Introduction
Retinal vein occlusion (RVO) is a type of ophthalmic
vascular lesion characterized by tortuous expansion and hemorrhage
along a retinal vein that may be visualized on funduscopic exams.
The prevalence of these lesions determined by epidemiological
studies varies between 0.1 to 0.5% in middle-aged and elderly
individuals. It is the second most common retinal vascular disease
after diabetic retinopathy (1,2). The
major pathogenesis of RVO is venous thrombosis (3). According to different occlusion sites,
Hayreh (4) proposed that RVO may be
divided into three categories: Central retinal vein occlusion
(CRVO), top or bottom hemi-(H)CRVO and branch retinal vein
occlusion. Depending on the presence of ocular fundus ischemia,
CRVO and HCRVO may be sub-divided into ischemic and non-ischemic
groups. If left untreated, RVO may lead to blindness. RVO is
affected by systemic diseases and is more likely to occur in
individuals with hypertension, hyperlipidemia (5), diabetes (6), coagulation disorders (7), anti-phospholipid antibody syndrome
(8) and migraine (9). The control of systemic risk factors may
effectively reduce the occurrence of RVO. At our center, fundus
fluorescein angiography (FFA) is commonly used to diagnose RVO. The
degree of retinal edema is observed by optical coherence tomography
(OCT), but elucidation of the underlying pathophysiology is rarely
achieved through neuroimaging. A previous study reported
significantly decreased functional connectivity in the occipital
visual cortex of early blind patients (10). If untreated, patients with RVO may
eventually become blind. Therefore, the investigation of
RVO-associated brain processes using modern imaging techniques may
lead to a better understanding of the underlying visual
mechanisms.
Recently, increasing attention has been paid to
resting-state functional magnetic resonance imaging (RS-fMRI) for
the study of ongoing neuronal processes during rest (11). As a reliable method to measure the
correlation amplitude, ALFF has proven to be a valuable technique
for investigating the intensity of spontaneous neural activity
(12), and it has been applied in
studies of neurophysiological activity and diseases occurring in
different brain regions. In 2007, Zang et al (13) first proposed the ALFF index. By
calculating the low-frequency amplitude of each individual element,
this method directly represents the intensity of blood oxygen level
dependent (BOLD), and reflects the level of spontaneous activity of
each brain area in the resting state from the perspective of
energy. The reduction of ALFF represents the decrease of the BOLD
signal in this brain region. To date, ALFF has been applied in
ophthalmology research on conditions including glaucoma (14,15),
amblyopia (16), strabismus
(17), high myopia (18), optic neuritis (19), eye trauma (20), blindness (21), retinal detachment (22), diabetic retinal diseases (23) and acute eye pain (24). To the best of our knowledge, the
present study is the first to explore ALFF in different brain
regions of patients with RVO.
Materials and methods
Participants
A total of 24 patients with RVO (12 males and 12
females; age range, 27–85 years; average age, 54 years) were
recruited from the First Affiliated Hospital of Nanchang University
(Nanchang, China) with the following inclusion criteria: i) Signs
of RVO on ophthalmoscopy; ii) indication of macular edema on OCT;
iii) FFA indicating occlusion of a retinal vein (Fig. 1); iv) no history of nervous system
diseases (e.g. cerebral hemorrhage, cerebral infarction or brain
atrophy); v) no history of psychiatric illness, myocardial
infarction and/or cerebral infarction disease; and vi) ability to
undergo MRI examination. The exclusion criteria for the RVO group
were as follows: i) History of ophthalmic surgery (intraocular or
extraocular surgery) within three months; ii) history of other eye
diseases (glaucoma, cataracts, infections, inflammation, congenital
pathology and hereditary eye diseases); iii) systemic diseases that
affect the appearance of the eye; iv) presence of a cardiac
pacemaker or other implanted metal implants that may represent a
counterindication for MRI scans; v) medical history of risk
factors, including hypertension, hyperlipidemia, diabetes,
coagulation disorders, anti-phospholipid antibody syndrome and
migraine headaches. Anti-phospholipid antibodies may have a role in
the development of atherosclerosis. Induction of a prothrombotic
vascular endothelial microenvironment may be involved in the
pathogenesis of RVO (25). The
aforementioned additional risk factors were excluded according to
data obtained from previous studies (8,9).
Furthermore, 24 healthy controls (HCs; 12 males and
12 females) of comparable age and educational status to those of
the RVO subjects were enrolled. The inclusion criteria were as
follows: i) No ocular disease history; ii) no history of nervous
system diseases (e.g. cerebral hemorrhage, cerebral infarction or
brain atrophy); iii) no history of psychiatric illness, myocardial
infarction and/or cerebral infarction disease; and iv) ability to
undergo MRI examination.
MRI parameters
The MRI scanning was performed using a Trio 3-Tesla
MRI scanner (Siemens AG). For these MRI examinations, each of the
subjects was instructed to relax, keep their eyes closed and
continue to breathe steadily until the end of the scan. The
functional data were obtained using a 3D metamorphic gradient
recalled-echo pulse sequence. First, 176 structural images with the
parameters set as follows: Acquisition matrix, 256×256; field of
view, 250×250 mm; echo time, 2.26 msec; repetition time, 1,900
msec; thickness, 1.0 mm; gap, 0.5 mm; flip angle, 9°. Subsequently,
240 functional images were obtained with the following settings:
Acquisition matrix, 64×64; field of view, 220×220 mm; thickness,
4.0 mm; gap, 1.2 mm; repetition time, 2,000 msec; echo time, 30
msec; flip angle, 90°, 29 axial.
