|
1
|
Hoggatt J, Speth JM and Pelus LM: Concise
review: Sowing the seeds of a fruitful harvest: hematopoietic stem
cell mobilization. Stem Cells. 31:2599–2606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jantunen E, Varmavuo V and Valtola J:
Plerixafor injection: A hematopoietic stem cell mobilizer in
non-Hodgkin lymphoma and multiple myeloma. Expert Rev Hematol.
9:723–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duong HK, Savani BN, Copelan E, Devine S,
Costa LJ, Wingard JR, Shaughnessy P, Majhail N, Perales MA, Cutler
CS, et al: Peripheral blood progenitor cell mobilization for
autologous and allogeneic hematopoietic cell transplantation:
Guidelines from the American Society for Blood and Marrow
Transplantation. Biol Blood Marrow Transplant. 20:1262–1273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosing C, Saliba RM, Ahlawat S, Körbling
M, Kebriaei P, Alousi A, De Lima M, Okoroji JG, McMannis J,
Qazilbash M, et al: Poor hematopoietic stem cell mobilizers: A
single institution study of incidence and risk factors in patients
with recurrent or relapsed lymphoma. Am J Hematol. 84:335–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pusic I, Jiang SY, Landua S, Uy GL, Rettig
MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein
KE, et al: Impact of mobilization and remobilization strategies on
achieving sufficient stem cell yields for autologous
transplantation. Biol Blood Marrow Transplant. 14:1045–1056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kessans MR, Gatesman ML and Kockler DR:
Plerixafor: A peripheral blood stem cell mobilizer.
Pharmacotherapy. 30:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hübel K, Liles WC, Broxmeyer HE, Rodger E,
Wood B, Cooper S, Hangoc G, Macfarland R, Bridger GJ, Henson GW, et
al: Leukocytosis and Mobilization of CD34+ Hematopoietic Progenitor
Cells by AMD3100, a CXCR4 Antagonist. Support Cancer Ther.
1:165–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russell N, Douglas K, Ho AD, Mohty M,
Carlson K, Ossenkoppele GJ, Milone G, Pareja MO, Shaheen D,
Willemsen A, et al: Plerixafor and granulocyte colony-stimulating
factor for first-line steady-state autologous peripheral blood stem
cell mobilization in lymphoma and multiple myeloma: Results of the
prospective PREDICT trial. Haematologica. 98:172–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilgin YM and de Greef GE: Plerixafor for
stem cell mobilization: The current status. Curr Opin Hematol.
23:67–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Clercq E: Mozobil®
(Plerixafor, AMD3100), 10 years after its approval by the US Food
and Drug Administration. Antivir Chem Chemother.
27:2040206619829382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hartmann T, Hübel K, Monsef I, Engert A
and Skoetz N: Additional plerixafor to granulocyte
colony-stimulating factors for haematopoietic stem cell
mobilisation for autologous transplantation in people with
malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev.
20:CD0106152015.
