Introduction
Lung cancer is the most common cause of
cancer-associated death (1). Every
year, ~234,030 patients are newly diagnosed with cancer in the
United States, while nearly ~154,050 patients succumb to lung
cancer, with the majority of mortalities resulting from metastasis
(1,2).
The brain is one of the most common sites of distant
metastasis of lung cancer, particularly in lung cancer cases
without lymph node involvement (3,4). The
incidence of brain metastasis from lung cancer (BMLC) has been
reported as 23–65% (5). The
prognosis of patients with BMLC is poor, with a median survival of
only 4–5 months (6). Over the past
decades, several genetic alterations associated with the occurrence
of BMLC were identified. For instance, vascular endothelial growth
factor (VEGF)-C, a member of the VEGF family (7), was associated with BMLC. Chen et
al (8) reported that high
expression of VEGF-C is positively associated with brain metastasis
in patients with lung cancer. Furthermore, the incidence of BMLC in
a VEGF-C-positive expression group was higher compared with that in
a negative expression group. In addition, inflammatory chemokines
have been identified to be associated with brain metastases. The
expression of C-X-C motif chemokine receptor (CXCR)4, receptor of
the CXC chemokine ligand 12 (CXCL12), in the primary tumor tissues
and distant metastatic lung tumors in the brain was reported to be
higher than that in lung cancer patients without distant metastasis
(9). In addition, epidermal growth
factor receptor (EGFR) mutations and anaplastic lymphoma kinase
(ALK) rearrangement are also thought to be associated with the BMLC
(10).
Previous studies that focused on the role of genes
in the occurrence of BMLC provide information on the molecular
mechanisms of BMLC; however, they have remained to be fully
elucidated. Recently developed gene expression profiling arrays may
be used to assess the expression of thousands of genes
simultaneously, providing a tool to comprehensively elucidate the
mechanisms of BMLC (11).
Traditionally, lymph node metastasis was considered
to be closely associated with spread of tumor cells as the origin
of distant metastasis (12).
Recently, a study on human colorectal cancer suggested that for
most patients, metastasis to lymph nodes and distant sites were of
independent origin, which suggested two different lineage
associations between lymphatic and distant metastases in colorectal
cancer (13). Consistent with this
result, the brain was the most frequent site of distant metastasis
in patients with lung cancer without lymph node metastasis
(4).
In the present study, based on the data of a gene
expression chip, the mRNA expression levels were compared between
lung cancer with brain metastasis and lung cancer with lymph node
metastasis. In addition, pathways and functional annotation was
used to determine associations among the differentially expressed
genes (DEGs). Furthermore, protein-protein interaction analysis was
used to determine modules and hub genes. The results of the present
study may enhance the current understanding of the mechanisms of
BMLC.
Materials and methods
Expression profile microarray
The dataset of GSE18549, downloaded from Gene
Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo), is based on the GPL570
platform (Affymetrix Human Genome U133 Plus 2.0 Array). GSE18549 is
a dataset containing three lymph node metastases and six brain
metastases from lung cancer.
Identification of DEGs
The GSE18549 dataset was divided into two groups,
namely the lymph node metastasis group and brain metastasis group.
R (version 3.4.4) was used to identify the DEGs. First, background
correction and normalization of the microarray data was performed
in R and the Limma package (14) was
then used to identify the DEGs. Multiple t-tests were used to
detect the DEGs with the cut-off criteria of log2|fold
change|>1 and adjusted P-value <0.05.
Gene ontology (GO) terms and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis
GO analysis is a common method to annotate genes and
contains three categories: Cellular component (CC), molecular
function (MF) and biological process (BP) (15). KEGG analysis may be used to determine
the pathways of DEGs between two groups (16). GO and KEGG analyses were performed
for the identified DEGs using the Database for Annotation,
Visualization and Integrated Discovery (DAVID, version 6.7;
http://david.abcc.ncifcrf.gov) (17). P<0.05 and the number of involved
genes of ≥2 were selected as cut-off criteria.
Construction of protein-protein
interaction (PPI) network and module analysis
The Search Tool for the Retrieval of Interacting
Genes (STRING; version 10.5) (18)
is an online tool for determining interactions among DEGs. The
screened DEGs were mapped using STRING, and a combined score of
>0.4 was considered to indicate significance. Cytoscape software
(version 3.6.1) was then used to visualize the PPI network
(19), and the Network Analyzer
(version 2.7), a Cytoscape plugin, was used to compute the basic
properties of the PPI network, including average clustering
coefficient distribution, closeness centrality, node degree
distribution and shortest path length distribution. In addition,
module analysis was performed by the plug-in Molecular Complex
Detection (MCODE; version 1.5.1) with the following settings:
Degree cutoff, 2; node score cutoff, 0.2; k-core, 2; and
maximum depth, 100, and the following criteria: MCODE score >4;
number of nodes >5. Finally, the hub genes in the PPI network
were determined, defined as those with a degree of connectivity of
>10.
Results
Identification of DEGs
First, the raw data of GSE18549 were normalized, as
presented in Fig. 1A and B.
Subsequently, the significant DEGs in BMLC compared with lung
cancer with lymph node metastasis were identified. A total of 190
DEGs were identified, which consisted of 129 downregulated genes
and 61 upregulated genes. A volcano plot of the differential
expression analysis is presented in Fig.
1C. In addition, the top 40 DEGs were displayed in a heat map
in Fig. 1D.