RS-fMRI data analysis
Functional data from different brain regions were
differentiated by using MRIcro software (REST; http://www.restfmri.net), and unqualified data were
eliminated. Qualified data were processed using software from
rs-fMRI (DPARSFA 2.3, http://rfmri.org/DPARSF), including digital image form
conversion, slice time, head action adjustment, spatial
standardization, and with a smooth Gaussian core 6×6×6
mm3 widescreen at half-peak. If during the scan, the
patient's head moved by >1.5 mm along the x-, y- or z-axis and
the angle range was >1.5 mm, the data were deemed ineligible. A
previous study indicated that the higher-order model is more
effective in eliminating head movement errors (26). Using linear regression to help
eliminate uncontrollable variables, signals from the central white
matter of the brain were excluded (27). The longer the scan, the more agitated
the patients became, accompanied by an increase in body movements,
including head movements, and therefore, brain function images were
extracted after corrections were performed to compensate for any
movement artifacts (13).
Brain-visual acuity and other eye
parameters correlation analysis
These other eye parameters include onset time of RVO
and central subfield retinal thickness. First, Brain areas with
different ALFF findings between groups were classified as regions
of interest using the resting-state fMRI data analysis toolkit
software. Second, the average ALFF values for the different brain
regions were calculated. Finally, a linear correlation analysis was
performed to define the correlation between the behavior of the RVO
group and the average ALFF value in the different brain regions
(P<0.05).
Statistical analysis
The clinical data, including the duration of the
onset of RVO, best-corrected VA and central subfield retinal
thickness were recorded and analyzed in the study with independent
sample t-test (SPSS 24.0; IBM Corp.). The differences in ALFF
values of the RVO and HC groups were collected and receiver
operatin characteristic (ROC) curves were plotted and analyzed. The
ALFF values in RVO patients were also compared with clinical
features, and the correlation was analyzed through the Pearson's
correlation analysis software and the scatter diagram was
generated. P<0.05 was considered to indicate statistical
significance.
Results
Demographics and clinical behavioral
results
As demonstrated in Table
I, there were no significant differences in body weight
(P=0.916), gender (P>0.999) or age (P=0.753) between the
subjects with RVO and the HCs. In addition, there were obvious
differences in best-corrected VA-right (P=0.001), best-corrected
VA-left (P=0.001), central subfield retinal thickness (P=0.012) and
cube average thickness (P=0.009) between the RVOs and the HCs
(Table I).
 | Table I.Demographics and clinical
measurements in the two groups. |
Table I.
Demographics and clinical
measurements in the two groups.
|
Characteristics | RVO (n=24) | HC (n=24) | T | P-value |
|---|
| Males/females | 12/12 | 12/12 | N/A | >0.99 |
| Age (years) | 54.04±4.93 | 56.76±5.87 | 0.097 | 0.753 |
| Body weight
(kg) | 66.46±6.12 | 68.11±5.98 | 0.154 | 0.916 |
|
Right-handedness | 24 | 24 | N/A | >0.99 |
| Duration of RVO
(days) | 66.67±24.28 | N/A | N/A | N/A |
| Best-corrected
VA |
| Right
eye | 0.16±0.07 | 0.95±0.12 | −0.432 | 0.001 |
| Left
eye | 0.22±0.10 | 0.98±0.22 | −0.396 | 0.001 |
| IOP (mmHg) |
| Right
eye | 14.69±1.11 | 16.54±1.23 | 0.078 | 0.673 |
| Left
eye | 14.62±0.99 | 15.18±2.32 | 0.085 | 0.731 |
| Central subfield
retinal thickness (µm) | 701.13±81.61 | 301.58±46.18 | 0.683 | 0.012 |
| Cube average
thickness (µm) | 746.83±86.67 | 312.34±44.52 | 0.531 | 0.009 |