|
|
12
|
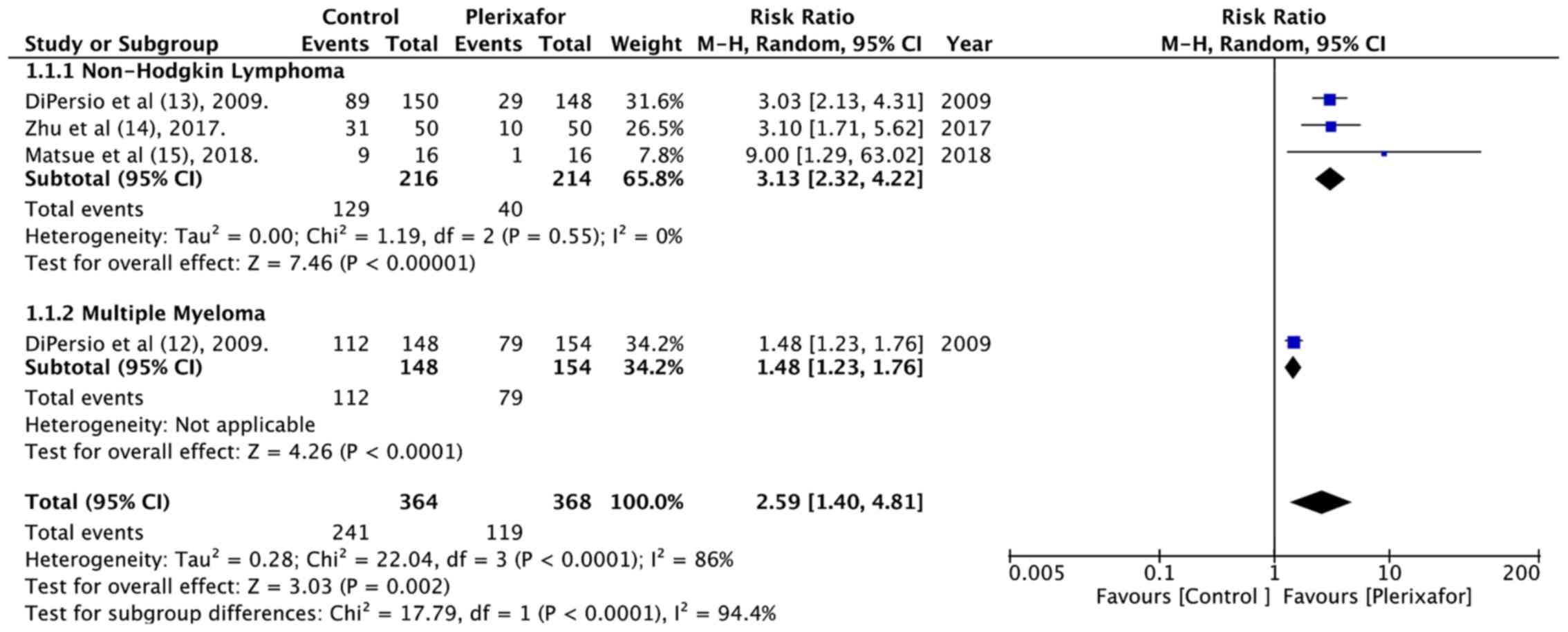
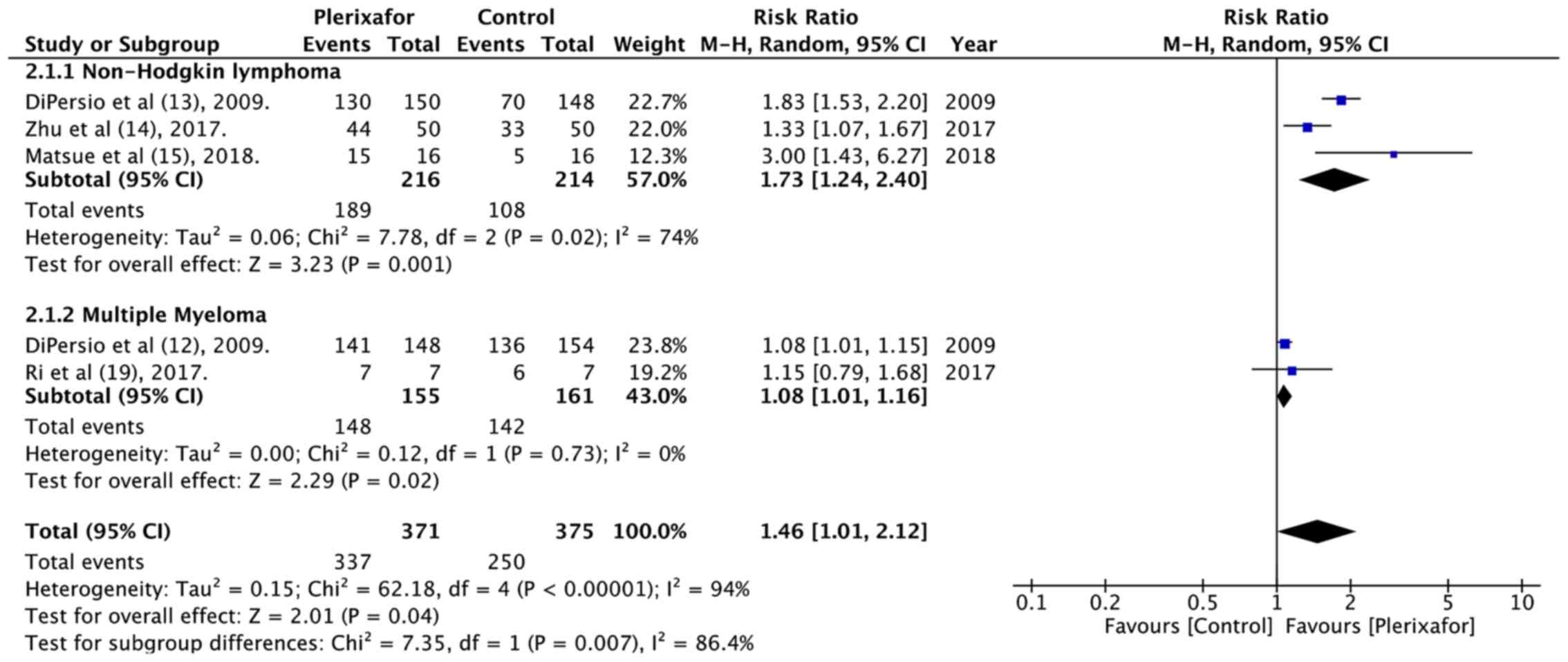
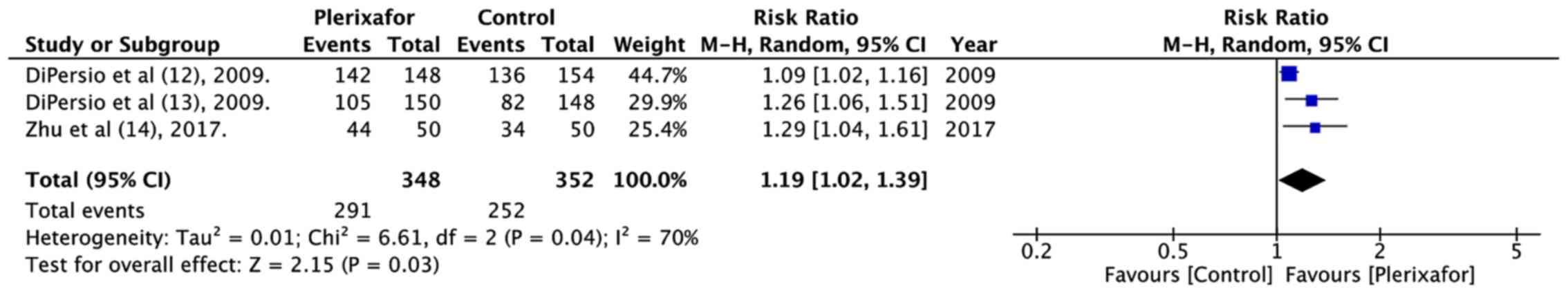
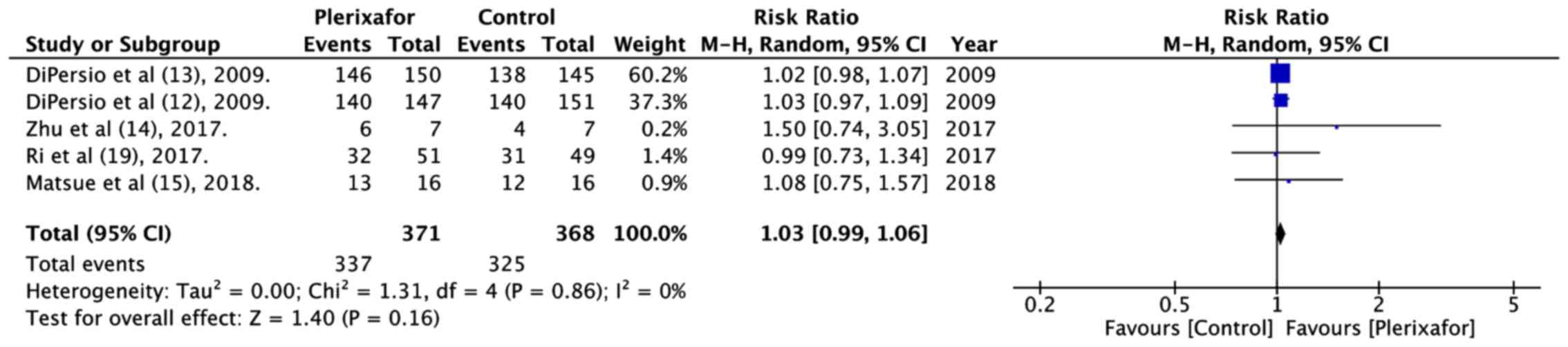
DiPersio JF, Stadtmauer EA, Nademanee A,
Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Früehauf
S, Horwitz M, et al 3102 Investigators, : Plerixafor and G-CSF
versus placebo and G-CSF to mobilize hematopoietic stem cells for
autologous stem cell transplantation in patients with multiple
myeloma. Blood. 113:5720–5726. 2009.PubMed/NCBI
|
|
13
|
DiPersio JF, Micallef IN, Stiff PJ,
Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger
G and Calandra G; 3101 Investigators, : Phase III prospective
randomized double-blind placebo-controlled trial of plerixafor plus
granulocyte colony-stimulating factor compared with placebo plus
granulocyte colony-stimulating factor for autologous stem-cell
mobilization and transplantation for patients with non-Hodgkin's
lymphoma. J Clin Oncol. 27:4767–4773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu J, Huang H, Chen H, Zhang X, Li Z, Wu
D, Zhou D, Song Y, Hu Y, Liang Y, et al: Plerixafor and
granulocyte-colony-stimulating factor for mobilization of
hematopoietic stem cells for autologous transplantation in Chinese
patients with non-Hodgkin's lymphoma: A randomized Phase 3 study.
Transfusion. 58:81–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsue K, Kumagai K, Sugiura I, Ishikawa
T, Igarashi T, Sato T, Uchiyama M, Miyamoto T, Ono T, Ueda Y, et
al: Plerixafor for mobilization and collection of haematopoietic
stem cells for autologous transplantation in Japanese patients with
non-Hodgkin lymphoma: A randomized phase 2 study. Int J Hematol.
