Introduction
Breast cancer (BC), the most frequent malignant
tumour in females, is a disease with a heterogenous genetic
background and morphology. The prediction of the biological
behaviour of a tumour and its response to therapy currently relies
on histological classification of the BC tumour. BC subtyping based
on molecular profiling, including intrinsic subtypes luminal A,
luminal B, human epidermal growth factor receptor 2
(HER2)-enriched, basal-like and normal-like, may lead to improved
prediction of the tumour's response to therapy (1–3). Novel
subgroups with features distinct from the previous ones have been
proposed, including the claudin-low and -high subtypes (4,5).
However, the additional value of this molecular classification in
clinical practice remains unclear (6).
The claudin family currently comprises 27 proteins
that form the integral backbone of tight junctions, which are key
intercellular connections responsible for maintaining tissue
homeostasis (7). The combination of
claudins, as well as the level of their expression, are tissue
specific and various tumours have been reported to exhibit
characteristic patterns (8–11). In non-cancerous breast tissue, the
duct epithelium expresses high levels of claudin-1, −3 and −4
(8). In breast tumours, the
downregulation or complete loss of claudin-1 and the preservation
or upregulation of claudin-3 and −4 were commonly observed in
previous studies (8–11). There are, however, some exceptions to
these findings, particularly the claudin-low and -high molecular
subtypes. The claudin-low subtype is characterized by low
expression of claudin-3, −4 and −7 and E-cadherin, and a high
frequency of triple-negative tumours [TNBC; negative oestrogen (ER)
and progesterone receptors (PR) and HER2]. The claudin-high subtype
is characterized by the high expression of claudin-1 and −4 and ER
negativity. The claudin-low and -high subtypes are associated with
aggressive tumours that tend to be chemosensitive, while other
therapy options like hormonal or anti-HER2 therapy are frequently
limited due to their ER- or triple-negativity (4,5,12,13).
Chemotherapy is one of the basic therapeutic
approaches to BC. There are selected patients who profit from
neoadjuvant chemotherapy, which is administered prior to surgery in
order to reduce the extent of primary tumour and lymph node
metastases, and therefore to reduce the extent of required surgery
(14). Since many patients undergo
adjuvant chemotherapy following the surgical resection of BC, a
question arises as to how a possible previous neoadjuvant
chemotherapy changes the expression profile of a tumour and whether
such changes influence the response to adjuvant chemotherapy.
Claudins have been previously investigated as
potential new predictive markers of BC (4,5,15). Additionally, due to their easily
accessible extracellular receptor domains and their involvement in
carcinogenesis, claudins are promising targets for novel
therapeutic tools, particularly for chemoresistant cancer (16–18). The
exact role of claudins in carcinogenesis remains unclear and seems
to be very complex, involving proliferation, differentiation and
apoptosis on multiple levels (9,10,19). An
increasing body of evidence suggests that they serve a role in the
epithelial-mesenchymal transition (EMT), during which epithelial
neoplastic cells gain a mesenchymal-like phenotype through
disruption of intercellular junctions, loss of cell polarity and
reorganisation of the cytoskeleton (12,20,21).
This process facilitates the growth of a tumour, its invasion into
surrounding tissues and metastasis. Furthermore, EMT is thought to
be partially responsible for cancer chemoresistance (22). During EMT, intercellular junctions
not only come apart but also undergo regulatory changes including a
cadherin switch, where epithelial cells loose E-cadherin expression
and begin to express N-cadherin, which is typically expressed in
tissues of mesodermal origin (23).
Even though the expression of E-cadherin is not always entirely
lost, a cadherin switch is generally considered to be a marker of
EMT (24,25). Claudin-1 facilitates collective
migration, by which neoplastic cells detach from the tumour mass at
the leading front and penetrate into the surrounding tissues either
through partial EMT mechanisms or independently of EMT (26). Claudin-3 and −4 maintain the
epithelial phenotype of a cell by modulating the expression of
major EMT markers, mainly maintaining the expression of E-cadherin,
which is usually highly expressed by the most common type of BC,
invasive carcinoma of no special type (NST, ductal) (21). Claudins serve a role in the
regulation of cancer stem cells or tumour-initiating cells with
self-renewal potential. Therefore, it is possible that they may
contribute to drug resistance and tumour recurrence after initial
therapy (12,20,22,27).
To the best of our knowledge, this is the first
study to investigate the routinely assessed markers of BC such as
ER, PR, HER2 and Ki-67, as well as the changes in expression of the
intercellular junction proteins claudin-1, −3 and −4, E- and
N-cadherin following neoadjuvant chemotherapy. An improved
understanding of their expression following chemotherapy may
contribute to the elucidation of their role in tumour response to
chemotherapy. Furthermore, the current study investigated the
associations between the expression of claudins, cadherins,
standard BC biomarkers, tumour grade and the extent of histological
regressive changes after chemotherapy that may reveal the role of
claudins in BC carcinogenesis and EMT.
Materials and methods
Tissue samples
Formalin-fixed, paraffin-embedded (FFPE) tissue
samples of invasive breast carcinoma NST from 62 patients who
underwent neoadjuvant chemotherapy between January 2006 and June
2015 were selected from the archive of the Institute of Pathology
of the General University Hospital and the First Faculty of
Medicine of The Charles University in Prague, Czech Republic. All
patients were females whose age at the first biopsy ranged from 23
to 70 years (mean, 53.5 years). The tumour tissue was obtained from
a diagnostic core needle biopsy before treatment, and from the
definitive resection of the residual tumour following neoadjuvant
chemotherapy using mostly anthracyclines or taxanes, in combination
with the HER2-antibody trastuzumab in HER2-positive tumours.