Differences in ALFF
Compared with the HCs, subjects with RVOs had
significantly higher ALFF values areas in the posterior lobe of the
left cerebellum, inferior temporal gyrus, anterior lobe of the
cerebellum, right cerebellar posterior/anterior lobe, lower area of
the medial frontal gyrus, the right precuneus, left middle frontal
gyrus, right angular gyrus and right superior frontal gyrus
(Figs. 2 and 3; Table
II).
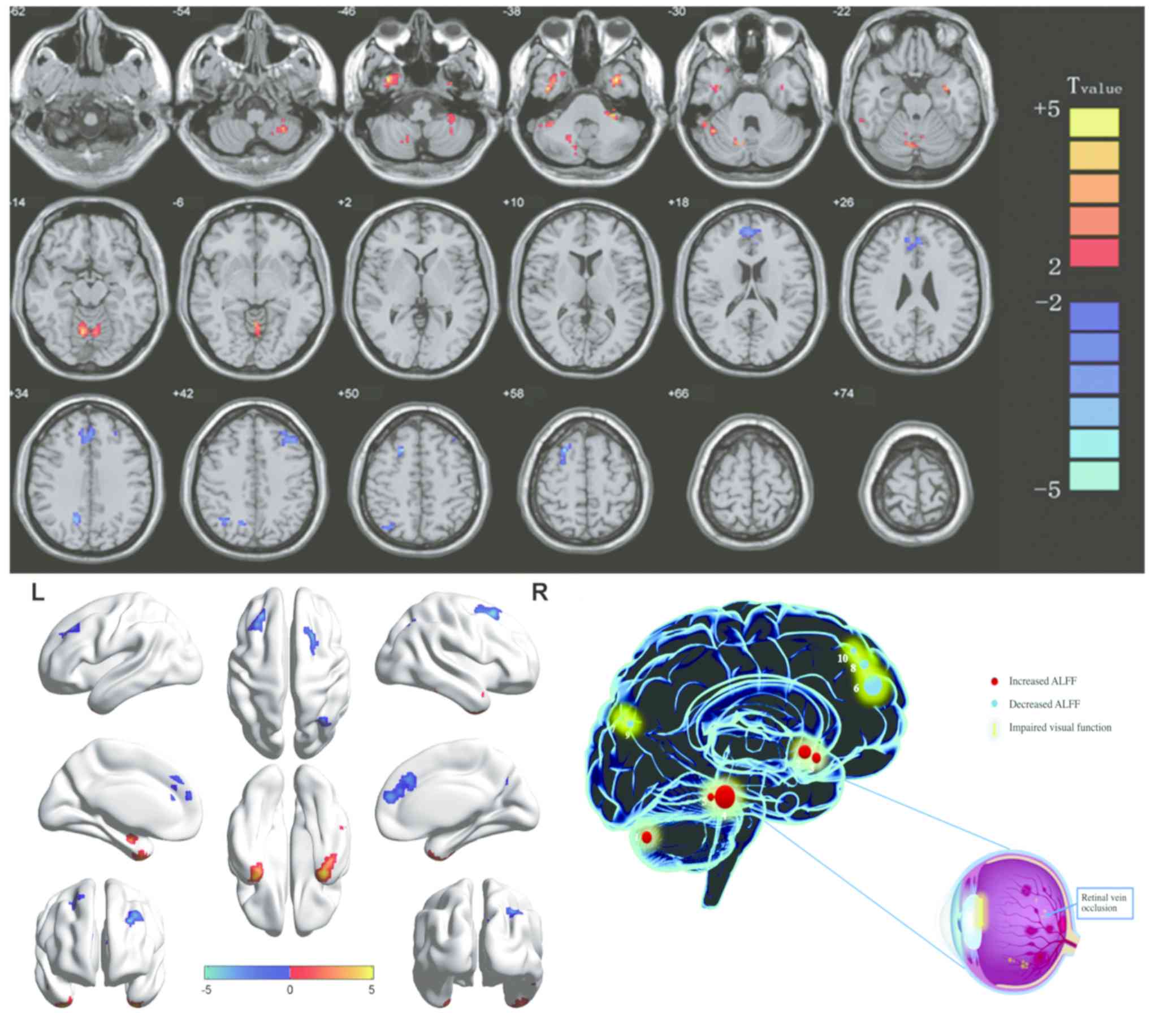 | Figure 2.Significant differences in
spontaneous brain activity between the retinal vein occlusion group
and healthy controls. The sizes of the spots denote the degree of
quantitative changes. The different brain regions were observed in
the left cerebellar posterior lobe, right inferior temporal gyrus,
left inferior temporal gyrus, bilateral cerebellar anterior lobe,
right cerebellar posterior/anterior lobe, bilateral medial frontal
gyrus, right precuneus, left middle frontal gyrus, right angular
gyrus and right superior frontal gyrus. The red areas denote that
patients with RVO exhibit higher ALFF in brain areas than HCs and
the blue areas denote brain regions with a lower ALFF [P<0.001
for multiple comparisons using Gaussian random field theory (z.2.3,
P<0.001, cluster >13 voxels, Alphasim corrected)]. ALFF,
amplitude of low-frequency fluctuation; L, left; R, right. RVO,
retinal vein occlusion; HCs, healthy controls; 1, left cerebellar
posterior lobe; 2, right inferior temporal gyrus; 3, right
cerebellar posterior/anterior lobe; 4, left inferior temporal
gyrus; 5, bilateral cerebellar anterior lobe; 6, right angular
gyrus; 7, bilateral medial frontal gyrus; 8, right superior frontal
gyrus; 9, left middle frontal gyrus; 10, right precuneus. |
 | Table II.Brain areas with significantly
different ALFF values between groups. |
Table II.
Brain areas with significantly
different ALFF values between groups.
|
|
| MNI
coordinates |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Brain area | BA | X | Y | Z | Peak voxels | T-value | P-values |
|---|
| RVO>HC |
| Left
cerebellar posterior lobe | 0 | −30 | −36 | −39 | 81 | 4.4823 | P<0.001 |
| Right
inferior temporal gyrus | 20 | 42 | −6 | −39 | 97 | 5.1104 | P<0.001 |
| Left
inferior temporal gyrus | 20 | −33 | 3 | −39 | 66 | 4.9095 | P<0.001 |
|
Bilateral cerebellar anterior
lobe | 0 | 9 | −63 | −12 | 175 | 4.8534 | P<0.001 |
| Right
cerebellar posterior/anterior lobe | 0 | 42 | −54 | −30 | 60 | 4.3742 | P<0.001 |
| RVO<HC |
|
Bilateral medial frontal
gyrus | 9 | 0 | 36 | 30 | 150 | −4.2564 | P<0.001 |
| Right
precuneus | 0 | 15 | −63 | 36 | 41 | −4.4224 | P<0.001 |
| Left
middle frontal gyrus | 9 | −30 | 39 | 39 | 69 | −4.8306 | P<0.001 |
| Right
angular gyrus | 7 | 33 | −66 | 45 | 48 | −4.0212 | P<0.001 |
| Right
superior frontal gyrus | 0 | 24 | 18 | 54 | 41 | −5.2273 | P<0.001 |
ROC curve analysis
The mean ALFF values of the two groups were analyzed
using ROC curves, with a larger the area under the curve (AUC)
indicating a higher diagnostic rate. The following AUCs were
determined for ALFF values (RVOs>HCs) in the different brain
regions: Left cerebellar posterior lobe, 0.897 (P<0.001); right
inferior temporal gyrus, 0.949 (P<0.001); left inferior temporal
gyrus, 0.926 (P<0.001); bilateral anterior lobes of the
cerebellum, 0.949 (P<0.001); right posterior/anterior lobe of
the cerebellum, 0.893 (P<0.001; Fig.
4A). For ALFF values (RVOs<HCs), the following AUCs were
determined: Bilateral medial frontal gyrus, 0.967 (P<0.001);
right precuneus, 0.849 (P=0.001); left middle frontal gyrus, 0.919
(P<0.001); right angular gyrus, 0.868 (P<0.001); and right
superior frontal gyrus, 0.904 (P<0.001; Fig. 4B).
