Introduction
Atopic dermatitis (AD) is caused by a disturbance of
the immune system. Therefore, to treat AD, it is necessary to
normalize the immune system in addition to treating the external
skin manifestations (1–3). Characteristic symptoms of AD are
pruritus, pus, erythema and chronic skin bacterial infection. Skin
barrier defects are recognized as one of the most important
features of AD (4–6). The abnormal differentiation of skin
epithelial cells causes skin barrier defects. These defects enable
the infiltration of allergens, which induce an inflammatory
reaction and systemic immunological reaction. These skin barrier
defects are usually caused by genetic and acquired factors
(7,8).
Keratinocytes are keratin-producing epidermal cells,
which account for ~90% of epidermal cells (9,10). The
main function of the epidermis is to provide a barrier that
protects the human body from environmental factors, including
pathogens, heat, ultraviolet rays and moisture loss. Thymic stromal
lymphopoietin (TSLP) present in keratinocytes stimulates dendritic
cells to increase the production of thymus and activation-regulated
chemokine [TARC; also known as chemokine (C-C motif) ligand 17,
CCL17] and macrophage-derived chemokine (MDC; also known as CCL22)
(11,12). TARC and MDC are typical type 2 helper
T cell (Th2 cell)-secreted chemokines that induce Th2 cell
recruitment at inflammatory sites (13). High concentrations of TARC, MDC and
TSLP have been detected in patients with AD (14). These biomarkers are known to be very
closely associated with atopic disease (15,16).
Cedrela odorata L. is a plant of the genus Cedrela
and is distributed across tropical climate regions, such as the
Amazon (17,18). Its wood is mainly used as raw
material for household furniture or musical instruments (19). Traditionally, C. odorata L. has been
utilized in folk remedies for diarrhea, fever, inflammation,
vomiting, hemorrhage, and indigestion (20,21).
However, since there is no literature regarding this plant in
relation to inflammation or AD, it was investigated in the present
study. The aim of the study was to examine the biochemical activity
of C. odorata L. ethanol extract (COEE) using HaCaT cells induced
with a mixture of tumor necrosis factor (TNF)-α and interferon
(IFN)-γ.
Materials and methods
Preparation of COEE
The leaves and shoots of C. odorata L. were
collected in Palo Verde National Park, Costa Rica, in 2014. A
voucher specimen (KRIB 0056657) has been deposited at the
International Biological Material Research Center (IBMRC) in the
Korea Research Institute of Bioscience and Biotechnology (Daejeon,
South Korea). The dried and refined leaves and shoots of C. odorata
(100 g) were extracted with 700 ml 95% ethanol for 2 h, three
times. The extract was percolated through filter paper (3 mm;
Whatman PLC, Kent, UK), condensed using a rotary evaporator (Büchi
AG, Flawil, Switzerland) and lyophilized using a freeze dryer
(Martin Christ Gefriertrocknungsanlagen, Osterode am Harz,
Germany).
Cell culture
The human keratinocyte HaCaT cell line was purchased
from the American Tissue Culture Center (Manassas, VA, USA). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc. Waltham, MA, USA) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin, and maintained using an incubator at
temperature 37°C with a 5% CO2 atmosphere while maintaining a
confluency of 60–80%.
MTT assay
Cell viability was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. HaCaT cells were seeded in 96-well plates (SPL Life
Sciences, Pocheon, Korea) at a density of 1×104 cells/well. After 6
h of incubation, COEE (1.25, 2.5, 5, 10 and 20 µg/ml) was
administered and the cells were incubated for 24 h at 37°C.
Untreated cells were defined as the control group. Next, 5 µl MTT
solution (5 mg/ml; Amresco, LLC, Solon, OH, USA) was added to the
cell supernatant, and the mixture was incubated for 4 h at 37°C.
After removing the medium, DMSO was added to dissolve the formazan.
A microplate reader was used to measure the absorbance at 570 nm,
and the untreated formazan value was set at 100%.
Cytokine assay
HaCaT cells were cultured in 96-well plates at a
density of 5×104 cells/well. After an incubation for 6 h at 37°C,
COEE (2.5, 5, 10 and 20 µg/ml) and Bay11-7082 (5 µM) were
administered. TNF-α/IFN-γ (10 ng/ml of each; TNF-α cat. no.