Patient and tumour characteristics are summarized in Table I. In mastectomy specimens, optimal
fixation was ensured by scoring the tissue with parallel incisions
(every 10 mm of tissue) immediately upon the delivery of the
specimen to the Institute of Pathology, allowing equal permeation
of the tissue by the fixative. This process prevented suboptimal
fixation of the specimen and any subsequent alterations in the
results of immunohistochemistry (IHC) reactions, specifically the
presence of false negativity. Such preparation, together with the
inking of the resection margins, enabled the preservation of the
anatomical context without hindering macroscopic evaluation of the
specimen. All specimens were fixed in 10% neutral buffered formalin
for 6–24 h at room temperature and embedded in paraffin wax.
Haematoxylin and eosin-stained 3 µm sections were subsequently
examined by a pathologist using a light microscope. The
histological grade was assessed in the two sample sets according to
the Nottingham grading system of Elston and Ellis (1). The effect of therapy in the residual
tumour tissue was scored according to the Chevallier
classification: i) Class I, no residual carcinoma; ii) class II,
carcinoma in situ; iii) class III, invasive carcinoma with
stromal fibrosis and class IV, no or minimal regressive changes in
the invasive carcinoma (28). Only
samples containing residual invasive cancer with either marked
(class III) or minor signs of regression after chemotherapy (class
IV) were selected. Patients with Chevallier class I and II tumour
regression following chemotherapy were not included in the current
study due to the absence of invasive cancer.
 | Table I.Clinicopathological characteristics
of 62 patients with invasive breast carcinoma, including the
comparison of tumour stage and grade prior to and following
neoadjuvant chemotherapy. |
Table I.
Clinicopathological characteristics
of 62 patients with invasive breast carcinoma, including the
comparison of tumour stage and grade prior to and following
neoadjuvant chemotherapy.
| Variable | N (%) |
|---|
| Age at the time of
core needle biopsy, years |
|
|
<40 | 10 (16) |
|
40–60 | 34 (55) |
|
>60 | 18 (29) |
| Extent of
surgery |
|
|
Mastectomy | 32 (52) |
|
Breast-conserving surgery | 30 (48) |
| Neoadjuvant
chemotherapy |
|
|
Doxorubicin,
cyclophosphamide | 5 (8) |
|
Doxorubicin, cyclophosphamide,
docetaxel | 34 (55) |
|
Doxorubicin, cyclophosphamide,
paclitaxel | 3 (5) |
|
Fluorouracil, epirubicin,
cyclophosphamide | 3 (5) |
|
Letrozole | 4 (6) |
|
Paclitaxel | 2 (3) |
| Other
combinations | 11 (18) |
| Neoadjuvant
anti-HER2 therapy (trastuzumab) |
|
|
Yes | 8 (13) |
| No | 54 (87) |
| Stage prior to
therapy |
|
| I | 4 (6) |
| II | 39 (63) |
|
III | 16 (26) |
| IV | 2 (3) |
|
Unknown | 1 (2) |
| Stage following
therapy |
|
| I | 15 (25) |
| II | 30 (48) |
|
III | 12 (19) |
| IV | 2 (3) |
|
Unknown | 3 (5) |
| Stage changes after
neoadjuvant chemotherapy |
|
|
Decreased | 31 (50) |
|
Increased | 11 (18) |
|
Unchanged | 17 (27) |
|
Unknown | 3 (5) |
| Chevallier
classa |
|
|
III | 48 (77) |
| IV | 14 (23) |
| Tumour grade prior
to therapy |
|
| G1 | 6 (10) |
| G2 | 33 (53) |
| G3 | 23 (37) |
| Tumour grade
following therapy |
|
| G1 | 6 (10) |
| G2 | 30 (48) |
| G3 | 26 (42) |
| Histological type
of breast cancer |
|
|
Invasive carcinoma of NST | 62 (100) |
| Minor histological
component in invasive carcinoma NSTb |
|
| Mixed
NST and lobular | 4 (6) |
| Mixed
NST and tubular | 1 (2) |
| NST
with neuroendocrine features | 1 (2) |
| NST
with micropapillary component | 1 (2) |
|
Total | 7 (11) |
Samples with a sufficient amount of residual tumour
were chosen for tissue microarray (TMA). Representative areas of
the tumour were marked and their respective FFPE blocks were used
for TMA construction based on the haematoxylin and eosin-stained
slides. Three or four tissue cores of 2.0 mm in diameter were
drilled from each donor block and implanted into the recipient
block using the tissue microarray instrument TMA Master (3DHISTECH
Ltd.).
IHC staining and evaluation
IHC evaluation of expression of all following
markers was conducted by a pathologist using a light microscope.
The IHC assessment of standard BC biomarkers included HER2, ER, PR
and Ki-67. Evaluation of HER2 was performed using HerceptTest
(complete kit, original dilution by manufacturer; Agilent
Technologies, Inc.; K5204) or rabbit monoclonal anti-human
anti-HER2 4B5 PATHWAY antibody (original dilution by manufacturer;
Ventana Medical Systems; 790-2991) according to the manufacturers'
instructions on the device Ventana BenchMark ULTRA (Ventana Medical
Systems). The evaluation of HER2 expression was performed according
to the World Health Organization 2012 scoring guidelines (1): i) Negative (score 0 and 1+); ii) weakly
positive (2+) and iii) strongly positive (3+) for HER2 protein
overexpression. Fluorescence in situ hybridization (FISH)
with ZytoLight® HER2/CEN 17 Dual Colour probe
(ZytoVision; Z-2077) was performed according to the manufacturer's
instructions on samples that were scored by IHC as weakly positive
(2+) and also on the samples that were scored as negative (0 and
1+) and ER and/or PR negative. For the FISH evaluation, ≥20 cells
located in the area of invasive cancer were counted using a
fluorescence microscope (magnification, ×100 and immerse oil). A
positive result was defined as the ratio of HER2: Centromere of
chromosome 17 being ≥2. For the evaluation of the other markers,
the following antibodies were used according to the manufacturer's
instructions: Mouse monoclonal anti-human antibody clone ER-6F11
for the ER receptor (1:50; Novocastra, Leica Microsystems;
NCL-L-ER-6F11), two clones PGR-312 and 16 for the PR receptor
(1:50; Leica Microsystems Inc.; NCL-PGR-312; ORG-8721) and a mouse
monoclonal anti-human clone Mib-1 for the evaluation of Ki-67
(1:50; Agilent Technologies, Inc.; M7240). After that, slides were
incubated in N-Histofine Simple Stain Max-Peroxidase (multi)
(original dilution by manufacturer, Nichirei Biosciences Inc.,
41415) for 30 min and then in chromogen DAB-3S (original dilution
by manufacturer, Nichirei Biosciences Inc., 415194S) for 5 min at
room temperature. The evaluation of these markers was expressed as
the percentage of positive cells. The data for ER and PR was
categorized into groups as either positive or negative, a positive
score was defined as ≥1% of tumour cells showing nuclear staining.