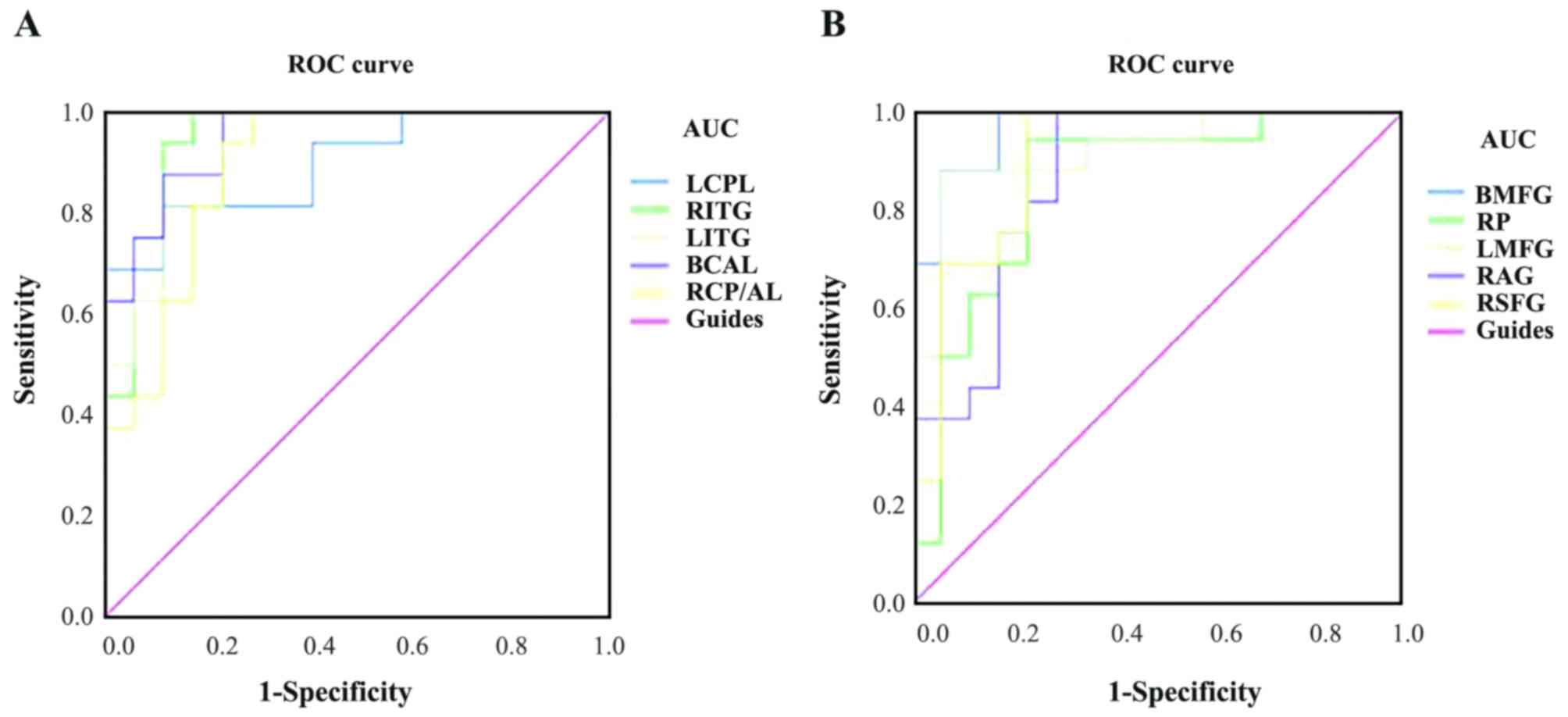 | Figure 4.ROC curve analysis of the mean ALFF
values for altered brain regions. (A) The AUCs of different brain
regions were as follows: LCPL, 0.897 (P<0.001; 95% CI:
0.790–1.000); RITG, 0.949 (P<0.001; 95% CI: 0.874–1.000); LITG,
0.926 (P<0.001; 95% CI: 0.841–1.000); BCAL, 0.949 (P<0.001;
95% CI: 0.881–1.000); RCP/AL, 0.893 (P<0.001; 95% CI:
0.783–1.000). (B) The AUCs of different brain regions were as
follows: BMFG, 0.967 (P<0.001; 95% CI: 0.916–1.000); RP, 0.849
(P=0.001; 95% CI: 0. 711–0.988); LMFG, 0.919 (P<0.001; 95% CI:
0.822–1.000); RAG, 0.868 (P<0.001; 95% CI: 0.742–0.993), RSFG,
0.904 (P<0.001; 95% CI: 0.798–1.000). ALFF, amplitude of
low-frequency fluctuation; ROC, receiver operating characteristic;
AUC, area under the ROC curve; LCPL, left cerebellar posterior
lobe; RITG, right inferior temporal gyrus; LITG, left inferior
temporal gyrus; BCAL, bilateral cerebellar anterior lobe; RCP/AL,
right cerebellar posterior/anterior lobe; BMFG, bilateral medial
frontal gyrus; RP, right precuneus; LMFG, left middle frontal
gyrus; RAG, right angular gyrus; RSFG, right superior frontal
gyrus. |
Correlation analysis of eye parameters
and ALFF value
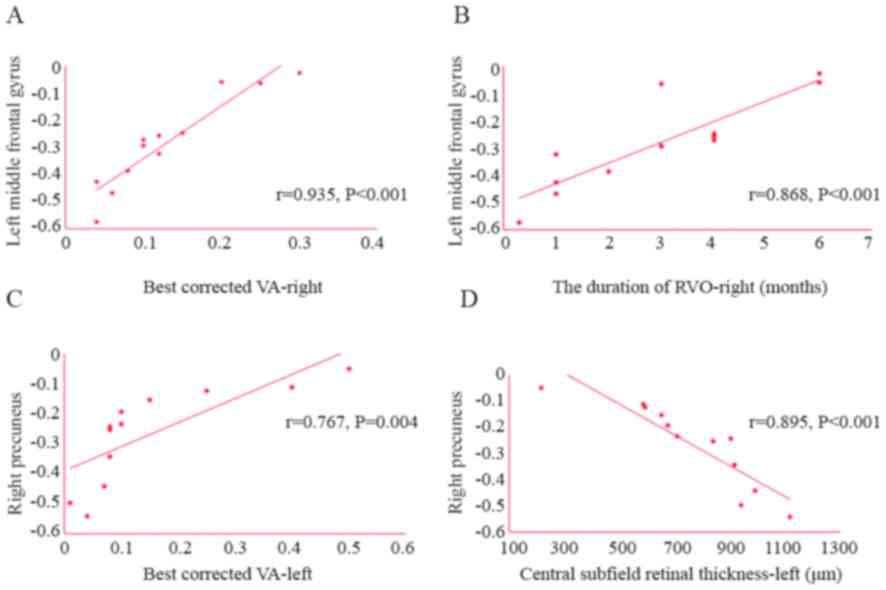
The best-corrected VA in the left eye was positively
correlated with the ALFF value of the right precuneus (r=0.767;
P=0.004) and the best-corrected VA in the right eye was positively
correlated with the ALFF value of the left middle frontal gyrus
(r=0.935; P<0.001). Furthermore, the central subfield retinal
thickness of the left eye was negatively correlated with the ALFF
value of the right precuneus (r=−0.895; P<0.001). The duration
of RVO in the right eye was also positively correlated with the
ALFF value of the left middle frontal gyrus (r=0.868; P<0.001;
Fig. 5). It can be inferred that
with the extension of the onset time of RVO and the improvement of
the best corrected VA in the right eye, the ALFF value in the left
middle frontal gyrus increases gradually. Furthermore, with the
improvement of the best corrected VA in the left eye, the ALFF
value of the right precuneus increased gradually. However, the
central subfield retinal thickness in the left eye was negatively
correlated with the ALFF value of right precuneus. That is, with
the increase of the central subfield retinal thickness in the left
eye, the ALFF value of right precuneus decreases gradually.
