Introduction
Acute pancreatitis (AP) is an inflammatory disease
that affects the pancreas. The pathogenesis of AP is complex and
involves multiple factors, such as hyperlipidemia, alcoholism and
biliary diseases (1). The prognosis
of mild AP is typically good, with a very low mortality rate;
however, severe AP may cause serious consequences and have a poor
prognosis (2). AP progression is
associated with the activation of pancreatin, cytokines and
chemokines (3,4). Therefore, it is of great clinical
significance to explore the inflammation mechanism and to find a
potential treatment that targets the inflammation process.
Rosiglitazone, currently the most effective
thiazolidinedione drug, is principally used for the treatment of
diabetes (5,6). Numerous studies have demonstrated that
rosiglitazone can increase insulin sensitivity and decrease insulin
resistance (5–7). However, in recent years, due to better
understanding of peroxisome proliferator activated receptor (PPAR)γ
and its ligands, rosiglitazone has been determined to have
significant effects on the inflammatory response, cell
differentiation and cell metabolism (8–10).
Previous research suggested that rosiglitazone exhibits
anti-inflammatory effects on osteoporosis, acute or chronic
gastrointestinal diseases and other systemic inflammatory response
syndromes (7,11–12).
MicroRNA (miRNA) is a non-coding, single-chain RNA
(18–25 nucleotides in length), which can bind to the
3′-untranslated region (UTR) of target genes and suppress the
translation or promote the degradation of genes (13). Although miRNA only accounts for ~1%
of the human genome, it regulates ~60% protein expression (14). Present studies determined that miRNAs
are involved in various cellular functions, such as proliferation,
differentiation and the inflammation response (13,14).
miRNA (miR)-26 is located in chromosome 19q14.12, which is closely
associated with tumor development by regulation of tumor cell
proliferation and apoptosis (15).
In addition, miR-26a is involved in the allergic inflammatory
reaction and the toll like receptor 4 (TLR-4)-mediated inflammatory
response (16,17). However, the specific role of miR-26a
in AP has not been fully elucidated.
The aim of the present study was to investigate the
regulatory effect of rosiglitazone on the progression of AP and
pancreas injury, and its underlying mechanism.
Materials and methods
Animal model
A total of 40 male Sprague Dawley rats (age, 6–8
weeks; weight, 180–200 g) were purchased from Model Animal Research
Center of Nanjing University (Nanjing, China). The rats were housed
in a temperature-controlled room (21±2°C) with 60–70% relative
humidity on a 12-h light: dark cycle (lights on at 06:00). All rats
had free access to water and food. The rats of specific pathogen
free level were randomly divided into three groups: The control
group (n=10), the AP model group (n=15) and the
rosiglitazone-treated group (n=15). AP model rats were anesthetized
by intraperitoneal injection of pentobarbital sodium (30 mg/kg)
then injected intraperitoneally with 50 µg/kg caerulein
(MedChemExpress) five times at 1-h intervals. Rats in the control
group were given the same volume (1 ml each time) of 0.9% NaCl.
Rats in the rosiglitazone-treated group were administered with
rosiglitazone (4 mg/kg, Sigma-Aldrich; Merck KGaA) by
intraperitoneal injection 1 h before the first injection of
caerulein. Peripheral blood was collected 6, 12 and 24 h after the
last injection of caerulein then the rats were sacrificed for
pancreatic tissues. Parts of the pancreatic tissues were stored in
liquid nitrogen, whilst the remainder were fixed for histological
analysis. All animals used in the experiment were obtained from the
Model Animal Research Center of Nanjing University. This experiment
was approved by Soochow University Ethics Committee (Soochow,
China).
ELISA
Blood samples were maintained at room temperature
for 20 min then centrifuged at 1,000 × g at 4°C for 15 min for
serum sample preparation. Serum contents of amylase (cat. no.
