: Trisomy of human chromosome
18: Molecular studies on parental origin and cell stage of
nondisjunction. Hum Genet. 97:218–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bugge M, Collins A, Petersen MB, Fisher J,
Brandt C, Hertz JM, Tranebjaerg L, de Lozier-Blanchet C, Nicolaides
P, Brøndum-Nielsen K, et al: Non-disjunction of chromosome 18. Hum
Mol Genet. 7:661–669. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hassold TJ, Sherman SL, Pettay D, Page DC
and Jacobs PA: XY chromosome nondisjunction in man is associated
with diminished recombination in the pseudoautosomal region. Am J
Hum Genet. 49:253–260. 1991.PubMed/NCBI
|
|
52
|
Lorda-Sanchez I, Binkert F, Maechler M,
Robinson WP and Schinzel AA: Reduced recombination and paternal age
effect in Klinefelter syndrome. Hum Genet. 89:524–530. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Martin RH, Ko E and Rademaker A:
Distribution of aneuploidy in human gametes: Comparison between
human sperm and oocytes. Am J Med Genet. 39:321–331. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Spriggs EL, Rademaker AW and Martin RH:
Aneuploidy in human sperm: The use of multicolor FISH to test
various theories of nondisjunction. Am J Hum Genet. 58:356–362.
1996.PubMed/NCBI
|
|
55
|
Martin RH: Mechanisms of nondisjunction in
human spermatogenesis. Cytogenet Genome Res. 111:245–249. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duncan A and Langlois S; SOGC Genetics
Committee; CCMG Prenatal Diagnosis Committee, : Use of array
genomic hybridization technology in prenatal diagnosis in Canada. J
Obstet Gynaecol Can. 33:1256–1259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Benn P, Borrell A, Chiu RW, Cuckle H,
Dugoff L, Faas B, Gross S, Huang T, Johnson J, Maymon R, et al:
Position statement from the chromosome abnormality screening
committee on behalf of the board of the international society for
prenatal diagnosis. Prenat Diagn. 35:725–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Langlois S and Duncan A; SOGC Genetics
Committee; CCMG Prenatal Diagnosis Committee, : Use of a DNA
method, QF-PCR, in the prenatal diagnosis of fetal aneuploidies. J
Obstet Gynaecol Can. 33:955–960. 2011. View Article : Google Scholar : PubMed/NCBI
|