Discussion
The current study assessed the association between
clinical behavior and brain changes in patients with RVO on
resting-state brain activity using the ALFF technique. The clinical
behaviors assessed include the duration of the onset of RVO,
best-corrected VA and central subfield retinal thickness. Compared
to the HCs, the subjects with RVO of the present study exhibited
distinctly increased ALFF values in the left posterior lobe of the
cerebellum, bilaterally in the inferior temporal gyri, bilaterally
in the anterior lobes of the cerebellum, as well as the right
posterior/anterior lobes of the cerebellum. By contrast, the ALFF
values of the medial frontal gyri, right precuneus, left middle
frontal gyrus, right angular gyrus and right superior frontal gyrus
were significantly decreased.
Reduced ALFF values in the subjects with RVO vs. HCs
were first analyzed. The middle frontal gyrus is thought to be
associated with mental and physical balance. Carter et al
(28) used fMRI to synchronously
track and delay the acquisition of explicit knowledge in a
conditioned reflex paradigm. Their experiments demonstrated that
activity in the frontal gyrus was associated with the accuracy of
clear emergency awareness in each trial. Leung et al
(29) used fMRI to study activity in
the MFG, revealing that, during more demanding tasks, it may
provide up to 24 sec of memory, and the signal changes in this area
are greater than those in other pre-frontal areas. Therefore, the
middle frontal gyrus constitutes an important part of the brain's
memory storage. Japee et al (30) investigated the function of the middle
frontal gyrus and concluded that it has an important role in
attention control. Talati and Hirsch (31) reported that the middle frontal gyrus
participates in advanced execution and decision making.
Furthermore, previous studies have indicated that patients with
depression have dysfunctional middle frontal gyri (32,33). In
adjustment disorder, stress is not proportional to physical and
mental stimulus. In other words, a more obvious the clinical
manifestation may not necessarily be proportional to the pressure
(34). The best-corrected VA in the
right eye was positively correlated with the ALFF value of the left
middle frontal gyrus (r=0.935, P<0.001). Therefore, it may be
hypothesized that, with the decrease of the ALFF value, the
best-corrected VA may also decrease, which means that the
dysfunction of the left middle frontal gyrus would increase. The
duration of RVO in the right eye was positively correlated with the
ALFF value of the left middle frontal gyrus (r=0.868, P<0.001).
With the extension of the course and progression of the disease,
the left middle frontal gyrus impairment of RVO patients became
aggravated. The angular gyrus has the function of integrating and
transmitting multi-organ sensory information in different ways.
Therefore, the role of the angular gyrus in isolation cannot be
determined. Yazar et al (35)
performed a study on 61 healthy individuals and 61 patients with
disease of the angular gyrus, indicating that the ability of
patients with angular gyrus disease to acquire context features in
diverse modes was reduced, and that the angular gyrus was required
for memory experiences. Yazar et al (36) then indicated that reduced stimulation
of the angular gyrus was associated with lower levels of subjective
recall.
The superior frontal gyrus is part of the frontal
lobe, dividing it into three sub-regions. A positive correlation
has been identified between the default network and the cognitive
control network in the anterior medial inferior region of the
frontal gyrus (37). There was a
positive correlation between the superior dorsolateral sub-area
default network and the cognitive execution network (38). Studies using fMRI experiments have
indicated that the superior frontal gyrus is involved in
self-awareness (39) and laughter
(40). The present study suggested
that ALFF values of RVO patients were lower in the right superior
frontal gyrus, which suggested that there was brain dysfunction in
this area.
The precise function of the precuneus remains
elusive. Three different functional modes may exist in the
precuneus: The anterior precuneus exhibits functional connectivity
with sensorimotor regions, the central precuneus appears to be a
cognitive/associative region, and the posterior precuneus displays
functional connectivity with adjacent visual cortical regions
(41). Uchimura et al
(42) reported that the right
precuneus is involved in visuospatial cognitive tasks. This is
similar to the result of another study, according to which the
right precuneus has a causal role in visual short-term memory
capacity, particularly in bilateral visual displays (43). Therefore, the precuneus is considered
‘an active region that can continuously collect information about
the surrounding world’ (44). Chen
et al (45) indicated that
responses in the right anterior cingulate gyrus were enhanced in
individuals with higher language creativity. Therefore, it may be
concluded that the size of the anterior right wedge is positively
correlated with the individual's linguistic creativity. Functional
fMRI studies have indicated that this region of the brain
participates in the integration of behavior, visual images and
consciousness (46). The present
study determined that the best-corrected VA in the left eye was
positively correlated with the ALFF value of the right precuneus
(r=0.767, P=0.004). Furthermore, the central subfield retinal
thickness in the left eye was negatively correlated with the ALFF
value of the right precuneus (r=−0.895, P<0.001). The average
thickness of the macular fovea was increased in RVO patients vs.