A high cell proliferation was defined as ≥20% of Ki-67-positive
tumour cells.
Examination of claudins and cadherins was performed
either on whole-tissue sections from the FFPE tissue blocks, which
were core needle biopsies and samples containing minimal residual
tumour tissue insufficient for the implementation of TMA, or on
TMAs. The tissue sections (4 µm) were deparaffinised in xylene at
room temperature and rehydrated in a graded alcohol series. Antigen
retrieval was performed using the heat-induced epitope retrieval
technique with a citrate buffer (pH 6.0 for claudin-3 and −4; pH
9.0 for claudin-1, E-cadherin and N-cadherin) at 98°C for 40 min.
Endogenous peroxidase was quenched by 3% hydrogen peroxide solution
in methanol at room temperature for 20 min prior to incubation with
the primary antibodies: Polyclonal rabbit anti-human anti-claudin-1
(1:100; Cell Marque Corporation; 359A) and anti-claudin-3 (1:800;
Abcam; ab15102), polyclonal goat anti-human anti-claudin-4 (1:100;
Santa Cruz Biotechnology, Inc.; sc-17664), monoclonal mouse
anti-human anti-E-cadherin (1:100; Thermo Fisher Scientific, Inc.;
18-0223) and anti-N-cadherin (1:300; Agilent Technologies, Inc.;
M3613). After that, slides for claudin-1, claudin-3 and E-cadherin
were incubated in N-Histofine Simple Stain Max-Peroxidase (multi)
(original dilution by manufacturer, Nichirei Biosciences Inc.;
41415) and for claudin-4 in N-Histofine Simple Stain Max-Peroxidase
(g) (original dilution by manufacturer, Nichirei Biosciences Inc.;
41416) for 30 min and then in chromogen DAB-3S (original dilution
by manufacturer, Nichirei Biosciences Inc.; 415194S) for 5 min at
room temperature. Slides for N-cadherin were stained by the kit
EnVision+ System-HRT (original dilution by manufacturer; Agilent
Technologies Inc.; K4006) according to the manufacturers'
instructions. Different semi-quantitative scales were used to
evaluate the expression of the claudins and cadherins in order to
distinguish particular subtypes with a possible impact on tumour
biology and/or response to chemotherapy. The evaluation of the
expression of claudins was based on a combined score, as previously
described (29,30): Scores 0–3 were used to classify the
percentage of positive tumour cells (0, 0%; 1, <25%; 2, 25–50%;
3, >50%) and the intensity of membrane staining (0, 0; 1, 1+; 2,
2+; 3,3+). These two scores were subsequently multiplied. In the
resulting overall score of 0–9, 0 was considered as negative, 1 or
2 as weakly positive, 3–6 as moderately positive and 9 as strongly
positive. Negative and weak positive staining were designated as
low expression, while moderate and strong positivity were
designated as high expression. For the identification of tumours
that could be classified as either molecular claudin-low or -high,
the IHC criteria suggested in previous studies were used (4,31,32). The
criteria for the claudin-low subgroup included triple negativity
and a low or absent expression of at least two of four of the
following intercellular junction proteins: Claudin-3, −4, −7 and
E-cadherin. Previous studies have added low or absent expression of
claudin-1 to these criteria (31,32). The
criteria for the claudin-high subgroup included ER negativity and a
high expression of claudin-1 and −4 (4). A three-tier scale was applied for the
evaluation of E-cadherin (0, 0%; 1, <70%; 2, ≥70%). A strong
membrane positivity in >70% of tumour tissue (score 2) was
considered as normal, lower positivity (score 1) or negativity
(score 0) were considered as aberrant (33). A four-tier scale was applied for the
evaluation of N-cadherin (0, 0%; 1, <25%; 2, 25–50%; 3,
>50%). The result was considered as positive when >1% of the
tumour cells exhibited membrane staining.
Statistical analysis
STATISTICA software (version 10; StatSoft, Inc.) was
used for data analysis. The Wilcoxon signed-rank test was used to
evaluate the differences in the expression of the markers (ordered
categorical) of interest in the paired tumour samples (prior and
following treatment). The χ2 test was used to reveal
associations between pathological characteristics (dichotomous
variables; Tables SI–III). All data used for statistical
calculations are in Table SIV.
However, the sample set in the current study is limited in size and
in the number of cases in the respective categories. Therefore, the
outcome of the statistical analysis should be interpreted with
caution. All tests were two-sided, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of the expression of
observed markers before and after therapy
The IHC assessment (Table SIV) of the paired tumour samples
(before and after chemotherapy) from 62 female patients with
invasive breast carcinoma NST revealed a lower expression of PR
(median before-/after treatment 25/2; Z=3.7; P<0.001) and Ki-67
(median 25/10; Z=2.7; P=0.01) following chemotherapy. No
significant differences were observed in the expression of
E-cadherin, N-cadherin, ER or HER2, or in the frequency of TNBC
(P>0.05) when comparing tumours prior to and following
treatment. In 4 cases, the expression of HER2 changed from negative
to positive, and in 3 of these the positive IHC result of 2+ was
confirmed by FISH (the remaining tumour had the positive result of
3+ determined by IHC; Fig. S1). The
tumour grade was not significantly affected following therapy
(P>0.05). The assessment of the histological grade of residual
tumours was often limited by the small amount of residual tumour
tissue and/or the cytopathic effects of the drugs. The current
study included a sample set made up of patients chosen for
neoadjuvant chemotherapy, and therefore it included tumours of a
higher grade when compared with other studies that analysed cohorts
of unsorted patients with BC (34).