108:524–534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins J and Green S: Cochrane Handbook
for Systemic Reviews of Interventions. Version 5. The Cochrane
Collaboration. 2011
|
|
18
|
Higgins J, Altman D and Sterne J; Cochrane
Statistical Methods Group and the Cochrane Bias Methods Group, :
Chapter 8: assessing risk of bias in included studies. Cochrane
Handbook for Systemic Reviews of Interventions, Version 5. The
Cochrane Collaboration. 2011
|
|
19
|
Ri M, Matsue K, Sunami K, Shimazaki C,
Hayashi A, Sunaga Y, Sasaki T and Suzuki K: Efficacy and safety of
plerixafor for the mobilization/collection of peripheral
hematopoietic stem cells for autologous transplantation in Japanese
patients with multiple myeloma. Int J Hematol. 106:562–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dugan MJ, Maziarz RT, Bensinger WI,
Nademanee A, Liesveld J, Badel K, Dehner C, Gibney C, Bridger G and
Calandra G: Safety and preliminary efficacy of plerixafor (Mozobil)
in combination with chemotherapy and G-CSF: An open-label,
multicenter, exploratory trial in patients with multiple myeloma
and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone
Marrow Transplant. 45:39–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin AP, Richards S, Haycox A, Houten R,
McLeod C, Braithwaite B, Clark JO, Bell J and Clark RE: Evaluating
the use of plerixafor in stem cell mobilisation - an economic
analysis of the PHANTASTIC trial. J Clin Apher. 31:434–442. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haverkos BM, Huang Y, Elder P, O'Donnell
L, Scholl D, Whittaker B, Vasu S, Penza S, Andritsos LA, Devine SM,
et al: A single center's experience using four different front line
mobilization strategies in lymphoma patients planned to undergo