300-01A; IFN-γ cat. no. 300-02; PeproTech, Inc., Rocky Hill, NJ,
USA) was applied 1 h later. The next day, the supernatant was
harvested. The inhibitory effect of COEE on the secretion of
interleukin (IL)-6, IL-8, TARC and MDC into the supernatants was
evaluated using the following ELISA kits: IL-6 (cat. no. 555220; BD
Biosciences, Santa Clara, CA, USA), IL-8 (cat. no. DY208), TARC
(cat. no. DY364) and MDC (cat. no. DY336; all R&D Systems,
Inc., Minneapolis, MN, USA). Samples were analyzed according to the
manufacturer's protocol.
RT-PCR analysis
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen, Thermo Fisher Scientific,
Inc.). Following isolation of the RNA, cDNA synthesis was performed
using a QuantiTect Reverse Transcription kit (cat. no. 205310;
Qiagen GmbH, Hilden, Germany). RNA, gDNA Wipeout Buffer and
RNase-free water were mixed and incubated at 42°C for 2 min. Then,
Quantiscript Reverse Transcriptase, Quantiscript RT Buffer and RT
River Mix were mix with the aforementioned reagents and incubated
at 42°C for 15 min. Finally, the mixture was incubated at 95°C for
3 min to inactivate Quantiscript Reverse Transcriptase. The
synthesized cDNA was amplified by PCR using a GoTaq®
Green Master mix (Promega Corporation, Madison, WI, USA) with 11
pmol of each primer. The sequences of the RT-PCR primers used in
the present study are listed in Table
I. β-actin was used as the reference gene. The thermocycling
conditions were as follows: Pre-denaturation at 94°C for 5 min,
then 25 cycles of denaturation at 94°C for 20 sec, annealing at
56°C for 20 sec and extension at 72°C for 45 sec. The reaction
products were separated by electrophoresis on a 1.5% agarose gel
and stained with RedSafe™ kits (Intron Biotechnology, Inc.,
Seongnam, Korea). Images were captured using an Olympus C4000 zoom
camera system (Olympus Corporation). The densitometry of the bands
were measured using ImageJ 1.50i software (National Institutes of
Health, Bethesda, MD, USA).
 | Table I.Sequences of the reverse
transcription PCR primers used in the current study. |
Table I.
Sequences of the reverse
transcription PCR primers used in the current study.
| Gene | Direction | Primer sequences
(human; 5′-3′) | Fragment size
(bp) |
|---|
| TARC | Forward | CAC GCA GCT CGA GGG
ACC AAT GTG | 222 |
|
| Reverse | TCA AGA CCT CTC AAG
GCT TTG CAG G |
|
| MDC | Forward | AGG ACA GAG CAT GGC
TCG CCT ACA GA | 362 |
|
| Reverse | TAA TGG CAG GGA GGT
AGG GCT CCT GA |
|
| IL-6 | Forward | GAC AGC CAC TCA CCT
CTT CA | 124 |
|
| Reverse | AGT GCC TCT TTGCTG
CTT TC |
|
| IL-8 | Forward | ATG ACT TCC AAG CTG
GCC GTG GCT | 299 |
|
| Reverse | TTA TGA ATT CTC AGC
CCT CTT CAA AAA |
|
| β-actin | Forward | CAT GTA CGT TGC TAT
CCA GGC | 250 |
|
| Reverse | CTC CTT AAT GTC ACG
CAC GAT |
|
Immunoblotting
Immunoblotting of the cells was performed as
previously described (22). The
HaCaT cells were pre-treated with the indicated concentrations of
COEE (2.5, 5. 10 and 20 µg/ml) for 1 h and stimulated with
TNF-α/IFN-γ (10 ng/ml each) for 20 min at 37°C. Immunoblots were
created using anti-nuclear factor (NF)-κB p65 (cat. no. sc-8242;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-phospho-NF-κB inhibitor α (anti-p-IκBα; cat. no. 2859),
anti-IκB-α (cat. no. 9242), anti-NF-κB p-p65 (cat. no. 3033) and
anti-β-actin (cat. no. 4967; all 1:1,000; all from Cell Signaling
Technology, Inc., Danvers, MA, USA). The secondary antibodies were
horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no.
sc-2030; 1:5,000 in 5% skimmed milk; Santa Cruz Biotechnology,
Inc.). The densitometry of the bands were measured using ImageJ
1.50i software.