The residual tumours following chemotherapy displayed either strong
or minor regressive changes with fibrosis corresponding to
Chevallier class III (48/62; 77%) or class IV (14/62; 23%),
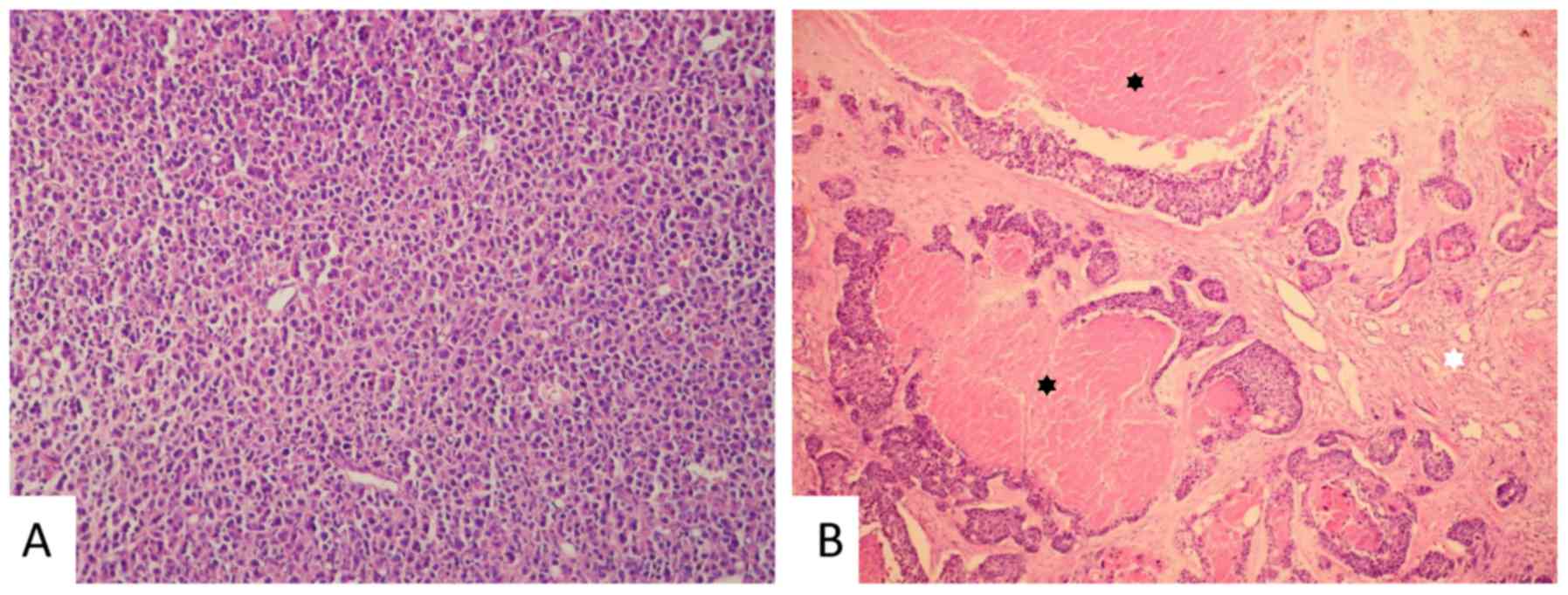
respectively (Fig. 1).
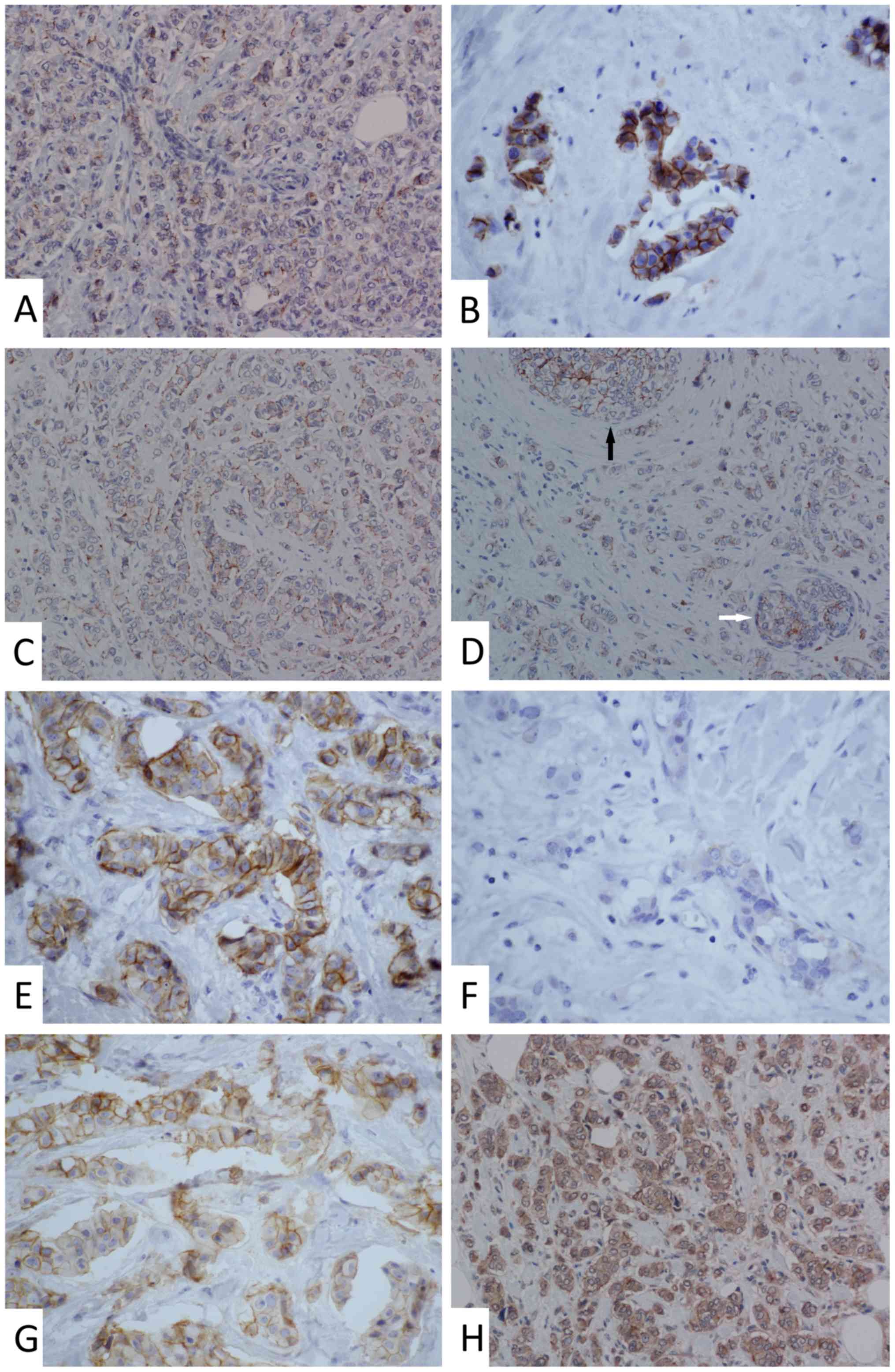
Membrane staining of claudin-1 was punctate and less
intense when compared with the more continuous moderate-to-strong
staining of claudin-3 and −4 found in the majority of the samples.
Cytoplasmic and nuclear staining of claudin-1 appeared in
