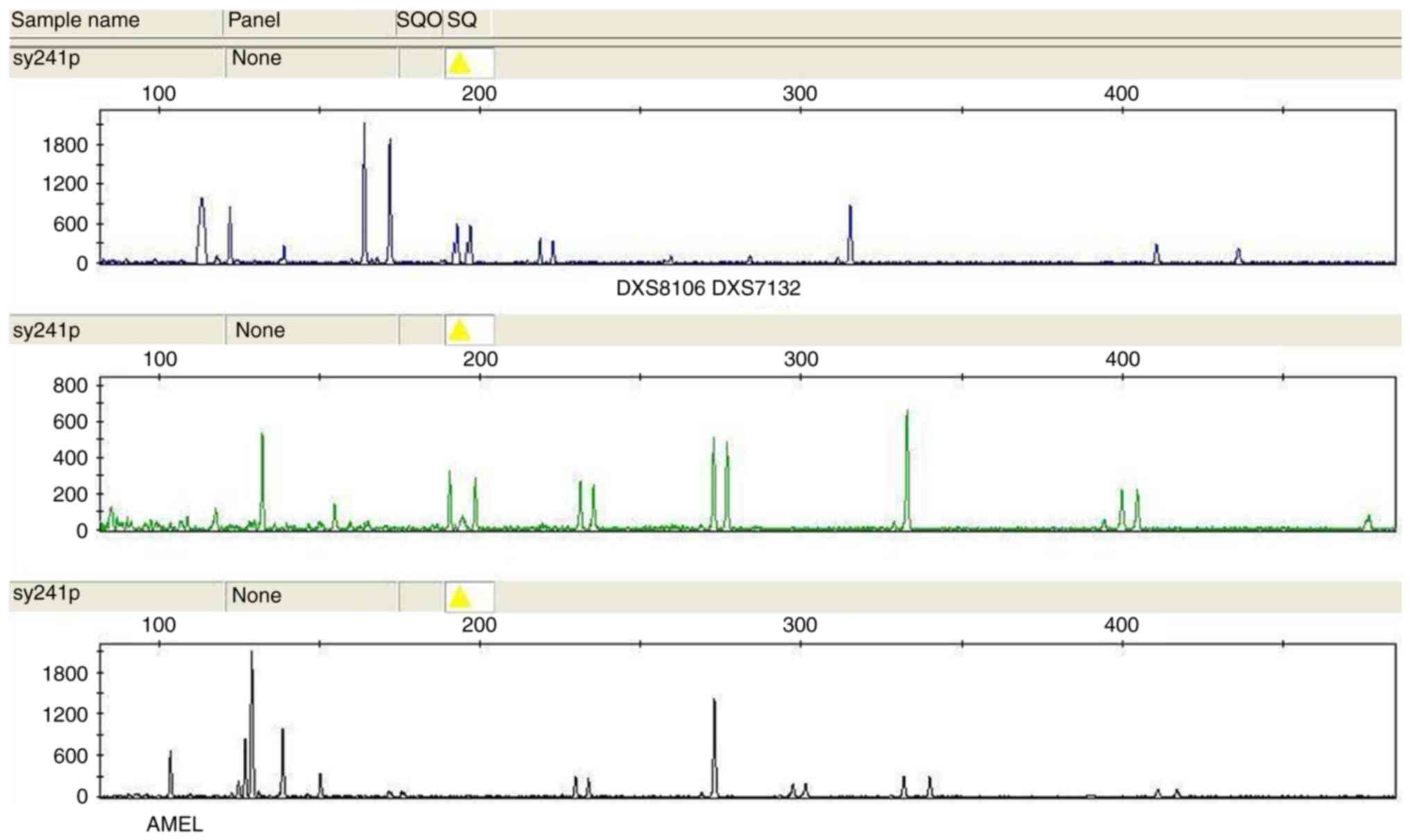
|
1
|
MacLennan M, Crichton JH, Playfoot CJ and
Adams IR: Oocyte development, meiosis and aneuploidy. Semin Cell
Dev Biol. 45:68–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin M, Besser LM, Kucik JE, Lu C, Siffel
C and Correa A; Congenital Anomaly Multistate Prevalence and
Survival Collaborative, : Prevalence of down syndrome among
children and adolescents in 10 regions of the United States.