SL-B23234), lipase (cat. no. LM04412B), tumor necrosis factor-α
(TNF-α; cat. no. QY-R2813), interleukin-6 (IL-6; cat. no. HS2102)
and transforming growth factor-β (TGF-β; cat. no. FS-E6931) were
determined using ELISA kits purchased from Guidechem. In brief, the
standard solution was added to each well and incubated for 2 h at
20°C. Then the liquid was removed and an anti-biotin antibody was
added for a 1-h incubation at 20°C. Each well was washed then
horseradish peroxidase-labeled streptavidin work solution was
added. Following a 1-h incubation at 20°C, substrates were added
for color development in the dark. Termination solution was added
15–30 min later, prior to detection of optical density.
AP assessment in rats
Pancreas tissues were fixed in 10% neutral buffered
formaldehyde for >24 h at room temperature. Tissue samples were
embedded in paraffin blocks and sliced into 5 µm sections for
hematoxylin and eosin (H&E) staining (hematoxylin, 5 min at
room temperature and eosin, 3 min at room temperature).
Histological analysis was performed using a light microscope
(magnification, ×400).
Histological scoring of pancreatic tissue was
performed to grade the severity and extent of acinar edema (0, no
edema; 1, inter lobular edema; 2, intralobular edema; and 3, inter
acinar edema), inflammation [0, no inflammation; 1, inflammatory
cells in ducts; 2, inflammatory cells in the parenchyma (<50% of
the lobules); and 3, inflammatory cells in the parenchyma (>50%
of the lobules)] and finally acinar cell necrosis (0, no necrosis;
1, <5% necrosis; 2, 5–20% necrosis; and 3, 20–50% necrosis).
Bioinformatic analysis
Bioinformatic analysis predicted that PTEN was the
potential target gene of miR-26a (http://www.targetscan.org). In detail, rat was
selected as species in the first search box and miR-26a-5p was put
into the microRNA name search box. PTEN was found in the table of
results.
Cell culture and transfection
Rat pancreatic AR42J cells (Type Culture Collection
of the Chinese Academy of Sciences) were cultured in DMEM-F12
containing 20% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 g/l streptomycin and
maintained in a 5% CO2 incubator. The culture medium was
changed every 2 days.
AR42J cells were inoculated into 24-well plates at
1×105 cells/well. When the cell density reached 70–80%,
miR-26a inhibitor (hsa-miR-26a-in; Hanbio Biotechnology Co., Ltd.),
miR-26a mimics (hsa-miR-26a-mi; Hanbio Biotechnology Co., Ltd),
phosphatase and tensin homolog (PTEN) small interfering (si)-RNA
and related non-targeting control (NC) siRNA (both GenePharma) were
transfected into cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The sequences were as follows: PTEN
siRNA forward, 5′-AACCCACCACAGCUAGAACTT-3′ and reverse,
5′-AAGUUCUAGCUGUGGUGGGTT-3′ and NC siRNA forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. Following incubation for 24–48 h,
cells were collected for the following experiments.
In vitro AP model
One day prior to model establishment, AR42J cells
were inoculated into 6-well plates with 1×105/ml and
treated with 10 nmol/l caerulein. Following caerulein treatment for
8 h at 37°C cells were then treated with 0, 0.01, 0.1 or 1 µM
rosiglitazone for 24 h.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA from cells was extracted with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and then
mixed with radioimmunoprecipitation assay (RIPA) lysis buffer
(Beyotime Institute of Biotechnology). The same volume of
chloroform was added into the mixture and centrifuged at 10,000 × g
at 4°C for 5 min. The aqueous phase was collected into Eppendorf
tubes. Following addition of 0.5 ml 99% isopropanol to the mixture,
the Eppendorf tubes were rinsed with ethanol to remove the residue
then samples were air dried at the room temperature. Total RNA was
reverse transcribed into cDNA using Takara PrimeScript™ RT Master
Mix kit (Takara Biotechnology Co., Ltd.). qPCR was performed using
SYBR® Green Master Mix (Takara Bio, Inc.). The
thermocycling conditions as: 40 cycles of 95°C for 30 sec, 95°C for
5 sec and 60°C for 31 sec. GAPDH served as the internal control for
PTEN, while U6 served as the internal control for miR-204. Primers
sequences were as follows: miR-26a-5p forward,
5′-GGATCCGCAGAAACTCCAGAGAGAAGGA-3′ and reverse,
5′-AAGCTTGCCTTTAGCAGAAAGGAGGTT-3′; PTEN forward,
5′-GTTTACCGGCAGCATCAAAT-3′ and reverse, 5′-CCCCCACTTTAGTGCACAGT-3′;
GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
The 2−ΔΔCq method was used to determine the relative
expression levels (18). mRNA
expression levels were normalized to GAPDH, whereas miRNA
expression levels were normalized to U6.