Luciferase assay
HaCaT cells were transfected with 0.1 µg pGL4.32
(luc2P/NF-κB-RE/Hygro) plasmids (Promega Corporation), using
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 24 h
after transfection, the cells were pretreated with COEE (2.5, 5, 10
and 20 µg/ml) and Bay11-7082 (5 µM) for 1 h at 37°C, stimulated
with TNF-α/IFN-γ for 20 h at 37°C, harvested and then assessed for
luc2P luciferase activity using the ONE-Glo™ luciferase reporter
assay system (Promega Corporation) according to the manufacturer's
instructions. Normalization was performed by comparison with
Renilla luciferase activity.
Statistical analysis
Data are presented as the mean ± SEM. Statistical
differences among groups were determined by one-way ANOVA with
repeated measures followed by Newman-Keuls testing using SPSS
version 14.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Cytotoxic effects of COEE in HaCaT
cells
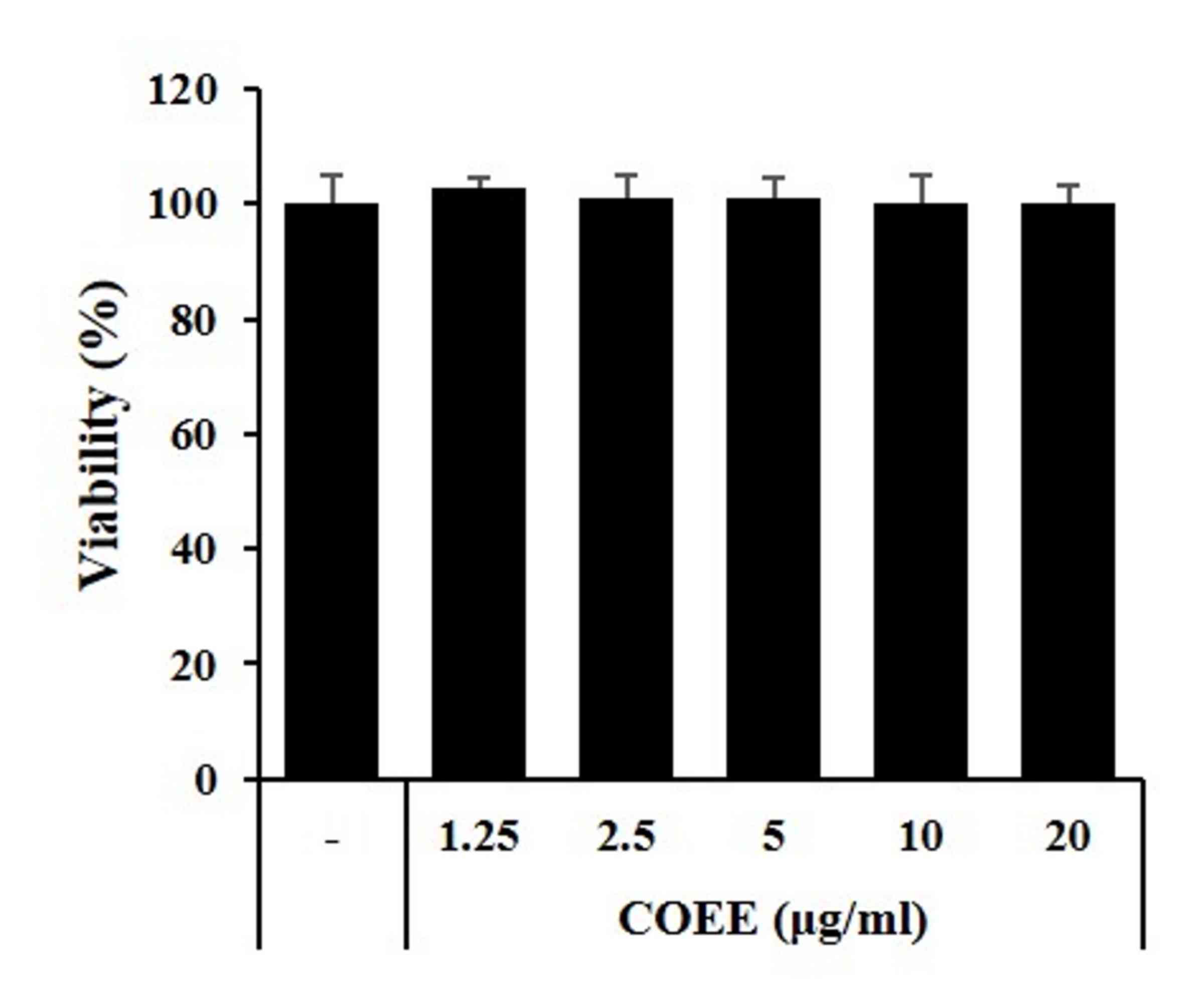
Whether COEE affects the viability of HaCaT cells
was analyzed using the MTT assay. As shown in Fig. 1, COEE did not exhibit cytotoxicity
and did not affect cell viability even when used at a high
concentration of 20 µg/ml for 24 h. This confirmed experimentally
that COEE does not exhibit toxicity in HaCaT cells at
concentrations ≤20 µg/ml.