GO terms and KEGG pathway enrichment
analysis
To gain insight into the GO categories of DEGs
between the lymph node metastasis group and brain metastasis group,
all DEGs were uploaded to the DAVID database. The results suggested
that downregulated DEGs were mainly enriched in the category BP,
including ‘immune response’, ‘cell activation’ and ‘leukocyte
activation’, while upregulated DEGs were significantly enriched in
‘cell division’, ‘DNA repair’ and ‘viral process’. In the category
CC, the downregulated DEGs were significantly enriched in ‘plasma
membrane’, ‘integral to plasma membrane’ and ‘intrinsic to plasma
membrane’, whereas the upregulated DEGs were significantly enriched
in ‘nucleoplasm’, ‘cytoplasm’ and ‘condensed nuclear chromosome’.
Furthermore, in the category MF, the downregulated genes were
mainly enriched in ‘cytokine binding’, ‘nucleotide receptor
activity’ and ‘purinergic nucleotide receptor activity’, and
conversely, upregulated genes were significantly enriched in
‘poly-(a)-RNA binding’, ‘chromatin binding’ and ‘ATP-dependent
helicase activity’. Further details on the results of the GO
enrichment analysis are provided in Table I.
 | Table I.GO analysis of differentially
expressed genes in the GSE18549 dataset. |
Table I.
GO analysis of differentially
expressed genes in the GSE18549 dataset.
| A, Downregulated
genes |
|---|
|
|---|
| Category/GO
term | P-value | Genes |
|---|
| Biological
Process |
| Immune
response |
2.52×10−20 | ITGAL, TNF, CCL2,
TRGC2, FASLG, CXCL6, TLR6, CLEC10A, CHIT1, FCRL4, CD96, SH2D1A,
LILRA4, POU2F2, MS4A1, LTB, CD27, CD28, CR1, CRTAM, SIT1, GBP5,
CR2, PTGER4, CMKLR1, CCL19, TNFRSF17, CD180, IGSF6, CYBB, CCR6,
IL18BP, KCNJ8, CCR2, LCP2 |
| Cell
activation |
1.38×10−12 | ITGAL, CRTAM, TNF,
CD3G, IKZF1, PLEK, IL21R, TLR6, SLAMF1, GIMAP1, CD48, P2RY12,
VCAM1, P2RX7, MS4A1, IRF4, LTB, CD28, LCP2 |
|
Leukocyte activation |
1.54×10−9 | ITGAL, CRTAM, CD3G,
IKZF1, IL21R, TLR6, SLAMF1, GIMAP1, CD48, VCAM1, P2RX7, MS4A1,
IRF4, LCP2, CD28 |
| Defense
response |
8.83×10−9 | ITGAL, CR1, TNF,
CCL2, CR2, CYSLTR1, CCL19, CXCL6, TLR6, CD180, CD48, CD84, SH2D1A,
CYBB, P2RX7, CCR6, TRAC, KCNJ8, CCR2, PLA2G2D, AOC3 |
|
Lymphocyte activation |
1.56×10−8 | CD48, VCAM1, ITGAL,
P2RX7, CRTAM, CD3G, IKZF1, IL21R, MS4A1, IRF4, SLAMF1, CD28,
GIMAP1 |
| Cellular
Component |
| Plasma
membrane |
4.92×10−11 | TRGC2, FASLG, TLR6,
DDR2, FCRL4, FCRL3, CD48, ART4, CD96, TRAC, SLC2A3, CXCR6, MS4A1,
CSF2RB, RECK, CRTAM, CD3G, SIT1, GBP5, PTGER4, GPR18, CMKLR1,
TNFRSF17, THY1, PRKCB, IGSF6, CD84, ARHGAP31, CCR6, CD34, CCR2,
PTGDR, ADAM12, AOC3, ITGAL, CD244, TNF, CYSLTR1, CD247, BRSK1,
CLEC10A, CSMD2, VCAM1, CD27, CD28, IL2RB, CR1, CR2, PLEK, SLAMF1,
CD180, P2RY12, P2RY13, P2RY10, P2RX7, CYBB, LYVE1, KCNJ8,
GFRA1 |
|
Integral to plasma
membrane |
1.14×10−8 | ITGAL, TNF,
CYSLTR1, TRGC2, FASLG, TLR6, DDR2, CD48, CD96, TRAC, CXCR6, MS4A1,
CSF2RB, CD27, CD28, IL2RB, CR1, SIT1, CD3G, CMKLR1, THY1, IGSF6,
CD84, CYBB, P2RX7, LYVE1, CCR6, KCNJ8, CCR2 |
|
Intrinsic to plasma
membrane |
1.86×10−8 | ITGAL, TNF,
CYSLTR1, TRGC2, FASLG, TLR6, DDR2, CD48, CD96, TRAC, CXCR6, MS4A1,
CSF2RB, CD27, CD28, IL2RB, CR1, SIT1, CD3G, CMKLR1, THY1, IGSF6,
CD84, CYBB, P2RX7, LYVE1, CCR6, KCNJ8, CCR2 |
| Plasma
membrane part |
2.11×10−7 | ITGAL, CD244, TNF,
CYSLTR1, CD247, TRGC2, FASLG, BRSK1, TLR6, DDR2, CD48, VCAM1, CD96,
TRAC, CXCR6, MS4A1, CSF2RB, CD27, CD28, IL2RB, CR1, SIT1, CD3G,