Pediatrics. 124:1565–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Centers for Disease Control, Prevention
(CDC), . Improved national prevalence estimates for 18 selected
major birth defects-United States, 1999–2001. MMWR Morb Mortal Wkly
Rep. 54:1301–1305. 2006.PubMed/NCBI
|
|
4
|
Roizen NJ and Patterson D: Down's
syndrome. Lancet. 361:1281–1289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cereda A and Carey JC: The trisomy 18
syndrome. Orphanet J Rare Dis. 7:812012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crider KS, Olney RS and Cragan JD:
Trisomies 13 and 18: Population prevalences, characteristics, and
prenatal diagnosis, metropolitan Atlanta, 1994–2003. Am J Med Genet
A 146A. 820–826. 2008. View Article : Google Scholar
|
|
7
|
Bridge JA: Advantages and limitations of
cytogenetic, molecular cytogenetic, and molecular diagnostic
testing in mesenchymal neoplasms. J Orthop Sci. 13:273–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Eggeling F, Freytag M, Fahsold R,
Horsthemke B and Claussen U: Rapid detection of trisomy 21 by
quantitative PCR. Hum Genet. 91:567–570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogilvie CM: Prenatal diagnosis for
chromosome abnormalities: Past, present and future. Pathol Biol
(Paris). 51:156–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leung WC, Lau ET, Lao TT and Tang MH:
Rapid aneuploidy screening (FISH or QF-PCR): The changing scene in
prenatal diagnosis? Expert Rev Mol Diagn. 4:333–337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Badenas C, Rodriguez-Revenga L, Morales C,
Mediano C, Plaja A, Pérez-Iribarne MM, Soler A, Clusellas N,
Borrell A, Sánchez MÁ, et al: Assessment of QF-PCR as the first
approach in prenatal diagnosis. J Mol Diagn. 12:828–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Paz-Gallardo MJ, Garcia FS, de
Haro-Munoz T, Padilla-Vinuesa MC, Zafra-Ceres M, Gomez-Capilla JA
and Gomez-Llorente C: Quantitative-fluorescent-PCR versus full
karyotyping in prenatal diagnosis of common chromosome aneuploidies
in Southern Spain. Clin Chem Lab Med. 53:1333–1338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rostami P, Valizadegan S, Ghalandary M,
Mehrjouy MM, Esmail-Nia G, Khalili S, Shahmoradi SS, Imanian H,
Hadavi V, Ghaderi-Sohi S, et al: Prenatal screening for
aneuploidies using QF-PCR and karyotyping: A comprehensive study in
Iranian population. Arch Iran Med. 18:296–303. 2015.PubMed/NCBI
|
|
14
|
Papoulidis I, Siomou E, Sotiriadis A,
Efstathiou G, Psara A, Sevastopoulou E, Anastasakis E, Sifakis S,
Tsiligianni T, Kontodiou M, et al: Dual testing with QF-PCR and
karyotype analysis for prenatal diagnosis of chromosomal
abnormalities. Evaluation of 13,500 cases with consideration of
using QF-PCR as a stand-alone test according to referral
indications. Prenat Diagn. 32:680–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ermito S, Dinatale A, Carrara S, Cavaliere
A, Imbruglia L and Recupero S: Prenatal diagnosis of limb
abnormalities: Role of fetal ultrasonography. J Prenat Med.
3:18–22. 2009.PubMed/NCBI
|
|
16
|
Xu AQ, Xia M, Liu JT, Yao FX, Zhang WM,
Hao N, Zhou J and Bian XM: Validation of quantitative
fluorescent-PCR for rapid prenatal diagnosis of common aneuploidies
in the Chinese population. Genet Mol Res. 12:6379–6388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emad A, Lamoureux J, Ouellet A and Drouin
R: Rapid aneuploidy detection of chromosomes 13, 18, 21, X and Y
using quantitative fluorescent polymerase chain reaction with few
microdissected fetal cells. Fetal Diagn Ther. 38:65–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Howe B, Umrigar A and Tsien F: Chromosome
preparation from cultured cells. J Vis Exp. 28:e502032014.
|
|
19
|
Guzel A, Yilmaz M, Demirhan O, Pazarbasi
A, Kocaturk-Sel S, Erkoc M, Inandiklioglu N, Ozgunen F and Sariturk
C: Rapid detection of fetal aneuploidies by quantitative
fluorescent-polymerase chain reaction for prenatal diagnosis in the
Turkish population. Balkan J Med Genet. 15:11–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramírez NJ, Belalcázar HM, Yunis JJ,
Quintero LN, Arboleda GH and Arboleda H: Parental origin,
nondisjunction, and recombination of the extra chromosome 21 in
Down syndrome: A study in a sample of the Colombian population.
Biomedica. 27:141–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gekas J, van den Berg DG, Durand A, Vallée
M, Wildschut HI, Bujold E, Forest JC, Rousseau F and Reinharz D:
Rapid testing versus karyotyping in down's syndrome screening:
Cost-effectiveness and detection of clinically significant
chromosome abnormalities. Eur J Hum Genet. 19:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allingham-Hawkins DJ, Chitayat D,
Cirigliano V, Summers A, Tokunaga J, Winsor E and Chun K:
Prospective validation of quantitative fluorescent polymerase chain
reaction for rapid detection of common aneuploidies. Genet Med.