autologous hematopoietic cell transplantation. Bone Marrow
Transplant. 52:561–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Partanen A, Valtola J, Ropponen A, Vasala
K, Penttilä K, Ågren L, Pyörälä M, Nousiainen T, Selander T,
Mäntymaa P, et al: Preemptive plerixafor injection added to
pegfilgrastim after chemotherapy in non-Hodgkin lymphoma patients
mobilizing poorly. Ann Hematol. 96:1897–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liles WC, Broxmeyer HE, Rodger E, Wood B,
Hübel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, et
al: Mobilization of hematopoietic progenitor cells in healthy
volunteers by AMD3100, a CXCR4 antagonist. Blood. 102:2728–2730.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Devine SM, Flomenberg N, Vesole DH,
Liesveld J, Weisdorf D, Badel K, Calandra G and DiPersio JF: Rapid
mobilization of CD34+ cells following administration of the CXCR4
antagonist AMD3100 to patients with multiple myeloma and
non-Hodgkin's lymphoma. J Clin Oncol. 22:1095–1102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flomenberg N, Devine SM, Dipersio JF,
Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K and Calandra
G: The use of AMD3100 plus G-CSF for autologous hematopoietic
progenitor cell mobilization is superior to G-CSF alone. Blood.
106:1867–1874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brave M, Farrell A, Ching Lin S, Ocheltree
T, Pope Miksinski S, Lee SL, Saber H, Fourie J, Tornoe C, Booth B,
et al: FDA review summary: Mozobil in combination with granulocyte
colony-stimulating factor to mobilize hematopoietic stem cells to
the peripheral blood for collection and subsequent autologous