COEE inhibits TNF-α/IFN-γ-induced IL-6
and IL-8 expression in HaCaT cells
Next, ELISA and RT-PCR assays were used to study the
inhibitory effect of COEE on the production of IL-6 and IL-8 in
HaCaT cells stimulated with TNF-α/IFN-γ. The RT-PCR results
confirmed that the levels of IL-6 and IL-8 were significantly
increased in the group treated with TNF-α/IFN-γ compared with those
in the untreated group. Similarly, when TNF-α/IFN-γ was added after
the introduction of COEE to HaCaT cells, the mRNA expression levels
of IL-6 and IL-8 decreased in an apparently concentration-dependent
manner compared with those in the group treated with TNF-α/IFN-γ
without COEE (Fig. 2A). Furthermore,
the ELISA results demonstrated that COEE inhibited the expression
of the IL-6 and IL-8 proteins in HaCaT cells stimulated with
TNF-α/IFN-γ compared with those in the cells treated with
TNF-α/IFN-γ without COEE (Fig. 2B and
C).
COEE inhibits TNF-α/IFN-γ-induced
TARC/CCL17 and MDC/CCL22 expression in HaCaT cells
Chemokines are significant mediators of the
inflammatory reaction and immune response. Exposure of
keratinocytes to TNF-α/IFN-γ induces the increased expression of
chemokines, leading to the infiltration of leukocytes into
inflammatory lesions in the skin (23,24). In
the present study, ELISA and RT-PCR were used to investigate the
suppressive effect of COEE on TARC and MDC production in
TNF-α/IFN-γ-stimulated HaCaT cells. The RT-PCR results confirmed
that TARC and MDC mRNA levels were significantly increased in the
cells treated with TNF-α/IFN-γ compared with those in the untreated
group. Similarly, when TNF-α/IFN-γ was added after the application
of COEE to the HaCaT cells, the mRNA expression levels of TARC and
MDC decreased compared with those in the group treated with
TNF-α/IFN-γ without COEE, and the reduction appeared to be
concentration-dependent (Fig. 3A).
Furthermore, the ELISA results indicated that COEE inhibited the
expression of the TARC and MDC proteins in HaCaT cells induced with
TNF-α/IFN-γ (Fig. 3B and C).
COEE inhibits the phosphorylation of
NF-κB p65 in HaCaT cells
The nuclear factor NF-κB signaling pathway is
considered a prototypical pro-inflammatory pathway, mainly due to
the role of NF-κB in the expression of pro-inflammatory genes, for
example, adhesion molecules, chemokines and cytokines (25). Therefore, NF-κB p65 phosphorylation
in TNF-α/IFN-γ-treated HaCaT cells was analyzed in the present
study. The western blotting results indicated that the
phosphorylation of IκBα and NF-κB p65 was significantly increased
by TNF-α/IFN-γ-treatment, whereas pretreatment with COEE attenuated
the TNF-α/IFN-γ-induced increase in p-IκBα and p-p65 levels
(Fig. 4).