approximately one-third of the samples. In ~20% of samples,
cytoplasmic staining of claudin-3 and −4 was observed. The
apicolateral polarity of claudin expression, which is common in
non-tumour breast epithelium (8),
was absent in the tumour tissue for all three examined claudins
(Fig. S2). The expression of
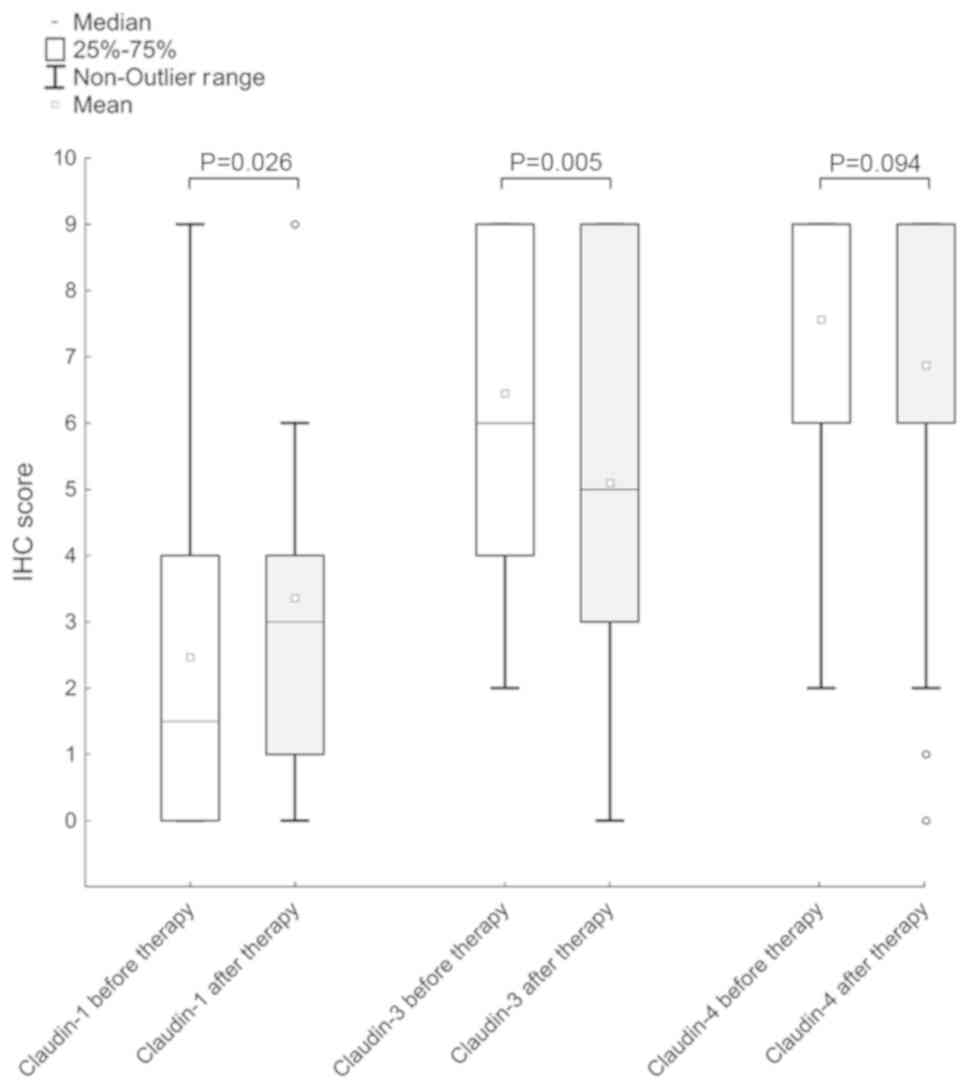
claudin-1 increased following neoadjuvant chemotherapy (median
before-/after treatment 1.5/3; Z=2.2; P=0.03), but a decrease in
claudin-3 expression was observed (median 6/5; Z=2.8; P=0.005).