transplantation. Oncology. 78:282–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan S, Palmer JM, Tsai N-C, Dagis A,
Nademanee A and Wang S: Engraftment and outcomes following
autologous stem cell transplantation in Hodgkin lymphoma patients
mobilized with plerixafor. Hematol Oncol. 35:281–287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milone G, Martino M, Leotta S, Spadaro A,
Zammit V, Cupri A, Avola G, Camuglia MG, Di Marco A, Scalzulli P,
et al: Cost-effectiveness of on-demand plerixafor added to
chemotherapy and granulocyte-colony stimulating factor for
peripheral blood stem cell mobilization in multiple myeloma. Leuk
Lymphoma. 59:42–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohty M, Azar N, Chabannon C, Le Gouill S,
Karlin L, Farina L, Milkovich G, Ostermann H, Glaß B, Noppeney R,
et al: Plerixafor in poor mobilizers with non-Hodgkin's lymphoma: A
multi-center time-motion analysis. Bone Marrow Transplant.
53:246–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemoli RM: New strategies for stem cell
mobilization. Mediterr J Hematol Infect Dis. 4:e20120662012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shpall EJ, Champlin R and Glaspy JA:
Effect of CD34+ peripheral blood progenitor cell dose on
hematopoietic recovery. Biol Blood Marrow Transplant. 4:84–92.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Micallef IN, Stiff PJ, Nademanee AP,
Maziarz RT, Horwitz ME, Stadtmauer EA, Kaufman JL, McCarty JM,
Vargo R, Cheverton PD, et al: Plerixafor Plus Granulocyte
Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma
and Multiple Myeloma: Long-Term Follow-Up Report. Biol Blood Marrow
Transplant. 24:1187–1195. 2018. View Article : Google Scholar : PubMed/NCBI
|