 | Figure 4.Effect of COEE on TNF-α/IFN-γ-induced
NF-κB activation in HaCaT cells. (A) Phosphorylation of p65 and
IκBα was analyzed by western blotting. (B) HaCaT cells were
transfected with the expression vector luciferase reporter plasmid
(0.1 µg). At 24 h after transfection, HaCaT cells were treated with
COEE for 1 h, and the luc2P/Renilla luciferase activity was then
measured. All data represent three independent experiments. Data
are presented as the mean values ± SEM of three samples. #P<0.01
vs. the untreated control. *P<0.05, **P<0.01 and
***P<0.001 vs. the TNF-α/IFN-γ group. COEE, Cedrela odorata L.
ethanol extract; TNF, tumor necrosis factor; IFN, interferon;
NF-κB, nuclear factor-κB; p65, NF-κB subunit p65; p, phospho; IFN,
interferon; IκBα, NF-κB inhibitor α. |
COEE and Bay11-7082 inhibit the
phosphorylation of NF-κB in HaCaT cells
Bay11-7082 inhibits IκBα phosphorylation in cells
and has been used to indicate the involvement of the canonical IκB
kinases and NF-κB in mechanistic analysis (26). A comparative experiment was conducted
in the present study, in which the efficacy of COEE (20 µg/ml) was
compared with that of Bay11-7082 (5 µM) in the inhibition of NF-κB
p65. Phosphorylation of IκBα and NF-κB p65 was significantly
increased by TNF-α/IFN-γ-treatment, while pretreatment with COEE
and Bay11-7082 decreased the levels of p-p65 and p-IκBα in
TNF-α/IFN-γ-treated HaCaT cells, as indicated by the results of
immunoblotting and the luciferase assay (Fig. 5).
COEE and Bay11-7082 inhibit the
expression of chemokines and cytokines in HaCaT cells
Using ELISA and RT-PCR, the suppressive effects of
COEE and Bay11-7082 on TARC, MDC, IL-6 and IL-8 production in HaCaT
cells stimulated with TNF-α/IFN-γ were investigated. The results
confirmed that the levels of TARC, MDC, IL-6 and IL-8 were
significantly increased in the group treated with TNF-α/IFN-γ
compared with those in the untreated group. However, when
TNF-α/IFN-γ was added after the introduction of COEE and Bay11-7082
to the HaCaT cells, the mRNA and protein expression levels of TARC,
MDC, IL-6 and IL-8 decreased in an apparently
concentration-dependent manner compared with those in the group
treated with TNF-α/IFN-γ without COEE (Fig. 6).
 | Figure 6.Effect of COEE and Bay11-7082 on
TNF-α/IFN-γ induced chemokines and cytokine activation in HaCaT
cells. HaCaT cells were pretreated with COEE (20 µg/ml), Bay11-7082
(5 µM) and then induced with TNF-α (10 ng/ml)/IFN-γ (10 ng/ml) for
24 h. At Protein concentrations of (A) TARC, (B) MDC, (C) IL-6 and
(D) IL-8. (E) mRNA expression of TARC, MDC, IL-6 and IL-8. Data are
presented as the mean values ± SEM of three samples. #P<0.01 vs.
the untreated control; *P<0.05, **P<0.01 and ***P<0.001
vs. the TNF-α/IFN-γ group. COEE, Cedrela odorata L. ethanol
extract; TNF, tumor necrosis factor; IFN, interferon; TARC, thymus
and activation-regulated chemokine; MDC, macrophage-derived
chemokine; IL, interleukin. |
Discussion
AD, also known as eczema, is a common chronic
inflammatory skin disease and is characterized by the infiltration
of inflammatory cells into the skin (27). Although AD is generally treated with
immunosuppressive drugs and anti-inflammatory drugs, these
treatments are often ineffective (28). This may cause patients to use
alternative treatment strategies, including traditional plant-based
remedies.
In the present study, in vitro experiments
were conducted to determine the effects of COEE on pro-inflammatory
chemokine secretion in keratinocytes. Keratinocytes serve a crucial
role in inflammatory skin disorders via the production of
pro-inflammatory cytokines and chemokines (29). These cells participate in the
pathogenesis of AD by secreting various chemokines, among which
TARC and MDC selectively attract Th2 cells that are predominant in
atopic inflammation (30). IL-8
amplifies the inflammatory response in AD by recruiting neutrophils
into the skin lesions (31).
Numerous researchers have reported that TNF-α/IFN-γ treatment
increases chemokine production in keratinocytes (32,33). The
TNF-α/IFN-γ combination activates several intracellular pathways,
including NF-κB pathways (34,35).