Western blot analysis
Cells in the logarithmic growth phase were collected
and digested by pancreatin, subsequently rinsed by PBS and fully
lysed by RIPA buffer (Beyotime Institute of Biotechnology).
Following oscillating incubation at 4°C for 10 min, cells were
decomposed using sonication at 20 kHz at 4°C for 2 min then
centrifuged at 5,000 × g for 10 min at 4°C for supernatant
preparation. Protein concentration was quantified using
bicinchoninic acid protein assay (Pierce; Thermo Fisher Scientific,
Inc.). Proteins (10 µg) were separated by 12% SDS-PAGE, followed by
an electrophoretic transfer onto polyvinylidene fluoride membranes
(EMD Millipore). Membranes were placed into 5% non-fat milk to
block non-specific binding at 25°C for 1 h. Membranes were then
incubated with primary antibodies including PTEN (1:500; cat. no.
ab32199; Abcam), phosphorylated (p)-PI3K (1:500; cat. no. ab182651;
Abcam), PI3K (1:500; cat. no. ab151549; Abcam), p-AKT (1:500; cat.
no. ab38449; Abcam), AKT (1:500; cat. no. ab8805; Abcam), GAPDH
(1:500; cat. no. ab8245; Abcam) overnight at 4°C. Finally, the
membranes were rinsed with PBS and incubated with goat horseradish
peroxidase-conjugated goat anti-rabbit IgG H&L secondary
antibody (1:1,000; cat. no. ab7090; Abcam) at 25°C for 1 h. Protein
bands were visualized following a 3-min incubation with enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.).
Quantity One (version 4.0; Bio-Rad Laboratories, Inc.) was used for
densitometric analysis.
Luciferase reporter gene assay
Cells were seeded and cultured into 24-well plates
at a density of 5×104 cells/well. The cells were
transfected with Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.). The pGL3-PTEN-3′UTR wild-type or mutant plasmid (0.5 µg/ml;
Hanbio Biotechnology Co., Ltd) was co-transfected with miR-26a
mimic (0.2 µg/ml) or miR-26a NC (0.2 µg/ml) and pRL-TK
Renilla plasmid (0.02 µg/ml; Promega Corporation) into the
cells. Following incubation for 48 h at room temperature, cells
were collected for analysis of the luciferase activities of both
firefly and Renilla using a Dual Luciferase®
Reporter Assay System (Promega Corporation). Firefly luciferase
activity was normalized by comparing the activity levels to pRL-TK
Renilla.
Statistical analysis
Statistical analysis was performed by Statistical
Product and Service Solutions v.19.0 (IBM Corp.). All data were
expressed as mean ± standard deviation. Statistical difference was
assessed using two-tailed Student t-test for comparisons amongst
two groups. Comparisons between multiple groups was performed using
one-way analysis of variance test followed by Least Significant
Difference post hoc test. P<0.05 was considered to indicate
statistical significance.