GBP5, PLEK, CMKLR1, SLAMF1, THY1, CD84, IGSF6, ARHGAP31, CYBB,
P2RX7, LYVE1, CCR6, CD34, KCNJ8, CCR2 |
|
Intrinsic to membrane |
3.72×10−7 | IL21R, TRGC2,
FASLG, TLR6, DDR2, FCRL4, FCRL3, CD48, ART4, CD96, TRAC, SLC2A3,
LILRA4, CXCR6, MS4A1, CSF2RB, MCOLN2, LTB, RECK, CRTAM, CD3G, SIT1,
PTGER4, GPR18, CMKLR1, TNFRSF17, PKHD1L1, THY1, MCTP1, IGSF6, CD84,
CCR6, CD34, PTGDR, CCR2, ADAM12, AOC3, ITGAL, CD244, TNF, CYSLTR1,
CD247, KMO, CLEC10A, CSMD2, VCAM1, FMO2, CD27, CD28, IL2RB, CR1,
CR2, SLAMF1, CD180, GIMAP1, P2RY12, P2RY13, CYBB, P2RY10, P2RX7,
LYVE1, RNF150, KCNJ8, ST8SIA4, GFRA1 |
| Molecular
Function |
|
Cytokine binding |
7.66×10−7 | IL2RB, CCR6,
IL18BP, CMKLR1, CCR2, IL21R, CXCR6, CSF2RB, GFRA1 |
|
Nucleotide receptor
activity |
1.03×10−4 | P2RY12, P2RY13,
P2RX7, P2RY10, GPR18 |
|
Purinergic nucleotide receptor
activity |
1.03×10−4 | P2RY12, P2RY13,
P2RX7, P2RY10, GPR18 |
|
Cytokine activity |
3.77×10−4 | TNF, CCL2, CCL19,
FASLG, CXCL6, IL33, GREM1, LTB |
|
Chemokine receptor
activity |
6.42×10−4 | CCR6, CMKLR1, CCR2,
CXCR6 |
|
| B, Upregulated
genes |
|
| Category/GO
term | P-value | Genes |
|
| Biological
process |
| Viral
process |
1.98×10−4 | DDX11, BTRC,
RBM15B, TSC2, BRD4, TPR, RCC1 |
| RNA
processing |
2.68×10−3 | DHX9, DDX54,
HNRNPDL, DHX30 |
| Cell
division |
1.76×10−2 | ANAPC1, BRCC3, TPR,
RCC1, SMC1A |
|
Positive regulation of
chromatin binding |
2.54×10−2 | KDM1A, DDX11 |
| DNA
repair |
2.95×10−2 | BRCC3, DDX11, TDP1,
SMC1A |
| Cellular
Component |
|
Nucleoplasm |
2.85×10−5 | ANAPC1, DHX9,
BRCC3, ZMYM3, TONSL, BTRC, RBM15B, SNIP1, HNRNPDL, RCC1, BMS1,
GTSE1, GPS2, KDM1A, DDX11, GTF2IRD1, BRD4, TPR, PPP4C, SMC1A,
HDAC8 |
|
Cytoplasm |
7.46×10−5 | SHROOM3, BTRC,
SNIP1, RCC1, TIPRL, ZIC2, DDX11, DLG3, BRD4, TPR, DHX30, PPP4C,
SAMD4B, DHX9, BRCC3, ZMYM3, PIK3C2A, TONSL, AMBRA1, HNRNPDL, MID1,
GCN1, GTF2IRD1, TDP1, TSC2, USP47, SPTBN1, SMC1A, HDAC8 |
|
Condensed nuclear
chromosome |
2.77×10−3 | BRD4, RCC1,
SMC1A |
|
Nucleus |
2.86×10−3 | BTRC, SNIP1, RCC1,
BMS1, ZIC2, KDM1A, DDX11, BRD4, DHX30, PPP4C, TPR, SAMD4B, DHX9,
PGAP2, BRCC3, PIK3C2A, CIZ1, HNRNPDL, GTF2IRD1, TDP1, TSC2, USP47,
SPTBN1, DDX54, SMC1A, HDAC8 |
|
Cytoplasmic microtubule |
8.97×10−3 | SPACA9, MID1,
GTSE1 |
| Molecular
Function |
| Poly(A)
RNA binding |
3.79×10−4 | DHX9, RBM15B,
SNIP1, SPTBN1, DDX54, HNRNPDL, TPR, DHX30, SMC1A, BMS1, GCN1,
SAMD4B |
|
Chromatin binding |
9.98×10−4 | KDM1A, DDX11, BRD4,
TPR, RCC1, DHX30, SMC1A |
|
ATP-dependent helicase
activity |
3.32×10−3 | DHX9, DDX11,
DHX30 |
| Protein
binding |
7.47×10−3 | REPS1, BTRC,
RBM15B, SNIP1, RCC1, TIPRL, GTSE1, KDM1A, DDX11, ILVBL, DLG3,
PEX13, BRD4, TPR, DHX30, PPP4C, DHX9, PGAP2, CHDH, BRCC3, SPACA9,
TONSL, CIZ1, AMBRA1, HNRNPDL, MID1, GPS2, ARHGAP32, TDP1, TSC2,
USP47, SPTBN1, SMC1A, HDAC8, GATC, IQCE |
| Nucleic
acid binding |
7.89×10−3 | DHX9, DDX11, CIZ1,
RBM15B, GPATCH1, DDX54, HNRNPDL, DHX30, ZIC2 |
As presented in Table
II, the KEGG pathway enrichment analysis of DEGs indicated that
the downregulated DEGs were mainly enriched in ‘cytokine-cytokine
receptor interaction’, ‘natural killer cell-mediated cytotoxicity’,
‘hematopoietic cell lineage’, ‘chemokine signaling pathway’ and
‘t-cell receptor signaling pathway’, and upregulated DEGs were
mainly enriched in ‘oocyte meiosis’.
 | Table II.KEGG pathway analysis of
differentially expressed genes in the GSE18549 dataset. |
Table II.