Furthermore, a reduction in the expression of claudin-4 following
chemotherapy was observed (mean, 7.56 vs. 6.87), but this
difference was not statistically significant (P>0.05; Figs. 2 and 3). Table II
summarizes simplified dichotomously categorized results from IHC
analysis of expression of claudins and other observed markers.
 | Table II.Immunohistochemical characteristics
of invasive breast carcinoma prior and following therapy. |
Table II.
Immunohistochemical characteristics
of invasive breast carcinoma prior and following therapy.
| Marker | Pre therapy, n
(%) | Post therapy, n
(%) |
|---|
| Claudin-1 |
|
High | 25 (40) | 34 (55) |
|
Low | 37 (60) | 28 (45) |
| Claudin-3 |
|
High | 59 (95) | 51 (82) |
|
Low | 3 (5) | 11 (18) |
| Claudin-4 |
|
High | 61 (98) | 58 (94) |
|
Low | 1 (2) | 4 (6) |
| E-cadherin |
|
Normal | 47 (76) | 44 (71) |
|
Aberrant | 15 (24) | 18 (29) |
| N-cadherin |
|
Negative | 47 (76) | 48 (77) |
|
Positive | 15 (24) | 14 (23) |
| ER |
|
Positive | 45 (73) | 48 (77) |
|
Negative | 17 (27) | 14 (23) |
| PR |
|
Positive | 39 (63) | 35 (56) |
|
Negative | 23 (37) | 27 (44) |
| HER2 |
|
Positive | 8 (13) | 12 (19) |
|
Negative | 54 (87) | 50 (81) |
| Ki-67 |
|
High | 43 (70) | 25 (40) |
|
Low | 19 (30) | 37 (60) |
| Triple-negative
breast cancer | 12 (19) | 10 (16) |
No tumour tissues with low expression of all three
claudins (claudin-1, −3 and −4) prior to therapy were observed in
the current study; however, four such cases (4/62; 6%) were
observed following therapy, all with simultaneous loss of
E-cadherin. Only one of these cases was TNBC and matched the
suggested criteria for IHC identification of claudin-low subtype.
Another case of TNBC in the post-therapy cohort (1/62; 2%) with low
expression of claudin-1 and −3 and reduced E-cadherin expression
matched the criteria only partially, as the present study did not
include the analysis of claudin-7. There were 11 cases (11/62; 18%)
pre- and 10 cases (10/62; 16%) post-therapy that matched the
suggested criteria for the claudin-high subtype. Upregulation of
HER2 was observed in 2 (2/11; 17%) claudin-high tumours pre- and in
1 tumour (1/10; 6%) post-therapy.
Association of the observed markers. A statistical
analysis of the association of the expression of claudins and
cadherins with other variables was performed (Tables SI–III). However, the results have to be
interpreted with caution due to the limited sample set. A negative
association between the expression of claudin-1 and ER
[χ2=5.79; degrees of freedom (df)=1; P=0.02] was
observed in tumours prior to chemotherapy but not in the residual
tumour tissue following neoadjuvant chemotherapy. Claudin-1 was
inversely associated with PR (χ2=4.66; df=1; P=0.03) and
HER2 (χ2=5.35; df=1; P=0.02) in the residual tumours
following therapy, but not in the core biopsies prior to therapy.
TNBC was associated with a high expression of claudin-1 in tumours
prior to (χ2=4.29; df=1; P=0.04) and following therapy
(χ2=5.95; df=1; P=0.02). High expression of claudin-1
was only associated with a high Ki-67 expression following therapy
(χ2=4.98; df=1; P=0.03). No association between
claudin-1 and either N-cadherin or E-cadherin was observed
(P>0.05; Table SI).
Following therapy, the expression of claudin-3 was
positively associated with the expression of E-cadherin
(χ2=7.77; df=1; P=0.005). No association between
claudin-3 and the standard BC biomarkers or N-cadherin was observed
(P>0.05). A statistical evaluation for claudin-3 before therapy
and claudin-4 in both sample sets was not performed due to the
unequal distribution of data in the cohorts with high and low
expression. Low expression of claudin-3 was only observed in 3
cases and low expression of claudin-4 was observed in 1 case before
therapy and 4 cases after therapy (Table SII).
The expression of N-cadherin had a negative
association with ER (χ2=6.68; df=1; P=0.01) and PR
expression (χ2=4.45; df=1; P=0.04) in tumours before
therapy and a positive association with HER2 (χ2=6.40;
df=1; P=0.01) in tumours after therapy. Positive N-cadherin
expression was more frequently observed in tumours with a higher
grade both before (χ2=4.45; df=1; P=0.04) and after
therapy (χ2=6.46; df=1; P=0.01). An association between
the expression of E-cadherin and standard BC biomarkers was not
observed (P>0.05). Interestingly, no association between the
expression of E-cadherin and N-cadherin was observed (P>0.05).
Although reduction or loss of E-cadherin was observed in 15 tumors
before (15/62, 24%) and 18 after therapy (18/62, 29%), and
N-cadherin positivity was observed in 15 tumors before (15/62, 24%)
and 14 after therapy (14/62, 23%), both of these features
simultaneously were detected only in 5 tumours pre- (5/62; 8%) and
4 post- (4/62; 6%) therapy (Table
SIII).
A larger extent of tumour regression after therapy
(Chevallier class III) was associated with higher Ki-67 before
treatment (χ2=5.97; df=1; P=0.02), when considering only
the histological characteristics of the primary tumour and not
other clinicopathological data (data not shown). Other markers did
not show any association with the histologically assessed tumour
regression after therapy (P>0.05). However, this finding was not
conclusive as tumours with a regression of Chevallier class I and
II were not included in the current study due to the absence of
invasive cancer in these samples.
Discussion
Claudins are involved in carcinogenesis and cancer
progression. Their involvement in the EMT and response to
chemotherapy has previously been investigated (12,20–22,26).