NF-κB pathways have been shown to be involved in the regulation of
chemokine and cytokine production in keratinocyte cells; they serve
a significant role in the immune response and regulate inflammatory
signaling (36,37). Therefore, experiments to investigate
the effect of COEE on the TNF-α/IFN-γ-stimulated expression of MDC
and TARC in HaCaT cells were conducted in the present study.
The NF-κB family includes critical transcription
factors that are activated by various stimuli, including TNF-α,
IFN-γ, IL-1 and lipopolysaccharide. Upon stimulation, NF-κB
complexes in the cytoplasm translocate into the nucleus, where they
participate in the expression of numerous pro-inflammatory genes
(22). NF-κB signaling pathways have
been shown to be involved in the regulation of TARC and MDC
production in HaCaT cells (38).
Furthermore, the promoters of TARC and MDC both contain
NF-κB-binding sites (39),
indicating that these transcription factors may be involved in the
modulation of TARC and MDC (38). In
the present study, the results indicated that COEE suppressed
signaling pathways leading to the activation of TARC and MDC by
NF-κB.
Treatment with COEE or the IκBα inhibitor Bay11-7082
reduced the TNF-α/IFN-γ-activated expression of pro-inflammatory
cytokines (IL-6 and IL-8) and chemokines (TARC and MDC) to baseline
values. These results indicate that COEE reduces the production of
the pro-inflammatory cytokines IL-6 and IL-8, and the expression of
the Th2 chemokines TARC and MDC in HaCaT cells via inhibition of
NF-κB pathways in HaCaT cells. These effects are hypothesized to be
closely associated with the suppression of NF-κB activation.
Therefore, it is suggested that COEE has the potential to be used
as a therapeutic drug for the treatment of AD.
In conclusion, the results of the present study
indicate that COEE inhibits the TNF-α/IFN-γ-stimulated expression
of TARC and MDC in HaCaT cells via the inhibition of NF-κB
pathways. The ability of COEE to suppress the formation of these
Th2 chemokines suggests that it may be able to inhibit the
infiltration of Th2 cells into skin lesions and thereby reduce skin
inflammation. Further investigation of the mechanism by which COEE
inhibits the release of these Th2 chemokines may provide insights
helpful in the design of targeted treatments for AD. However,
additional studies using in vivo skin inflammation models
are required to support the potential of COEE in the clinical
treatment of AD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Bio &
Medical Technology Development Program of the National Research
Foundation (NRF) and funded by the Korean government (MSIT; grant
no. NRF-2016K1A1A8A01939075) and the Korea Research Institute of
Bioscience and Biotechnology Research Initiative Program of the
Republic of Korea (grant no. KGM5521911). The authors thank the
National Biodiversity Institute and Tempisque Conservation Area for
preparing the plant materials.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HSL and JWP analyzed the data and wrote the
manuscript. OKK, YL, JHK, SYK, NZ and KR prepared the Cedrela
odorata L., and analyzed and edited the manuscript. SC, SRO, and
KSA designed the study and edited the manuscript. All authors
critically revised the article and have approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elbadawy HM, Borthwick F, Wright C, Martin
PE and Graham A: Cytosolic StAR-related lipid transfer domain 4
(STARD4) protein influences keratinocyte lipid phenotype and
differentiation status. Br J Dermatol. 164:628–632. 2011.PubMed/NCBI
|
|
2
|
Hong SW, Choi EB, Min TK, Kim JH, Kim MH,
Jeon SG, Lee BJ, Gho YS, Jee YK, Pyun BY and Kim YK: An important
role of α-hemolysin in extracellular vesicles on the development of
atopic dermatitis induced by Staphylococcus aureus. PLoS One.
9:e1004992014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elmariah SB and Lerner EA: The missing
link between itch and inflammation in atopic dermatitis. Cell.