KEGG pathway analysis of
differentially expressed genes in the GSE18549 dataset.
| KEGG pathway | P-value | Genes |
|---|
| Downregulated
genes |
|
|
|
Cytokine-cytokine receptor
interaction |
3.90×10−7 | IL2RB, CCL2, TNF,
IL21R, FASLG, TNFRSF17, CCL19, CXCL6, CCR6, CXCR6, CCR2, CSF2RB,
LTB, CD27 |
| Natural
killer cell-mediated cytotoxicity |
2.36×10−5 | CD48, ITGAL, CD244,
SH2D1A, TNF, CD247, FASLG, PRKCB, LCP2 |
|
Hematopoietic cell
lineage |
1.08×10−3 | CR1, TNF, CR2,
CD3G, CD34, MS4A1 |
|
Chemokine signaling
pathway |
6.98×10−3 | CCR6, CCL2, CCR2,
CXCR6, CCL19, CXCL6, PRKCB |
| T-cell
receptor signaling pathway |
1.70×10−2 | TNF, CD3G, CD247,
CD28, LCP2 |
| Upregulated
genes |
|
|
| Oocyte
meiosis |
3.89×10−2 | ANAPC1, BTRC,
SMC1A |
Construction of PPI network and module
analysis
The STRING database was used to predict the
interaction between the 190 DEGs, and the PPI was visualized using
Cytoscape software. Initially, basic properties of the network were
computed. The network contained 90 nodes and 205 interaction edges,
where the average degree of connectivity (i.e., average number of
neighbors) was 4.56 (Fig. 2A).
Subsequently, the Network Analyzer tool was used to compute the
basic properties of the PPI network, including the degree
distribution, clustering coefficient, average shortest path and the
closeness centrality of the PPI network (Fig. 3). The analysis indicated that the
degree distribution of PPI network nodes followed the power-law of
network, and had small-world network characteristics, including a
short average shortest path (20,21). In
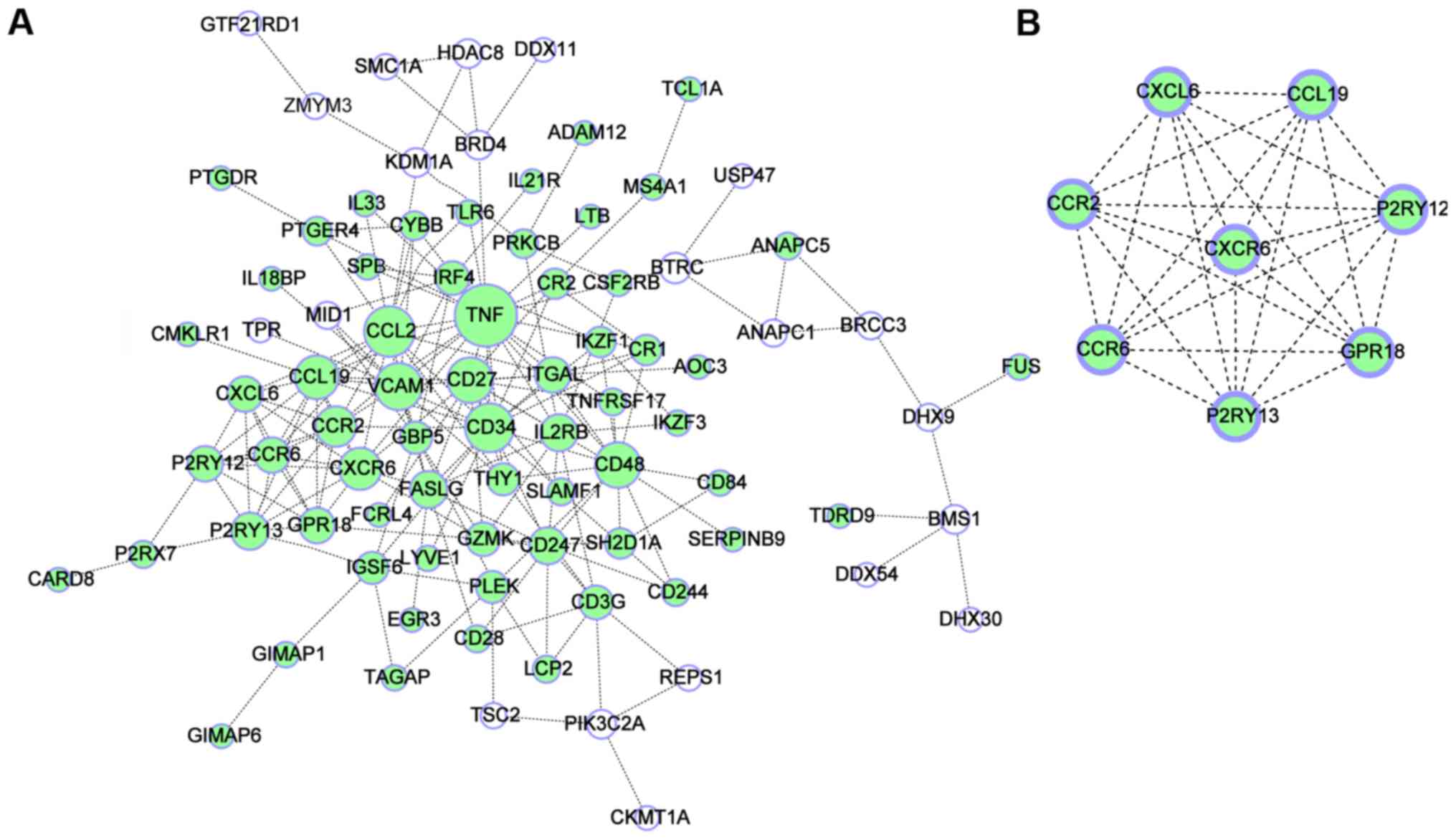
addition, one significant module consisting of 8 nodes and 28 edges
was obtained from the PPI network of DEGs using MCODE (Fig. 2B). As provided in Table III, enrichment analysis suggested
that the genes in this significant module were mainly associated
with functional terms in the category BP, including
‘G-protein-coupled receptor protein signaling pathway’, ‘cell
surface receptor-linked signal transduction’, ‘taxis’, ‘chemotaxis’
and ‘locomotory behavior’. In the category CC, the genes in this
significant module were significantly enriched in ‘plasma
membrane’, and in the category MF, the genes were mainly enriched
in ‘C-C chemokine receptor activity’, ‘C-C chemokine binding’,
‘chemokine receptor activity’, ‘chemokine binding’ and ‘nucleotide
receptor activity, G-protein coupled’. Furthermore, results from
KEGG analysis demonstrated that the genes in this significant
module were associated with ‘chemokine signaling pathway’ and
‘cytokine-cytokine receptor interaction’ (Table IV). Finally, the hub genes with a
degree of connectivity of >10 were identified, including tumor
necrosis factor (TNF), C-C motif chemokine ligand 2 (CCL2), CD34,
vascular cell adhesion molecule 1 (VCAM1), CD48, CD27, C-C motif
chemokine ligand 19 (CCL19), C-X-C motif chemokine receptor 6
(CXCR6) and C-C motif chemokine receptor 2 (CCR2).