HCs. The increase in thickness of the central subfield retinal
reflects the severity of RVO, so the ALFF value reduction of the
right precuneus may be associated with the severity of RVO.
Increased ALFF values in the subjects with RVO vs.
HCs were then analyzed. The inferior temporal gyrus is located on
the outer and lower surfaces of the temporal neocortex. The
temporal cortex is composed of three parts, of which the inferior
temporal gyrus (20, 21 and 37 region) is associated to visual
information processing, and is particularly important for promoting
cognitive processing and emotional regulation (47). Stoeter et al (48) suggested that somatic pain disorder
was associated with increased temporal lobe activation, which was
consistent with the present result that ALFF values were
significantly increased in the sub-temporal region. This may be
associated with the activation of multiple cortical regions when
patients are anxious that a serious eye disease may lead to
blindness (49).
Anatomically, the cerebellar is located in the
posterior fossa. Cerebellar function includes cognition,
coordination and balance, and fine regulation of the eye (50). Ataxia occurs when the cerebellar is
damaged. According to studies, the functions of the cerebellar
include cognition and memory (51),
and dysfunction of the cerebellar is associated with Alzheimer's
disease (52), bipolar disorder
(53), depression (54) and schizophrenia (55). This is consistent with the present
study, as RVO patients were generally anxious over their condition
while they underwent fMRI scanning. Therefore, it is likely that
the high ALFF value of the inferior lobe of the cerebellar was the
result of anxiety in these patients. The ALFF method has been
successfully applied to patients with ophthalmological diseases
(Table III) and is expected to
have huge prospects for development. In the present study, the mean
ALFF values of specific ROIs were collected and subjected to ROC
curve analyses. The accuracy was considered excellent if the AUC
was >0.8. In the present ROC curve analysis, excellent AUC
values were obtained for all ROIs, including the right precuneus
and the left middle frontal gyrus, indicating that ALFF methodology
may provide promising biological indicators for distinguishing
patients with RVO from HCs.
 | Table III.Amplitude of low-frequency
fluctuation method applied in ophthalmological diseases. |
Table III.
Amplitude of low-frequency
fluctuation method applied in ophthalmological diseases.
| First author
(year) | Disease | (Refs.) |
|---|
| Liu (2014) | Glaucoma | (14) |
| Huang (2015) | Glaucoma | (15) |
| Shao (2015) | Optic neuritis | (19) |
| Huang (2016) | Strabismus | (17) |
| Tan (2017) | Amblyopia | (16) |
| Huang (2016) | High myopia | (18) |
| Tan (2016) | Open-globe
injury | (20) |
| Li (2016) | Monocular
blindness | (21) |
| Wang (2017) | Diabetic
retinopathy | (23) |
| Xin (2017) | Retinal
detachment | (22) |
| Pan(2018) | Acute eye pain | (24) |
Of note, the present study had several limitations.
First, the small sample size may have affected the experimental
results. Furthermore, the best corrected visual acuity is easily
influenced by subjective factors. In addition, the spontaneous
activity in different brain regions was affected by different
scanning times. At present, it remains elusive whether lowering
blood pressure, blood lipid and/or blood sugar levels may improve
vision or complications of RVO, and this may be worthy of further
study. In the future, more objective indicators may be used to
record and analyze data, and the determination of spontaneous
activity changes in different brain regions in RVO patients may
also be improved. This may provide a theoretical basis for further
study of the pathophysiological changes and treatment of RVO.
In conclusion, the present study was the first, to
the best of our knowledge, to report that brain activity disorders
occur in RVO patients. In the future, ALFF may provide guidance in
the early detection of the neuropathological mechanisms of RVO and
provide a basis for clinical diagnosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660158), the
Natural Science Key Project of Jiangxi Province (grant no.
20161ACB21017), the Youth Science Foundation of Jiangxi Province
(grant nos. 20151BAB215016 and 20161BAB215198), the Key Research
Foundation of Jiangxi Province (grant no. 20181BBG70004), the
Teaching Reform of Degree and Graduate Education Research Project
of Jiangxi Province (grant no. JXYJG-2018-013) and the Health
Development Planning Commission Science TCM Foundation of Jiangxi
Province (grant no. 2018060).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS and QZ designed the current study. QY and BL
recruited healthy controls. YQS performed MRI scanning. YM, PZ and
WS collected and analyzed the data. YW wrote the manuscript. All
the authors read and approved the final manuscript.
Ethical approval and consent to
participate
The study methods and protocols were approved by the
Medical Ethics Committee of the First Affiliated Hospital of
Nanchang University (Nanchang, China) and followed the principles
of the Declaration of Helsinki. All subjects were notified of the
objectives and content of the study and latent risks, and then
provided written informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
This study did not receive any industrial support.
The authors have no competing interests to declare regarding this
study.
References
|
1
|
Klein R, Klein BE, Moss SE and Meuer SM:
The epidemiology of retinal vein occlusion: The beaver dam eye
study. Trans Am Ophthalmol Soc. 98:133–143. 2000.PubMed/NCBI
|
|
2
|
Ho M, Liu DT, Lam DS and Jonas JB: Retinal
vein occlusions, from basics to the latest treatment. Retina.