155:267–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuhlbrigge RC, Kieffer JD, Armerding D and
Kupper TS: Cutaneous lymphocyte antigen is a specialized form of
PSGL-1 expressed on skin-homing T cells. Nature. 389:978–981. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boguniewicz M and Leung DY: Atopic
dermatitis: A disease of altered skin barrier and immune
dysregulation. Immunol Rev. 242:233–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oyoshi MK, He R, Kumar L, Yoon J and Geha
RS: Cellular and molecular mechanisms in atopic dermatitis. Adv
Immunol. 102:135–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ou LS and Huang JL: Cellular aspects of
atopic dermatitis. Clin Rev Allergy Immunol. 33:191–198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Lasse S, Lee P, Nakasaki M, Chen SW,
Yamasaki K, Gallo RL and Jamora C: Development of atopic
dermatitis-like skin disease from the chronic loss of epidermal
caspase-8. Proc Natl Acad Sci USA. 107:22249–22254. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Metral E, Bechetoille N, Demarne F, Damour
O and Rachidi W: Keratinocyte stem cells are more resistant to UVA
radiation than their direct progeny. PLoS One. 13:e02038632018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orazizadeh M, Hashemitabar M, Bahramzadeh
S, Dehbashi FN and Saremy S: Comparison of the enzymatic and
explant methods for the culture of keratinocytes isolated from
human foreskin. Biomed Rep. 3:304–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi XF, Kim DH, Yoon YS, Li JH, Jin D, Teng
YC, Kim SK and Lee KJ: Fluvastatin inhibits expression of the
chemokine MDC/CCL22 induced by interferon-gamma in HaCaT cells, a
human keratinocyte cell line. Br J Pharmacol. 157:1441–1450. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilson SR, Thé L, Batia LM, Beattie K,
Katibah GE, McClain SP, Pellegrino M, Estandian DM and Bautista DM:
The epithelial cell-derived atopic dermatitis cytokine TSLP
activates neurons to induce itch. Cell. 155:285–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirata H, Arima M, Cheng G, Honda K,
Fukushima F, Yoshida N, Eda F and Fukuda T: Production of TARC and
MDC by naive T cells in asthmatic patients. J Clin Immunol.
23:34–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ying S, O'Connor B, Ratoff J, Meng Q,
Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH and
Corrigan C: Thymic stromal lymphopoietin expression is increased in
asthmatic airways and correlates with expression of Th2-attracting
chemokines and disease severity. J Immunol. 174:8183–8190. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YJ: Thymic stromal lymphopoietin:
Master switch for allergic inflammation. J Exp Med. 203:269–273.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leyva-Castillo JM, Hener P, Jiang H and Li
M: TSLP produced by keratinocytes promotes allergen sensitization
through skin and thereby triggers atopic march in mice. J Invest
Dermatol. 133:154–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veitch NC, Wright GA and Stevenson PC:
Four new tetranortriterpenoids from Cedrela odorata associated with
leaf rejection by exopthalmus jekelianus. J Nat Prod. 62:1260–1263.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Navarro C, Ward S and Hernandez M: The
tree Cedrela odorata (Meliaceae): A morphologically subdivided
species in Costa Rica. Rev Biol Trop. 50:21–29. 2002.PubMed/NCBI
|
|
19
|
Thu PQ, Quang DN and Dell B: Threat to
cedar, Cedrela odorata, plantations in Vietnam by the weevil,
Aclees sp. J Insect Sci. 10:1922010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Millan-Orozco L, Corredoira E and San José
Mdel C: In vitro rhizogenesis: Histoanatomy of Cedrela odorata
(Meliaceae) microcuttings. Rev Biol Trop. 59:447–453.
2011.PubMed/NCBI
|
|
21
|
Wu WB, Zhang H, Dong SH, Sheng L, Wu Y, Li
J and Yue JM: New triterpenoids with protein tyrosine phosphatase
1B inhibition from Cedrela odorata. J Asian Nat Prod Res.
16:709–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JW, Kwon OK, Yuniato P, Marwoto B,
Lee J, Oh SR, Kim JH and Ahn KS: Amelioration of an LPS-induced
inflammatory response using a methanolic extract of Lagerstroemia
ovalifolia to suppress the activation of NF-κB in RAW264.7
macrophages. Int J Mol Med. 38:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JH, Jin SW, Park BH, Kim HG, Khanal
T, Han HJ, Hwang YP, Choi JM, Chung YC, Hwang SK, et al: Cultivated
ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic
dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced
TARC activation in HaCaT cells. Food Chem Toxicol. 56:195–203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung MR, Lee TH, Bang MH, Kim H, Son Y,
Chung DK and Kim J: Suppression of thymus- and activation-regulated
chemokine (TARC/CCL17) production by
3-O-β-D-glucopyanosylspinasterol via blocking NF-κB and STAT1
signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes.