Results
Rosiglitazone reduces serum levels of
amylase and cytokines
Rats were intraperitoneally injected with caerulein
for the establishment of an AP rat model. Results demonstrated that
serum expressions of amylase, lipase, TNF-α, IL-6 and TGF-β were
significantly increased in the AP model group compared with control
group, whilst the levels decreased in the rosiglitazone-treated
group (Fig. 1A-E). Pathological
examination of the pancreas indicated that caerulein induced
infiltration of immune cells and pancreas injury (Fig. 1F). Rosiglitazone pretreatment
remarkably and significantly reduced the level of pancreas injury
at all timepoints compared with caerulein treatment (Fig. 1G).
 | Figure 1.Rosiglitazone prevents the progression
of AP. (A) Serum expression of amylase, (B) lipase, (C) TNF-α, (D)
IL-6 and (E) TGF-β were detected following establishment of an AP
model and pretreatment with rosiglitazone. (F) Representative
H&E staining images of pancreas tissue in control, caerulein
and rosiglitazone treatment groups, black arrow showed the
infiltration of immune cells. (G) H&E staining
immunohistochemical scores in the pancreas. Each experiment was
repeated three times. *P<0.05. AP, acute pancreatitis; TNF-α,
tumor necrosis factor-α; IL-6, interleukin-6; TGF-β, transforming
growth factor-β; H&E, hematoxylin and eosin. |
Rosiglitazone suppresses miR-26a
expression
AR42J cells were pretreated with different
concentrations of rosiglitazone (0, 0.01, 0.1 and 1 µM). Results
revealed that miR-26a expression in AR42J cells was significantly
decreased in what appears to be a dose-dependent manner by
rosiglitazone compared with the untreated group (Fig. 2A) whilst PTEN mRNA expression was
significantly increased in what appears to be a dose-dependent
manner compared with the untreated group (Fig. 2B). In addition, expression levels of
miR-26a in pancreatic tissues and serum of rats was determined.
miR-26a expression was significantly increased in the AP model
group but decreased with rosiglitazone pretreatment compared with
control group (Fig. 2C and D).
Results indicated that rosiglitazone may regulate AP via
miR-26a.
miR-26a regulates PTEN expression
whilst PTEN has no effect on miR-26a
Bioinformatic analysis predicted that PTEN was the
potential target gene of miR-26a (http://www.targetscan.org). Transfection efficacy of
constructed plasmids was first verified by RT-qPCR as miR-26a
mimics significantly increased and miR-26a inhibitor significantly
decreased expression compared with the miR-26a NC group (Fig. 3A). It was identified that miR-26a
overexpression significantly suppressed PTEN expression and miR-26a
inhibition significantly increased PTEN levels compared with the
miR-26a NC group (Fig. 3B). Similar
results were produced when detecting PTEN protein levels (Fig. 3C and D). The luciferase reporter gene
assay demonstrated that miR-26a mimics significantly decreased the
luciferase activity of cells co-transfected with PTEN-WT compared
with those co-transected with miR-26a NC, which suggested that
miR-26a could directly bind to PTEN and inhibit its expression
(Fig. 3E). The expression of PTEN
was significantly decreased in cells transfected with si-PTEN
compared with those transfected with NC siRNA (Fig. 3F). By contrast, the expression of
miR-26a was not changed significantly following PTEN knockdown
(Fig. 3G).