36:432–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sivaprasad S, Amoaku WM and Hykin P: The
royal college of ophthalmologists guidelines on retinal vein
occlusions: Executive summary. Eye (Lond). 29:1633–1638. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayreh SS: Retinal vein occlusion. Indian
J Ophthalmol. 42:109–132. 1994.PubMed/NCBI
|
|
5
|
Lee JY, Yoon YH, Kim HK, Yoon HS, Kang SW,
Kim JG, Park KH and Jo YJ; Korean RVO Study, : Baseline
characteristics and risk factors of retinal vein occlusion: A study
by the Korean RVO study group. J Korean Med Sci. 28:136–144. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogers S, McIntosh RL, Cheung N, Lim L,
Wang JJ, Mitchell P, Kowalski JW, Nguyen H and Wong TY;
International Eye Disease Consortium, : The prevalence of retinal
vein occlusion: Pooled data from population studies from the United
States, Europe, Asia, and Australia. Ophthalmology. 117:313–319.e1.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuhli-Hattenbach C, Inge S, Lüchtenberg M
and Hattenbach LO: Coagulation disorders and the risk of retinal
vein occlusion. Thromb Haemost. 103:299–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stem MS, Talwar N, Comer GM and Stein JD:
A longitudinal analysis of risk factors associated with central
retinal vein occlusion. Ophthalmology. 120:362–370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tilleul J, Glacet-Bernard A, Coscas G,
Soubrane G and Souied EH: Underlying conditions associated with the
occurrence of retinal vein occlusion. J Fr Ophtalmol. 34:318–324.
2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Yu C, Liang M, Li J, Tian L, Zhou
Y, Qin W, Li K and Jiang T: Whole brain functional connectivity in
the early blind. Brain. 130:2085–2096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biswal BB: Resting state fMRI: A personal
history. Neuroimage. 62:938–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Logothetis NK, Pauls J, Augath M, Trinath
T and Oeltermann A: Neurophysiological investigation of the basis
of the fMRI signal. Nature. 412:150–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ,
Liang M, Tian LX, Jiang TZ and Wang YF: Altered baseline brain
activity in children with ADHD revealed by resting-state functional
MRI. Brain Dev. 29:83–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Liu Z, Li J, Liu Z, Tang Z, Xie X,
Yang D, Wang N, Tian J and Xian J: Altered amplitude of
low-frequency fluctuation in primary open-angle glaucoma: A
resting-state fMRI study. Invest Ophthalmol Vis Sci. 56:322–329.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang X, Zhong YL, Zeng XJ, Zhou F, Liu
XH, Hu PH, Pei CG, Shao Y and Dai XJ: Disturbed spontaneous brain
activity pattern in patients with primary angle-closure glaucoma
using amplitude of low-frequency fluctuation: A fMRI study.
Neuropsychiatr Dis Treat. 11:1877–1883. 2015.PubMed/NCBI
|
|
16
|
Tang A, Chen T, Zhang J, Gong Q and Liu L:
Abnormal spontaneous brain activity in patients with anisometropic
amblyopia using resting-state functional magnetic resonance
imaging. J Pediatr Ophthalmol Strabismus. 54:303–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang X, Li SH, Zhou FQ, Zhang Y, Zhong
YL, Cai FQ, Shao Y and Zeng XJ: Altered intrinsic regional brain
spontaneous activity in patients with comitant strabismus: A
resting-state functional MRI study. Neuropsychiatr Dis Treat.
12:1303–1308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang X, Zhou FQ, Hu YX, Xu XX, Zhou X,
Zhong YL, Wang J and Wu XR: Altered spontaneous brain activity
pattern in patients with high myopia using amplitude of
low-frequency fluctuation: A resting-state fMRI study.
Neuropsychiatr Dis Treat. 12:2949–2956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao Y, Cai FQ, Zhong YL, Huang X, Zhang
Y, Hu PH, Pei CG, Zhou FQ and Zeng XJ: Altered intrinsic regional
spontaneous brain activity in patients with optic neuritis: A
resting-state functional magnetic resonance imaging study.
Neuropsychiatr Dis Treat. 11:3065–3073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan G, Huang X, Ye L, Wu AH, He LX, Zhong
YL, Jiang N, Zhou FQ and Shao Y: Altered spontaneous brain activity
patterns in patients with unilateral acute open globe injury using
amplitude of low-frequency fluctuation: A functional magnetic
resonance imaging study. Neuropsychiatr Dis Treat. 12:2015–2020.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong
YL, Jiang N and Shao Y: Altered spontaneous brain activity pattern
in patients with late monocular blindness in middle-age using
amplitude of low-frequency fluctuation: A resting-state functional
MRI study. Clin Interv Aging. 11:1773–1780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang X, Li D, Li HJ, Zhong YL, Freeberg
S, Bao J, Zeng XJ and Shao Y: Abnormal regional spontaneous neural
activity in visual pathway in retinal detachment patients: A
resting-state functional MRI study. Neuropsychiatr Dis Treat.
13:2849–2854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang ZL, Zou L, Lu ZW, Xie XQ, Jia ZZ, Pan
CJ, Zhang GX and Ge XM: Abnormal spontaneous brain activity in type
2 diabetic retinopathy revealed by amplitude of low-frequency
fluctuations: A resting-state fMRI study. Clin Radiol.
72:340.e1–e7. 2017. View Article : Google Scholar
|
|
24
|
Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q,
Freeberg S, Zhu PW, Ye L, Ma MY, Huang X and Shao Y: Altered
intrinsic brain activities in patients with acute eye pain using
amplitude of low-frequency fluctuation: A resting-state fMRI study.
Neuropsychiatr Dis Treat. 14:251–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Janssen MC, den Heijer M, Cruysberg JR,
Wollersheim H and Bredie SJ: Retinal vein occlusion: A form of
venous thrombosis or a complication of atherosclerosis? A
meta-analysis of thrombophilic factors. Thromb Haemost.
93:1021–1026. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HJ, Dai XJ, Gong HH, Nie X, Zhang W and
Peng DC: Aberrant spontaneous low-frequency brain activity in male
patients with severe obstructive sleep apnea revealed by
resting-state functional MRI. Neuropsychiatr Dis Treat. 11:207–214.