However, their exact role and relevant regulatory mechanisms remain
unclear. The expression pattern of several members of the claudin
family has been described in numerous types of tumours including
breast, ovarian, pancreatic or prostate cancer and can be employed
in the diagnostic process namely in gynecological and renal
carcinomas or mesothelioma (29,35,36).
Analysis of claudin expression has been suggested to enhance the
molecular classification of BC and may therefore affect the
indication for chemotherapy in the future, although the use of
molecular classification in clinical practice remains questionable
(1,6). A large number of patients with BC
selected for neoadjuvant therapy receive chemotherapy twice during
treatment. The selective pressure of chemotherapy on cancer cells
may change the expression profile of a tumour and thereby lead to a
loss of sensitivity to anticancer drugs (37,38).
Previous studies have compared the expression of the
standard IHC BC markers in diagnostic core needle biopsies and
surgical specimens in patients who did and did not receive
neoadjuvant chemotherapy. In patients who did not receive
neoadjuvant chemotherapy, a high concordance was found for all four
markers (39–41). However, the majority of studies
focusing on patients after chemotherapy revealed substantial
changes in these markers (42–51). We
observed a decrease of cell proliferation (Ki-67) and PR
expression, and only insignificant changes of ER after therapy.
This result is in accordance with previous studies that reported
similar IHC evaluation of the expression of standard BC markers
(43,49–51). The
decrease of Ki-67 expression after chemotherapy may be due to the
antiproliferative effects of common anticancer drugs (47,48,52). The
data obtained in the current study suggested that HER2 expression
was unchanged after chemotherapy, which was also reported in a
previous study (42). However, this
finding is not in agreement with previous studies that described
either downregulation (45) or
upregulation of HER2 after therapy (53). In other studies, the IHC evaluation
of the HER2 status pre- and post- therapy revealed stronger
discrepancies, while the status of gene amplification assessed by
FISH was reported as rather stable (44,46). In
the present study, both techniques (IHC and FISH) were used
according to the American Society of Clinical Oncology guidelines
(54), with concordant results.
However, in certain tumours the areas of HER2 overexpression are
only focal and might be missed in the core needle biopsy (55), which may result in the ambiguity of
the results reported in literature.
The present study revealed a significant
upregulation of claudin-1 and a downregulation of claudin-3 after
chemotherapy. Furthermore, claudin-4 expression was downregulated
but not significantly. The expression of claudin-1 is frequently
decreased or lost in cancer cells when compared with the luminal
cells of non-tumour breast tissue (30), a feature that was also observed in
the current study. Claudin-1 may act as a tumour suppressor or
tumour enhancer, depending on cancer type and other not yet well
understood conditions (12,26). As the role of claudin-1 is possibly
not limited to tight junctions, cytoplasmic or nuclear expression
is a common finding (26,56). As a tumour suppressor, its reduced
expression may facilitate the EMT and collective migration and may
contribute to chemoresistance (26,57,58).
However, the current study revealed an increased expression of
claudin-1 after therapy, suggesting that other mechanisms may be
involved. Our data also showed an association between high
expression of claudin-1 and increased rates of cell
proliferation.
In the current study, a high expression of claudin-1
was more frequently observed in TNBC irrespective of the
administered treatment, which is in concordance with studies that
described a higher expression of claudin-1 in basal-like BC, which
includes mainly TNBC (30,59). Furthermore, the data obtained in the
current study suggested that a high expression of claudin-1 was
more common in ER-negative breast tumours before treatment, and in
PR-negative or HER2-negative tumours after treatment. A higher
expression of claudin-1 in ER-negative compared with ER-positive BC
has been previously described (26,60).
Additionally, in the current study, positive expression of
N-cadherin was more frequently observed in ER- or PR-negative BC
prior to treatment, and in HER2-positive BC following treatment.
The upregulation of N-cadherin contributes to the invasive
phenotype and metastatic potential of moderately-to-poorly
differentiated breast carcinomas, which often lose the expression
of hormonal receptors and/or overexpress HER2 (61,62). The
current study did not reveal an association between claudin-1 and
N-cadherin expression, despite the fact that they share some common
features in relation to standard BC markers, namely, expression of
ER and PR.
The expression of claudin-3 and −4 is high in the
luminal cells of non-tumour breast tissue, and typically remains
high in BC (8–11). Concordantly, a high expression of
these claudins was found in the majority of breast tumour samples
in the current study, despite a decrease in expression after
chemotherapy. Claudin-3 and −4 are considered to maintain the
epithelial phenotype of epithelial cells by modulating the
expression of major EMT proteins, specifically by maintaining the
expression of E-cadherin (21). The
results obtained in the current study suggest an association
between the expression of E-cadherin and claudin-3.
Considering the interactions between claudins and
cadherins, the reduced expression of claudin-3 and −4 in neoplastic
cells may increase the resistance to chemotherapy. The results
obtained in the current study support this theory, as a reduction
of the expression of both these markers after therapy was observed.
However, previous studies reported conflicting results and may be
difficult to interpret in relation to the current study as the
majority of those studies focused on ovarian cancer and
platinum-based chemotherapy (57,58,63).
While platinum-based chemotherapy may be used in the treatment of
BC, drugs such as anthracyclines and taxanes are more commonly
indicated. Nevertheless, a summary of the aforementioned studies
suggests the possibility that a reduced expression of claudin-3 and
−4 increases the resistance to chemotherapy, although the influence
of factors such as the type of cancer or chemotherapy used cannot
be excluded (12).
Despite the fact that high expression of claudin-3,
claudin-4 and E-cadherin is common in invasive breast carcinoma
NST, their association with standard BC markers remains unclear.
Previous studies detected a slightly higher expression of claudin-3
in ER-positive compared with ER-negative tumours, a higher
expression of claudin-4 in ER-negative and basal-like tumours
(TNBC) and a more frequent aberrant expression of E-cadherin in
ER-negative tumours. However, these associations were not observed
in other studies (30,33,60,64,65). The
data obtained in the current study were insufficient for
statistical evaluation of the association between claudin-4 and any
other marker, and only partially sufficient for the evaluation of
claudin-3. No associations between claudin-3 and the hormonal
receptors, HER2 and Ki-67 were observed. Similarly, E-cadherin was
not found to be associated with any of the standard BC markers.