13:140–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Comas C, Echevarria M, Carrera M and Serra
B: Rapid aneuploidy testing versus traditional karyotyping in
amniocentesis for certain referral indications. J Matern Fetal
Neonatal Med. 23:949–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hills A, Donaghue C, Waters J, Waters K,
Sullivan C, Kulkarni A, Docherty Z, Mann K and Ogilvie CM: QF-PCR
as a stand-alone test for prenatal samples: The first 2 years'
experience in the London region. Prenat Diagn. 30:509–517.
2010.PubMed/NCBI
|
|
25
|
Speevak MD, McGowan-Jordan J and Chun K:
The detection of chromosome anomalies by QF-PCR and residual risks
as compared to G-banded analysis. Prenat Diagn. 31:454–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hulten MA, Dhanjal S and Pertl B: Rapid
and simple prenatal diagnosis of common chromosome disorders:
Advantages and disadvantages of the molecular methods FISH and
QF-PCR. Reproduction. 126:279–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caine A, Maltby AE, Parkin CA, Waters JJ
and Crolla JA; UK Association of Clinical Cytogeneticists (ACC), :
Prenatal detection of down's syndrome by rapid aneuploidy testing
for chromosomes 13, 18, and 21 by FISH or PCR without a full
karyotype: A cytogenetic risk assessment. Lancet. 366:123–128.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Donaghue C, Mann K, Docherty Z and Ogilvie
CM: Detection of mosaicism for primary trisomies in prenatal
samples by QF-PCR and karyotype analysis. Prenat Diagn. 25:65–72.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mackie FL, Hemming K, Allen S, Morris RK
and Kilby MD: The accuracy of cell-free fetal DNA-based
non-invasive prenatal testing in singleton pregnancies: A
systematic review and bivariate meta-analysis. BJOG. 124:32–46.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mansfield ES: Diagnosis of down syndrome
and other aneuploidies using quantitative polymerase chain reaction
and small tandem repeat polymorphisms. Hum Mol Genet. 2:43–50.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adinolfi M, Pertl B and Sherlock J: Rapid
detection of aneuploidies by microsatellite and the quantitative
fluorescent polymerase chain reaction. Prenat Diagn. 17:1299–1311.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmidt W, Jenderny J, Hecher K, Hackelöer
BJ, Kerber S, Kochhan L and Held KR: Detection of aneuploidy in
chromosomes X, Y, 13, 18 and 21 by QF-PCR in 662 selected
pregnancies at risk. Mol Hum Reprod. 6:855–860. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cirigliano V, Voglino G, Cañadas MP,
Marongiu A, Ejarque M, Ordoñez E, Plaja A, Massobrio M, Todros T,
Fuster C, et al: Rapid prenatal diagnosis of common chromosome
aneuploidies by QF-PCR. Assessment on 18,000 consecutive clinical
samples. Mol Hum Reprod. 10:839–846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cirigliano V, Sherlock J, Conway G,
Quilter C, Rodeck C and Adinolfi M: Rapid detection of chromosomes
X and Y aneuploidies by quantitative fluorescent PCR. Prenat Diagn.
19:1099–1103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benn P, Cuckle H and Pergament E:
Non-invasive prenatal testing for aneuploidy: Current status and
future prospects. Ultrasound Obstet Gynecol. 42:15–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benachi A, Letourneau A, Kleinfinger P,
Senat MV, Gautier E, Favre R, Bidat L, Houfflin-Debarge V, Querol
V, Bouyer J, et al: Performance and indications of noninvasive
prenatal testing using cell free circulating fetal DNA (cffDNA) for
the detection of fetal trisomy 21, 18 and 13 in France. J Gynecol
Obstet Biol Reprod (Paris). 45:633–640. 2016.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hernández-Gómez M, Ramirez-Arroyo E,
Meléndez-Hernández R, Garduño-Zaraza LM and Mayén-Molina DG: Non
invasive prenatal test (NIPT) in maternal blood by parallel massive
sequencing. Initial experience in Mexican women and literature
review. Ginecol Obstet Mex. 83:277–288. 2015.(In Spanish).