2015.PubMed/NCBI
|
|
27
|
Yan CG, Cheung B, Kelly C, Colcombe S,
Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX and Milham
MP: A comprehensive assessment of regional variation in the impact
of head micromovements on functional connectomics. Neuroimage.
76:183–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carter RM, O'Doherty JP, Seymour B, Koch C
and Dolan RJ: Contingency awareness in human aversive conditioning
involves the middle frontal gyrus. Neuroimage. 29:1007–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leung HC, Gore JC and Goldman-Rakic PS:
Sustained mnemonic response in the human middle frontal gyrus
during on-line strage of spatial memoranda. J Cognit Neurosci.
14:659–671. 2002. View Article : Google Scholar
|
|
30
|
Japee S, Holiday K, Satyshur MD, Mukai I
and Ungerleider LG: A role of right middle frontal gyrus in
reorienting of attention: A case study. Front Syst Neurosci.
9:232015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Talati A and Hirsch J: Functional
specialization within the medial frontal gyrus for perceptual
go/no-go decisions based on ‘what,’ ‘when,’ and ‘where’ related
information: An fMRI study. J Cogn Neurosci. 17:981–993. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CC, Yu SC, McQuoid DR, Messer DF,
Taylor WD, Singh K, Boyd BD, Krishnan KR, MacFall JR, Steffens DC
and Payne ME: Reduction of dorsolateral prefrontal cortex gray
matter in late-life depression. Psychiatry Res. 193:1–6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nelson JD, Craig JP, Akpek EK, Azar DT,
Belmonte C, Bron AJ, Clayton JA, Dogru M, Dua HS, Foulks GN, et al:
TFOS DEWS II introduction. Ocul Surf. 15:269–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gnanavel S and Robert RS: Diagnostic and
statistical manual of mental disorders, fifth edition, and the
impact of events scale-revised. Chest. 144:1974–1975. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yazar Y, Bergström ZM and Simons JS:
Continuous theta burst stimulation of angular gyrus reduces
subjective recollection. PLoS One. 9:e1104142014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yazar Y, Bergström ZM and Simons JS:
Reduced multimodal integration of memory features following
continuous theta burst stimulation of angular gyrus. Brain Stimul.
10:624–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martino J, Gabarrós A, Deus J, Juncadella
M, Acebes JJ, Torres A and Pujol J: Intrasurgical mapping of
complex motor function in the superior frontal gyrus. Neuroscience.
179:131–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Owen AM: The role of the lateral frontal
cortex in mnemonic processing: The contribution of functional
neuroimaging. Exp Brain Res. 133:33–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goldberg I, Harel M and Malach R: When the
brain loses its self: Prefrontal inactivation during sensorimotor
processing. Neuron. 50:329–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fried I, Wilson CL, MacDonald KA and
Behnke EJ: Electric current stimulates laughter. Nature.
391:6501998. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Margulies DS, Vincent JL, Kelly C, Lohmann
G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP and
Petrides M: Precuneus shares intrinsic functional architecture in
humans and monkeys. Proc Natl Acad Sci USA. 106:20069–20074. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uchimura M, Nakano T, Morito Y, Ando H and
Kitazawa S: Automatic representation of a visual stimulus relative
to a background in the right precuneus. Eur J Neurosci.
42:1651–1659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kraft A, Dyrholm M, Kehrer S, Kaufmann C,
Bruening J, Kathmann N, Bundesen C, Irlbacher K and Brandt SA: TMS
over the right precuneus reduces the bilateral field advantage in
visual short term memory capacity. Brain Stimul. 8:216–223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Raichle ME, MacLeod AM, Snyder AZ, Powers
WJ, Gusnard DA and Shulman GL: A default mode of brain function.
Proc Natl Acad Sci USA. 98:676–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen QL, Xu T, Yang WJ, Li YD, Sun JZ,
Wang KC, Beaty RE, Zhang QL, Zuo XN and Qiu J: Individual
differences in verbal creative thinking are reflected in the
precuneus. Neuropsychologia. 75:441–449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cavanna AE and Trimble MR: The precuneus:
A review of its functional anatomy and behavioural correlates.
Brain. 129:564–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Noppeney U and Price CJ: Retrieval of
visual, auditory, and abstract semantics. Neuroimage. 15:917–926.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stoeter P, Bauermann T, Nickel R, Corluka
L, Gawehn J, Vucurevic G, Vossel G and Egle UT: Cerebral activation
in patients with somatoform pain disorder exposed to pain and
stress: An fMRI study. Neuroimage. 36:418–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schunck T, Mathis A, Erb G, Namer IJ,
Demazières A and Luthringer R: Effects of lorazepam on brain
activity pattern during an anxiety symptom provocation challenge. J
Psychopharmacol. 24:701–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schmahmann JD: Disorders of the
cerebellum: Ataxia, dysmetria of thought, and the cerebellar
cognitive affective syndrome. J Neuropsychiatry Clin Neurosci.
16:367–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Desmond JE and Fiez JA: Neuroimaging
studies of the cerebellum: Language, learning and memory. Trends
Cogn Sci. 2:355–362. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Thomann PA, Schläfer C, Seidl U, Santos
VD, Essig M and Schröder J: The cerebellum in mild cognitive
impairment and Alzheimer's disease-a structural MRI study. J
Psychiatr Res. 42:1198–1202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Baldaçara L, Nery-Fernandes F, Rocha M,
Quarantini LC, Rocha GG, Guimarães JL, Araújo C, Oliveira I,
Miranda-Scippa A and Jackowski A: Is cerebellar volume related to
bipolar disorder? J Affect Disord. 135:305–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bledsoe JC, Semrud-Clikeman M and Pliszka
SR: Neuroanatomical and neuropsychological correlates of the
cerebellum in children with attention-deficit/hyperactivity
disorder-combined type. J Am Acad Child Adolesc Psychiatry.
50:593–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Andreasen NC, Paradiso S and O'Leary DS:
‘Cognitive dysmetria’ as an integrative theory of schizophrenia: A
dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr
Bull. 24:203–218. 1998. View Article : Google Scholar : PubMed/NCBI
|