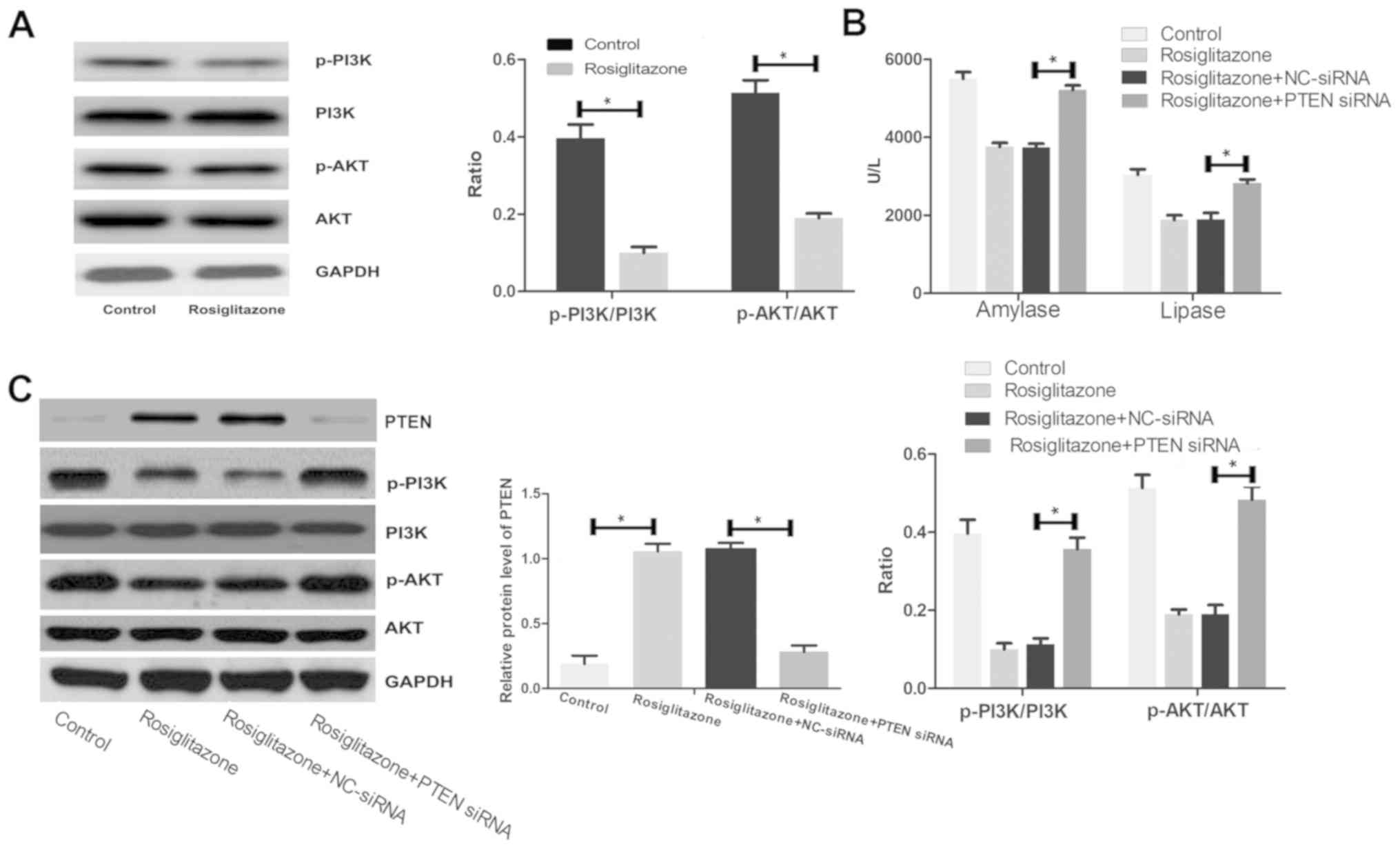
Rosiglitazone suppresses the PI3K/AKT
signaling pathway
A previous study demonstrated that the biological
functions of PTEN were mediated by the PI3K/AKT signaling pathway
(19). Therefore, the effect of
rosiglitazone on the PI3K/AKT signaling pathway were investigated.
Rosiglitazone pretreatment significantly suppressed the
phosphorylation of key proteins involved in the PI3K/AKT signaling
pathway compared with the control group (Fig. 4A). The serum levels of amylase and
lipase were significantly increased in the PTEN knockdown group
compared with the rosiglitazone + NC-siRNA group, indicating that
PTEN knockdown reversed the beneficial effect of rosiglitazone on
serum levels of amylase and lipase (Fig.
4B). In addition, the ratios of p-PI3K/PI3K and p-AKT/AKT were
significantly increased in the PTEN knockdown group compared with
the rosiglitazone + NC-siRNA group, indicating that PTEN knockdown
reversed the inhibitory effect of rosiglitazone on the PI3K/AKT
signaling pathway (Fig. 4C). These
results suggest that rosiglitazone regulated the PI3K/AKT signaling
pathway via PTEN.