 | Table III.GO analysis of genes in the
significant module. |
Table III.
GO analysis of genes in the
significant module.
| Category/GO
term | P-value | Genes |
|---|
| Biological
Process |
|
|
|
G-protein coupled receptor
protein signaling pathway |
2.67×10−8 | P2RY12, P2RY13,
CCR6, GPR18, CCR2, CXCR6, CCL19, CXCL6 |
| Cell
surface receptor linked signal transduction |
9.06×10−7 | P2RY12, P2RY13,
CCR6, GPR18, CCR2, CXCR6, CCL19, CXCL6 |
|
Taxis |
5.49×10−5 | CCR6, CCR2, CCL19,
CXCL6 |
|
Chemotaxis |
5.49×10−5 | CCR6, CCR2, CCL19,
CXCL6 |
|
Locomotory behavior |
2.71×10−4 | CCR6, CCR2, CCL19,
CXCL6 |
| Cellular
Component |
|
|
| Plasma
membrane |
2.69×10−2 | P2RY12, P2RY13,
CCR6, GPR18, CCR2, CXCR6 |
| Molecular
Function |
|
|
| C-C
chemokine receptor activity |
2.98×10−5 | CCR6, CCR2,
CXCR6 |
| C-C
chemokine binding |
2.98×10−5 | CCR6, CCR2,
CXCR6 |
|
Chemokine receptor
activity |
7.43×10−5 | CCR6, CCR2,
CXCR6 |
|
Chemokine binding |
8.69×10−5 | CCR6, CCR2,
CXCR6 |
|
Nucleotide receptor activity,
G-protein coupled |
1.00×10−4 | P2RY12, P2RY13,
GPR18 |
 | Table IV.KEGG pathway analysis of genes in the
significant module. |
Table IV.
KEGG pathway analysis of genes in the
significant module.
| KEGG pathway | Count | P-Value | Genes |
|---|
| hsa04062:Chemokine
signaling pathway | 5 |
8.61×10−6 | CCR6, CCR2, CXCR6,
CCL19, CXCL6 |
|
hsa04060:Cytokine-cytokine receptor
interaction | 5 |
3.31×10−5 | CCR6, CCR2, CXCR6,
CCL19, CXCL6 |
Discussion
Brain metastasis is a frequent complication in
patients suffering from advanced lung cancer (3). It has been estimated that 50% of the
patients diagnosed with lung cancer will develop metastatic brain
lesions, which results in a dismal prognosis (22). Recently, a series of biomarkers have
been identified to be associated with the development of brain
metastasis, such as integrins, cell adhesion molecules, cadherins,
VEGF, chemokines, matrix metalloproteinase, EGFR mutations and ALK
translocations (23). However, the
biology of brain metastasis is still poorly understood, as a
result, the specific and effective strategies used to control or
treat for brain metastasis are currently unavailable.
In the present study, a microarray dataset was
analyzed to screen the DEGs between lung cancers with lymph node
metastasis and brain metastasis. A total of 190 DEGs were obtained,
which included 129 downregulated genes and 61 upregulated genes. To
further investigate the functions of the DEGs, GO functional
annotation and KEGG pathway enrichment analysis were used based on
the DAVID database. The GO analysis demonstrated that downregulated
DEGs were mainly associated with ‘immune response’, ‘cell
activation’ and ‘leukocyte activation’, which was in accordance
with previous studies. For instance, secondary brain cancers
frequently exhibit high expression of programmed cell death 1
(PD-1) ligand 1, which may be inhibited by novel treatments that
activate the immune system (24,25).