Biochem Biophys Res Commun. 427:236–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strickson S, Campbell DG, Emmerich CH,
Knebel A, Plater L, Ritorto MS, Shpiro N and Cohen P: The
anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent
signalling network by targeting the ubiquitin system. Biochem J.
451:427–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voorhees T, Chang J, Yao Y, Kaplan MH,
Chang CH and Travers JB: Dendritic cells produce inflammatory
cytokines in response to bacterial products from Staphylococcus
aureus-infected atopic dermatitis lesions. Cell Immunol. 267:17–22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Megna M, Napolitano M, Patruno C, Villani
A, Balato A, Monfrecola G, Ayala F and Balato N: Systemic treatment
of adult atopic dermatitis: A review. Dermatol Ther (Heidelb).
7:1–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JW, Park HA, Kwon OK, Park JW, Lee G,
Lee HJ, Lee SJ, Oh SR and Ahn KS: NPS 2143, a selective
calcium-sensing receptor antagonist inhibits
lipopolysaccharide-induced pulmonary inflammation. Mol Immunol.
90:150–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kakinuma T, Nakamura K, Wakugawa M, Mitsui
H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K and
Tamaki K: Thymus and activation-regulated chemokine in atopic
dermatitis: Serum thymus and activation-regulated chemokine level
is closely related with disease activity. J Allergy Clin Immunol.
107:535–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JW, Shin IS, Ha UH, Oh SR, Kim JH and
Ahn KS: Pathophysiological changes induced by Pseudomonas
aeruginosa infection are involved in MMP-12 and MMP-13 upregulation
in human carcinoma epithelial cells and a pneumonia mouse model.
Infect Immun. 83:4791–4799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim SJ, Kim M, Randy A, Nam EJ and Nho CW:
Effects of Hovenia dulcis Thunb. extract and methyl vanillate on
atopic dermatitis-like skin lesions and TNF-α/IFN-γ-induced
chemokines production in HaCaT cells. J Pharm Pharmacol.
68:1465–1479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JW, Lee HS, Lim Y, Kwon OK, Kim JH,
Paryanto I, Yunianto P, Choi S, Oh SR and Ahn KS: Rhododendron
album Blume extract inhibits TNF-α/IFN-γ-induced chemokine
production via blockade of NF-κB and JAK/STAT activation in human
epidermal keratinocytes. Int J Mol Med. 41:3642–3652.
2018.PubMed/NCBI
|
|
34
|
Park JW, Kwon OK, Kim JH, Oh SR, Kim JH,
Paik JH, Marwoto B, Widjhati R, Juniarti F, Irawan D and Ahn KS:
Rhododendron album Blume inhibits iNOS and COX-2 expression in
LPS-stimulated RAW264.7 cells through the downregulation of NF-κB
signaling. Int J Mol Med. 35:987–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JW, Lee IC, Shin NR, Jeon CM, Kwon
OK, Ko JW, Kim JC, Oh SR, Shin IS and Ahn KS: Copper oxide
nanoparticles aggravate airway inflammation and mucus production in
asthmatic mice via MAPK signaling. Nanotoxicology. 10:445–452.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi JK and Kim SH: Inhibitory effect of
galangin on atopic dermatitis-like skin lesions. Food Chem Toxicol.
68:135–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang JH, Yoo JM, Cho WK and Ma JY:
Anti-inflammatory effects of Sanguisorbae Radix water extract on
the suppression of mast cell degranulation and STAT-1/Jak-2
activation in BMMCs and HaCaT keratinocytes. BMC Complement Altern
Med. 16:3472016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwon DJ, Bae YS, Ju SM, Goh AR, Youn GS,
Choi SY and Park J: Casuarinin suppresses TARC/CCL17 and MDC/CCL22
production via blockade of NF-κB and STAT1 activation in HaCaT
cells. Biochem Biophys Res Commun. 417:1254–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakayama T, Hieshima K, Nagakubo D, Sato
E, Nakayama M, Kawa K and Yoshie O: Selective induction of
Th2-attracting chemokines CCL17 and CCL22 in human B cells by
latent membrane protein 1 of Epstein-Barr virus. J Virol.
78:1665–1674. 2004. View Article : Google Scholar : PubMed/NCBI
|