Discussion
During the initial stage of AP, TNF-α is the main
regulatory factor responsible for triggering the inflammatory
cascade. Activation of the immune system elevates inflammatory
signaling, and further leads to cell injury and necrosis. The
TLR-4-mediated inflammatory reaction can active multiple cytokines
(20,21). The present study demonstrated that
expression levels of amylase, lipase, TNF-α, IL-6 and TGF-β were
significantly increased following caerulein treatment, which
suggested successful establishment of the AP model and pancreas
injury. Rats pretreated with intraperitoneal injection of
rosiglitazone significantly attenuated the inflammatory response
and pancreas injury. Therefore, the present study next explored the
underlying mechanism of rosiglitazone.
Previous studies have identified that miRNAs serve
important roles in the progression of inflammatory diseases. Wu
et al (22) reported that
miRNA regulates macrophage polarity and thus controls the
inflammatory reaction. In addition, miRNA is associated with
various inflammatory diseases. For example, miR-365 directly
suppresses the expression of histone deacetylase 4 and contributes
to the development of rheumatoid arthritis (23). Sorbin and SH3 domain containing
2-mediated cardiac dysfunction during sepsis is regulated by
miR-21-3p (24). Since miRNA can
regulate the expressions of several critical components and
cytokines, it has become an important diagnostic and therapeutic
target for rheumatoid arthritis (25). In the present study, rosiglitazone
suppressed miR-26a expression, thus resulting in the elevated
expression of the target gene PTEN.
PTEN and the PTEN-mediated pathway are involved in
the occurrence and development of various diseases (26). Previous studies have demonstrated
that the biological function of PTEN involved regulation of cell
survival, cell proliferation and inflammation via the P13K/AKT
signaling pathway (27,28). Inflammatory mediators can lead to the
activation and chemotaxis of immune cells via the PI3K pathway
(29). The present study
demonstrated that decreased expression of PTEN reduced the
inhibitory effect of miR-26a on the PI3K/AKT pathway, thereby
regulating inflammation. However, the underlying mechanism of
rosiglitazone suppression on the PI3K/AKT pathway remains poorly
understood. Future work will use the PI3K/AKT inhibitor wortmannin
to further investigate the underlying mechanism
In conclusion, rosiglitazone prevented AP
progression through suppressing miR-26a expression, which elevated
expression of PTEN. PTEN has been implicated in the development of
various diseases therefore research into the gene can provide
potential novel strategies for treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contribution
YC and CQ designed the study and performed the
experiments. YC, WX and XL established the animal models. YC and DW
collected the data. YC and WX analyzed the data. YC and CQ prepared
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Soochow University
Ethics Committee (Soochow, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banks PA and Freeman ML; Practice
Parameters Committee of the American College of Gastroenterology, :
Practice guidelines in acute pancreatitis. Am J Gastroenterol.
101:2379–2400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS; AcutePancreatitis
Classification Working Group, : Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kylanpaa ML, Repo H and Puolakkainen PA:
Inflammation and immunosuppression in severe acute pancreatitis.
World J Gastroenterol. 16:2867–2872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrov M: Nutrition, inflammation, and
acute pancreatitis. ISRN Inflamm. 2013:3414102013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kahn SE, Haffner SM, Heise MA, Herman WH,
Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B,
et al: Glycemic durability of rosiglitazone, metformin, or
glyburide monotherapy. N Engl J Med. 355:2427–2443. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fryer LG, Parbu-Patel A and Carling D: The
anti-diabetic drugs rosiglitazone and metformin stimulate
amp-activated protein kinase through distinct signaling pathways. J
Biol Chem. 277:25226–25232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramakers JD, Verstege MI, Thuijls G, Te
VA, Mensink RP and Plat J: The ppargamma agonist rosiglitazone
impairs colonic inflammation in mice with experimental colitis. J
Clin Immunol. 27:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji XX, Ji XJ, Li QQ, Lu XX and Luo L:
Rosiglitazone reduces apoptosis and inflammation in
lipopolysaccharide-induced human umbilical vein endothelial cells.