PubMed/NCBI
|
|
38
|
Koumbaris G, Kypri E, Tsangaras K,
Achilleos A, Mina P, Neofytou M, Velissariou V, Christopoulou G,
Kallikas I, González-Liñán A, et al: Cell-free DNA analysis of
targeted genomic regions in maternal plasma for non-invasive
prenatal testing of trisomy 21, trisomy 18, trisomy 13, and fetal
sex. Clin Chem. 62:848–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sills ES, Li X, Frederick JL, Khoury CD
and Potter DA: Determining parental origin of embryo aneuploidy:
Analysis of genetic error observed in 305 embryos derived from
anonymous donor oocyte IVF cycles. Mol Cytogenet. 7:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Templado C, Uroz L and Estop A: New
insights on the origin and relevance of aneuploidy in human
spermatozoa. Mol Hum Reprod. 19:634–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hassold T and Hunt P: To err (meiotically)
is human: The genesis of human aneuploidy. Nat Rev Genet.
2:280–291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vranekovic J, Bozovic IB, Grubic Z, Wagner
J, Pavlinić D, Dahoun S, Bena F, Culić V and Brajenović-Milić B:
Down syndrome: Parental origin, recombination, and maternal age.
Genet Test Mol Biomarkers. 16:70–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoon PW, Freeman SB, Sherman SL, Taft LF,
Gu Y, Pettay D, Flanders WD, Khoury MJ and Hassold TJ: Advanced
maternal age and the risk of down syndrome characterized by the
meiotic stage of chromosomal error: A population-based study. Am J
Hum Genet. 58:628–633. 1996.PubMed/NCBI
|
|
44
|
Petersen MB and Mikkelsen M:
Nondisjunction in trisomy 21: Origin and mechanisms. Cytogenet Cell
Genet. 91:199–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghosh S, Bhaumik P, Ghosh P and Dey SK:
Chromosome 21 non-disjunction and down syndrome birth in an Indian
cohort: Analysis of incidence and aetiology from family linkage
data. Genet Res (Camb). 92:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morris JK and Savva GM: The risk of fetal
loss following a prenatal diagnosis of trisomy 13 or trisomy 18. Am
J Med Genet A 146A. 827–832. 2008. View Article : Google Scholar
|
|
47
|
Won RH, Currier RJ, Lorey F and Towner DR:
The timing of demise in fetuses with trisomy 21 and trisomy 18.
Prenat Diagn. 25:608–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hill LM: The sonographic detection of
trisomies 13, 18, and 21. Clin Obstet Gynecol. 39:831–850. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eggermann T, Nothen MM, Eiben B, Hofmann
D, Hinkel K, Fimmers R and Schwanitz G: Trisomy of human chromosome
18: Molecular studies on parental origin and cell stage of
nondisjunction. Hum Genet. 97:218–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bugge M, Collins A, Petersen MB, Fisher J,
Brandt C, Hertz JM, Tranebjaerg L, de Lozier-Blanchet C, Nicolaides
P, Brøndum-Nielsen K, et al: Non-disjunction of chromosome 18. Hum
Mol Genet. 7:661–669. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hassold TJ, Sherman SL, Pettay D, Page DC
and Jacobs PA: XY chromosome nondisjunction in man is associated
with diminished recombination in the pseudoautosomal region. Am J
Hum Genet. 49:253–260. 1991.PubMed/NCBI
|
|
52
|
Lorda-Sanchez I, Binkert F, Maechler M,
Robinson WP and Schinzel AA: Reduced recombination and paternal age
effect in Klinefelter syndrome. Hum Genet. 89:524–530. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Martin RH, Ko E and Rademaker A:
Distribution of aneuploidy in human gametes: Comparison between
human sperm and oocytes. Am J Med Genet. 39:321–331. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Spriggs EL, Rademaker AW and Martin RH:
Aneuploidy in human sperm: The use of multicolor FISH to test
various theories of nondisjunction. Am J Hum Genet. 58:356–362.
1996.PubMed/NCBI
|
|
55
|
Martin RH: Mechanisms of nondisjunction in
human spermatogenesis. Cytogenet Genome Res. 111:245–249. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duncan A and Langlois S; SOGC Genetics
Committee; CCMG Prenatal Diagnosis Committee, : Use of array
genomic hybridization technology in prenatal diagnosis in Canada. J
Obstet Gynaecol Can. 33:1256–1259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Benn P, Borrell A, Chiu RW, Cuckle H,
Dugoff L, Faas B, Gross S, Huang T, Johnson J, Maymon R, et al:
Position statement from the chromosome abnormality screening
committee on behalf of the board of the international society for
prenatal diagnosis. Prenat Diagn. 35:725–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Langlois S and Duncan A; SOGC Genetics
Committee; CCMG Prenatal Diagnosis Committee, : Use of a DNA
method, QF-PCR, in the prenatal diagnosis of fetal aneuploidies. J
Obstet Gynaecol Can. 33:955–960. 2011. View Article : Google Scholar : PubMed/NCBI
|