Furthermore, degraded white-matter tract integrity in the areas
with high T-cell densities are thought to provide active
microenvironments for brain metastases (26); in addition, immune checkpoint
inhibitors targeting PD-1 and cytotoxic T lymphocyte-associated
protein 4 are becoming a frontline therapy in melanoma, which also
suggests that the immune response is involved in the development of
brain metastases (27). On the
contrary, the upregulated DEGs were involved in ‘DNA repair’ and
‘viral process’, which were also consistent with previous data.
Overexpression of DNA repair genes BRCA1-associated RING domain 1
and RAD51 recombinase frequently occur in brain metastases from
breast cancer (28). The DNA-damage
response pathway was also determined to be involved in
leptomeningeal metastasis of non-small cell lung cancer (29). Furthermore, high rates of DNA repair
mutations were identified in brain metastases from prostate cancer
(30). In addition, the prevalence
of human cytomegalovirus proteins and nucleic acids is high in
primary and metastatic tumors, indicating that this virus may drive
the development of metastatic brain tumors (31).
The results of the KEGG analysis indicated that the
downregulated DEGs were mainly enriched in ‘chemokine signaling
pathway’. Previous studies demonstrated that inflammatory
chemokines and their receptors regulate tumor cell migration and
participate in tumor growth, metastasis, angiogenesis and invasion
through the interaction between mesenchymal cells and neoplastic
cells (32,33). Of note, the upregulated DEGs were
mainly associated with ‘Oocyte meiosis’. However, there is no
evidence to support that this pathway was associated with BMLC or
brain metastasis from other types of tumor.
Furthermore, the protein interactions among the
screened DEGs were predicted. In the PPI network, 9 hub genes with
the highest degree of connectivity were selected, which included
TNF, CCL2, CD34, VCAM1, CD48, CD27, CCL19, CXCR6 and CCR2. TNF
encodes a multifunctional proinflammatory cytokine that belongs to
the TNF superfamily. TNF-α has an important role in the adhesion of
non-small cell lung cancer cells to brain endothelium mediated by
CD62E (34). A previous study
suggested that microRNA (miR)-509 has a critical role in brain
metastasis of breast cancer by modulating the Ras homolog family
member C/TNF-α network (35). Among
the above genes, half of the hub genes, including CCL2, CCL19, CCR2
and CXCR6, were involved in the chemokine signaling pathway. CCL2,
a small cytokine that belongs to the CC chemokine family, is
anchored in the plasma membrane of endothelial cells by
glycosaminoglycan side chains of proteoglycans; CCL2 exhibits a
chemotactic activity for monocytes and basophils. miR-19a contained
in astrocyte-derived exosomes reversibly downregulates phosphatase
and tension homolog in tumor cells, resulting in secretion of CCL2,
which is able to recruit brain metastasis-promoting myeloid cells
(36). Conceivably, CCR2, as one of
the receptors for CCL2, mediated the roles of CCL2 in breast cancer
metastasis (37). Similar to CCL2,
CCL19 is another member of the CC motif chemokine superfamily and
functions as a tumor suppressor in lung cancer; CCL19-expressing
fibroblastic stromal cells were indicated to inhibit lung carcinoma
growth by promoting local anti-tumor T-cell responses (38). CCL19 exhibited anti-tumor effects by
promoting interferon-γ-dependent responses in a lung cancer model
(39); however, the roles of CCL19
in BMLC have remained to be determined. CXCR6, belonging to family
A of the G protein-coupled receptor superfamily, was identified to
be associated with B-lineage maturation and antigen-driven B-cell
differentiation. As a binding partner, CXCR6 mediates
CXCL16-associated signaling. Recently, several studies indicated
that CXCL16/CXCR6 signaling drives metastasis of different cancer
types by promoting a protumor inflammatory environment or
attracting cancer cells (40,41).
Another group of hub genes, CD, are a defined subset
of cellular surface receptors, which include CD48, CD27 and CD34.
CD48, also known as B-lymphocyte activation marker or signaling
lymphocytic activation molecule 2, encodes a member of the CD2
subfamily of immunoglobulin-like receptors. Activation of CD48 by
injecting anti-CD48 monoclonal antibodies resulted in the
inhibition of tumor metastasis of melanoma (42). CD27, a member of the TNF-receptor
superfamily, is required for generation and long-term maintenance
of T-cell immunity; Pagès et al (43) discovered that the expression of CD27
was associated with early metastasis in colorectal cancer. CD34, a
single-pass membrane protein, is highly glycosylated and
phosphorylated by protein kinase C and expressed on human
hematopoietic progenitor cells and the small-vessel endothelium of
a variety of tissue types (44);
Upregulation of CD34-positive lymphatic/vascular endothelial
progenitor cells are associated with metastasis of ovarian cancer
(45).
VCAM1 is an Ig-like cell adhesion molecule expressed
by cytokine-activated endothelium (46). One study based on RNA sequencing
analysis confirmed that the expression of VCAM1 was upregulated in
brain tissue harboring metastases, which suggested that this gene
may contribute to the establishment of brain metastases from breast
cancer or melanoma (47); however,
the functions VCAM1 in BMLC have so far remained elusive.
In conclusion, although the present study had
certain limitations, including the small number of cases and the
lack of validation in clinical samples, the present analysis
identified distinct key genes and pathways closely associated with
BMLC, which may contribute to the current knowledge of the complex
mechanisms of BMLC. Of note, the present results warrant
confirmation by further investigations.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural
Science Foundation of China (grant no. 81501959), The Natural
Science Foundation of Liaoning Province (grant no. 20180530080),
The Technological Project of Liaoning Province (grant no.