Med Sci Monit. 24:6200–6207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding F, Qiu J, Li Q, Hu J, Song C, Han C,
He H and Wang J: Effects of rosiglitazone on proliferation and
differentiation of duck preadipocytes. In Vitro Cell Dev Biol Anim.
52:174–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng Y, Li S, Wang M, Cheng C and Liu R:
Peroxisome proliferator activated receptor gamma (PPARgamma)
agonist rosiglitazone ameliorate airway inflammation by inhibiting
toll-like receptor 2 (TLR2)/Nod-like receptor with pyrin domain
containing 3 (NLRP3) inflammatory corpuscle activation in asthmatic
Mice. Med Sci Monit. 24:9045–9053. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levi Z, Shaish A, Yacov N, Levkovitz H,
Trestman S, Gerber Y, Cohen H, Dvir A, Rhachmani R, Ravid M and
Harats D: Rosiglitazone (PPARgamma-agonist) attenuates
atherogenesis with no effect on hyperglycaemia in a combined
diabetes-atherosclerosis mouse model. Diabetes Obes Metab. 5:45–50.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hassumi MY, Silva-Filho VJ, Campos-Junior
JC, Vieira SM, Cunha FQ, Alves PM, Alves JB, Kawai T, Gonçalves RB
and Napimoga MH: PPAR-gamma agonist rosiglitazone prevents
inflammatory periodontal bone loss by inhibiting
osteoclastogenesis. Int Immunopharmacol. 9:1150–1158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin S and Gregory RI: Microrna biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: Microrna signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: Mir-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of ezh2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwon Y, Kim Y, Eom S, Kim M, Park D, Kim
H, Noh K, Lee H, Lee YS, Choe J, et al:
Microrna-26a/-26b-cox-2-mip-2 loop regulates allergic inflammation
and allergic inflammation-promoted enhanced tumorigenic and
metastatic potential of cancer cells. J Biol Chem. 290:14245–14266.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar A, Bhatia HS, de Oliveira AC and
Fiebich BL: Microrna-26a modulates inflammatory response induced by
toll-like receptor 4 stimulation in microglia. J Neurochem.
135:1189–1202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-AKT pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Yu CX, Song B, Cai W, Liu C and
Guan QB: Free fatty acids mediates human umbilical vein endothelial
cells inflammation through toll-like receptor-4. Eur Rev Med
Pharmacol Sci. 22:2421–2431. 2018.PubMed/NCBI
|
|
21
|
Sah RP, Garg P and Saluja AK: Pathogenic
mechanisms of acute pancreatitis. Curr Opin Gastroenterol.
28:507–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu XQ, Dai Y, Yang Y, Huang C, Meng XM, Wu
BM and Li J: Emerging role of microRNAS in regulating macrophage
activation and polarization in immune response and inflammation.
Immunology. 148:237–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Guan Y, Tian S, Wang Y, Sun K and
Chen Q: Mechanical and IL-1β responsive miR-365 contributes to
osteoarthritis development by targeting histone deacetylase 4. Int
J Mol Sci. 17:4362016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Bei Y, Shen S, Huang P, Shi J,
Zhang J, Sun Q, Chen Y, Yang Y, Xu T, et al: miR-21-3p controls
sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol
Cell Cardiol. 94:43–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma AR, Sharma G, Lee SS and
Chakraborty C: Mirna-regulated key components of cytokine signaling
pathways and inflammation in rheumatoid arthritis. Med Res Rev.
36:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki A, Yamaguchi MT, Ohteki T, Sasaki
T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M,
et al: T cell-specific loss of Pten leads to defects in central and
peripheral tolerance. Immunity. 14:523–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paez J and Sellers WR: PI3K/PTEN/AKT
pathway. A critical mediator of oncogenic signaling. Cancer Treat
Res. 115:145–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weichhart T and Saemann MD: The
PI3K/AKT/MTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67 (Suppl 3):iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|