20170540392), The Innovation Foundation for the University Students
(grant no. 201510160000013) and The Biological Anthropology
Innovation Team Project of JZMU (grant no. JYLJ201702).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
FR and PW designed the experiments, and XZ and NW
collected and analyzed the data. TC and HG wrote the manuscript. YL
and HC downloaded the gene expression profile from the GEO. All
authors critically reviewed the content and approved the final
version for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chuang CH, Greenside PG, Rogers ZN, Brady
JJ, Yang D, Ma RK, Caswell DR, Chiou SH, Winters AF, Grüner BM, et
al: Molecular definition of a metastatic lung cancer state reveals
a targetable CD109-Janus kinase-Stat axis. Nat Med. 23:291–300.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamura T, Kurishima K, Nakazawa K,
Kagohashi K, Ishikawa H and Hizawa N: Specific organ metastases and
survival in metastatic non-small-cell lung cancer. Mol Clin Oncol.
3:217–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yano T, Yokoyama H, Inoue T, Asoh H,
Tayama K, Takai E and Ichinose Y: The first site of recurrence
after complete resection in non-small-cell carcinoma of the lung.
Comparison between pN0 disease and pN2 disease. J Thorac Cardiovasc
Surg. 108:680–683. 1994.PubMed/NCBI
|
|
5
|
Chen W, Zhang S and Zou X: Estimation and
projection of lung cancer incidence and mortality in China.
Zhongguo Fei Ai Za Zhi. 13:488–493. 2010.(In Chinese). PubMed/NCBI
|
|
6
|
Sandler A, Hirsh V, Reck M, von Pawel J,
Akerley W and Johnson DH: An evidence-based review of the incidence
of CNS bleeding with anti-VEGF therapy in non-small cell lung
cancer patients with brain metastases. Lung Cancer. 78:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linardou H, Kalogeras KT, Kronenwett R,
Kouvatseas G, Wirtz RM, Zagouri F, Gogas H, Christodoulou C,
Koutras AK, Samantas E, et al: The prognostic and predictive value
of mRNA expression of vascular endothelial growth factor family
members in breast cancer: A study in primary tumors of high-risk
early breast cancer patients participating in a randomized Hellenic
Cooperative Oncology Group trial. Breast Cancer Res. 14:R1452012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen G, Liu XY, Wang Z and Liu FY:
Vascular endothelial growth factor C: The predicator of early
recurrence in patients with N2 non-small-cell lung cancer. Eur J
Cardiothorac Surg. 37:546–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen G, Wang Z, Liu XY and Liu FY:
High-level CXCR4 expression correlates with brain-specific
metastasis of non-small cell lung cancer. World J Surg. 35:56–61.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han CH and Brastianos PK: Genetic
characterization of brain metastases in the era of targeted
therapy. Front Oncol. 7:2302017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarca AL, Romero R and Draghici S:
Analysis of microarray experiments of gene expression profiling. Am
J Obstet Gynecol. 195:373–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong SY and Hynes RO: Lymphatic or
hematogenous dissemination: How does a metastatic tumor cell
decide? Cell Cycle. 5:812–817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naxerova K, Reiter JG, Brachtel E, Lennerz
JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak
MA, et al: Origins of lymphatic and distant metastases in human
colorectal cancer. Science. 357:55–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Law CW, Alhamdoosh M, Su S, Smyth GK and
Ritchie ME: RNA-seq analysis is easy as 1–2–3 with limma, Glimma
and edgeR. F1000Res. 5:14082016. View Article : Google Scholar
|
|
15
|
Gene Ontology Consortium: The gene
ontology (GO) project in 2006. Nucleic Acids Res. 34:D322–D326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strogatz SH: Exploring complex networks.
Nature. 410:268–276. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori T, Ikeda DD, Fukushima T, Takenoshita
S and Kochi H: NIRF constitutes a nodal point in the cell cycle
network and is a candidate tumor suppressor. Cell Cycle.
10:3284–3299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Preusser M, Winkler F, Valiente M,
Manegold C, Moyal E, Widhalm G, Tonn JC and Zielinski C: Recent
advances in the biology and treatment of brain metastases of
non-small cell lung cancer: Summary of a multidisciplinary
roundtable discussion. ESMO Open. 3:e0002622018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitsett TG, Inge LJ, Dhruv HD, Cheung PY,
Weiss GJ, Bremner RM, Winkles JA and Tran NL: Molecular
determinants of lung cancer metastasis to the central nervous
system. Transl Lung Cancer Res. 2:273–283. 2013.PubMed/NCBI
|
|
24
|
Duchnowska R, Pęksa R, Radecka B, Mandat
T, Trojanowski T, Jarosz B, Czartoryska-Arłukowicz B, Olszewski WP,
Och W, Kalinka-Warzocha E, Kozłowski W, et al: Immune response in
breast cancer brain metastases and their microenvironment: The role
of the PD-1/PD-L axis. Breast Cancer Res. 18:432016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berghoff AS, Venur VA, Preusser M and
Ahluwalia MS: Immune checkpoint inhibitors in brain metastases:
From biology to treatment. Am Soc Clin Oncol Educ Book.
35:e116–e122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zakaria R, Platt-Higgins A, Rathi N, Radon
M, Das S, Das K, Bhojak M, Brodbelt A, Chavredakis E, Jenkinson MD
and Rudland PS: T-cell densities in brain metastases are associated
with patient survival times and diffusion tensor MRI changes.
Cancer Res. 78:610–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taggart D, Andreou T, Scott KJ, Williams
J, Rippaus N, Brownlie RJ, Ilett EJ, Salmond RJ, Melcher A and
Lorger M: Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain
metastases depends on extracranial disease and augmentation of
CD8+ T cell trafficking. Proc Natl Acad Sci USA.
115:E1540–E1549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woditschka S, Evans L, Duchnowska R, Reed
LT, Palmier D, Qian Y, Badve S, Sledge G Jr, Gril B, Aladjem MI, et
al: DNA double-strand break repair genes and oxidative damage in
brain metastasis of breast cancer. J Natl Cancer Inst.
106:diu1452014. View Article : Google Scholar
|
|
29
|
Fan Y, Zhu X, Xu Y, Lu X, Xu Y, Wang M, Xu
H, Ding J, Ye X, Fang L, et al: Cell-cycle and DNA-damage response
pathway is involved in leptomeningeal metastasis of non-small cell
lung cancer. Clin Cancer Res. 24:209–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pritchard CC, Mateo J, Walsh MF, De Sarkar
N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R,
et al: Inherited DNA-repair gene mutations in men with metastatic
prostate cancer. N Engl J Med. 375:443–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taher C, Frisk G, Fuentes S, Religa P,
Costa H, Assinger A, Vetvik KK, Bukholm IR, Yaiw KC, Smedby KE, et
al: High prevalence of human cytomegalovirus in brain metastases of
patients with primary breast and colorectal cancers. Transl Oncol.
7:732–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng ZH, Shi YX, Yuan M, Xiong D, Zheng
JH and Zhang ZY: Chemokines and their receptors in lung cancer
progression and metastasis. J Zhejiang Univ Sci B. 17:342–351.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reckamp KL, Strieter RM and Figlin RA:
Chemokines as therapeutic targets in renal cell carcinoma. Expert
Rev Anticancer Ther. 8:887–893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jassam SA, Maherally Z, Smith JR, Ashkg K,
Roncaroli F, Fillmore HL and Pilkington GJ: TNF-α enhancement of
CD62E mediates adhesion of non-small cell lung cancer cells to
brain endothelium via CD15 in lung-brain metastasis. Neuro Oncol.
18:679–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing F, Sharma S, Liu Y, Mo YY, Wu K,
Zhang YY, Pochampally R, Martinez LA, Lo HW and Watabe K: miR-509
suppresses brain metastasis of breast cancer cells by modulating
RhoC and TNF-α. Oncogene. 34:4890–4900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang
Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al:
Microenvironment-induced PTEN loss by exosomal microRNA primes
brain metastasis outgrowth. Nature. 527:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu X and Kang Y: Chemokine (C-C motif)
ligand 2 engages CCR2+ stromal cells of monocytic origin to promote
breast cancer metastasis to lung and bone. J Biol Chem.
284:29087–29096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng HW, Onder L, Cupovic J, Boesch M,
Novkovic M, Pikor N, Tarantino I, Rodriguez R, Schneider T, Jochum
W, et al: CCL19-producing fibroblastic stromal cells restrain lung
carcinoma growth by promoting local antitumor T-cell responses. J
Allergy Clin Immunol. 142:1257–1271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hillinger S, Yang SC, Zhu L, Huang M,
Duckett R, Atianzar K, Batra RK, Strieter RM, Dubinett SM and
Sharma S: EBV-induced molecule 1 ligand chemokine (ELC/CCL19)
promotes IFN-gamma-dependent antitumor responses in a lung cancer
model. J Immunol. 171:6457–6465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chung B, Esmaeili AA, Gopalakrishna-Pillai
S, Murad JP, Andersen ES, Kumar Reddy N, Srinivasan G, Armstrong B,
Chu C, Kim Y, et al: Human brain metastatic stroma attracts breast
cancer cells via chemokines CXCL16 and CXCL12. NPJ Breast Cancer.
3:62017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH,
Sun J, Yi Y, Sun JY, Shi GM, Ding ZB, et al: CXCR6 upregulation
contributes to a proinflammatory tumor microenvironment that drives
metastasis and poor patient outcomes in hepatocellular carcinoma.
Cancer Res. 72:3546–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Johnson LA, Vaidya SV, Goldfarb RH and
Mathew PA: 2B4 (CD244)-mediated activation of NK cells reduces
metastases of B16F10 melanoma in mice. Anticancer Res.
23:3651–3655. 2003.PubMed/NCBI
|
|
43
|
Pagès F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simmons DL, Satterthwaite AB, Tenen DG and
Seed B: Molecular cloning of a cDNA encoding CD34, a sialomucin of
human hematopoietic stem cells. J Immunol. 148:267–271.
1992.PubMed/NCBI
|
|
45
|
Qiu H, Cao L, Wang D, Xu H and Liang Z:
High levels of circulating CD34+/VEGFR3+ lymphatic/vascular
endothelial progenitor cells is correlated with lymph node
metastasis in patients with epithelial ovarian cancer. J Obstet
Gynaecol Res. 39:1268–1275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lawson C, Ainsworth M, Yacoub M and Rose
M: Ligation of ICAM-1 on endothelial cells leads to expression of
VCAM-1 via a nuclear factor-kappaB-independent mechanism. J
Immunol. 162:2990–2996. 1999.PubMed/NCBI
|
|
47
|
Sato R, Nakano T, Hosonaga M, Sampetrean
O, Harigai R, Sasaki T, Koya I, Okano H, Kudoh J, Saya H and Arima
Y: RNA sequencing analysis reveals interactions between breast
cancer or melanoma cells and the tissue microenvironment during
brain metastasis. Biomed Res Int. 2017:80329102017. View Article : Google Scholar : PubMed/NCBI
|