Claudin-low BC is more frequent among residual
tumours after chemotherapy, which is also supported by our
experience from the current study (12,66).
However, the suggested IHC criteria for the identification of this
subgroup do not fully overlap with the molecular claudin-low
subtype. Since earlier studies presented claudin-low tumours as
mostly TNBC, this feature has been included in the IHC criteria
(5,15). Later, however, it was demonstrated
that this molecular subtype contains a proportion of ER-positive
and non-TNBC tumours, suggesting a large heterogeneity of this
subtype (66). The relevance of the
claudin-low and -high subgroups remains to be established (1,6).
Assessment of the tumour grade post-chemotherapy has
its limitations and must be considered with caution. The results
obtained in the current study do not indicate marked changes in
tumour differentiation after therapy. The only marker from the
studied proteins that reliably associated with tumour grade was
N-cadherin, the expression of which is associated with increased
invasiveness of poorly differentiated tumours (61,62). The
results obtained in the current study did not reveal an association
between tumour grade and E-cadherin, the expression of which is
more frequently reduced in poorly differentiated carcinomas when
compared with well-differentiated invasive breast carcinomas NST
(62,67). However, this feature may have been
obscured by the limited number of well-differentiated tumours
(grade 1) in the current study.
Previous studies reported a wide range (0–45%) of
aberrant E-cadherin expression in invasive breast carcinoma NST
(33,62,67).
Positive expression of N-cadherin in invasive breast carcinoma NST
reaching 50% has been reported, which is twice that observed in the
current study; however, the studies reporting this only took into
account moderately and/or poorly differentiated tumours (61,62). The
involvement of both cadherins in the chemotherapy response may be
due to their role in the EMT (68,69). The
downregulation of E-cadherin and the upregulation of N-cadherin
increase chemoresistance (68,69).
However, in the current study, the changes in expression of the two
cadherins following the therapy were not statistically significant.
The upregulation of N-cadherin does not have to be accompanied by
the downregulation of E-cadherin, despite the already described
phenomenon of cadherin switch (24,25).
Furthermore, a significant dependence was not observed in the
current study. Although E-cadherin downregulation was observed in
approximately one-quarter of the examined tumours, and N-cadherin
positive expression was observed in a similar proportion of the
tumours, only one-third of the tumours exhibited these features
simultaneously.
In summary, the current study described significant
changes in the expression of claudin-1 and −3 but not in the
expression of claudin-4, E- and N-cadherin in BC following
chemotherapy. Moreover, the current study revealed a number of
associations between the expressed markers, recently described in
other studies (21,26,30,59,60–62),
which suggested that such phenomena may frequently occur in BC. The
present study revealed that high expression of claudin-1 was
observed more frequently in ER- and triple-negative tumours. The
association of claudin-1 expression with Ki-67 and HER2 requires
further investigation. The association between claudin-3 and
E-cadherin corresponds with their role in maintaining epithelial
phenotype. The higher frequency of N-cadherin positive expression
in poorly differentiated tumours corresponded with the loss of
hormonal receptors and HER2 upregulation. The current study was
limited by the value of the statistical analyses performed due to
the small sample size. Further validations on larger cohorts of
patients are required to elucidate the underlying regulatory
mechanisms.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mr. Zachary H. K.
Kendall (Institute for History of Medicine and Foreign Languages,
First Faculty of Medicine, Charles University, Prague, Czech
Republic) for the English language corrections.
Funding
This study was supported by the Ministry of Health
(Conceptual Development of Research Organization 64165, General
University Hospital in Prague, Prague, Czech Republic), by Charles
University (Project Progress Q28/LF1, UNCE 204065 and SVV 260367),
by the European Regional Development Fund (project no. BBMRI-CZ;
grant no. EF16_013/0001674) and by European Regional Developmental
Fund, Operational Program Prague-Competitiveness (Research
Laboratory of tumour Diseases; grant no. CZ.2.16/3.1.00/24509).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and CP conceived and designed the study. HS, NH,
IT, MB and BM collected patient material and clinical data,
evaluated the data and performed the analyses. HS, IT and MB
drafted the manuscript. MB and CP proofread the manuscript. HS, NH,
BM, MB, CP and IT critically reviewed the manuscript and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the General University Hospital in Prague (Prague,
Czech Republic) in compliance with the Helsinki Declaration.
Additional informed consent signed by patients was not required as
the project was approved by Ethics Committee and the data was used
for scientific purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ER
|
oestrogen receptor
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
FISH
|
fluorescence in situ hybridization
|
|
IHC
|
immunohistochemistry
|
|
NST
|
(invasive breast carcinoma of) no
special type
|
|
PR
|
progesterone receptor
|
|
TMA
|
tissue microarray
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO classification of tumours of the
breastFourth. IARC; Lyon: 2012
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumour subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Myal Y, Leygue E and Blanchard AA: Claudin
1 in breast tumorigenesis: Revelation of a possible novel ‘claudin
high’ subset of breast cancers. J Biomed Biotechnol.
2010:9568972010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis IO, Carder P, Hales S, Lee A, Pinder
S, Rakha E and Stephenson T: Pathology reporting of breast disease
in surgical excision speciemens incorporating tha dataset for
histological reporting of breast cancer. Royal College of
Pathologists. 2016.
|
|
7
|
Mineta K, Yamamoto Y, Yamazaki Y, Tanaka
H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K and
Tsukita S: Predicted expansion of the claudin multigene family.
FEBS Lett. 585:606–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kulka J and Tökés AM: Claudin expression
in breast tumours. Hum Pathol. 36:859–860. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding L, Lu Z, Lu Q and Chen YH: The
claudin family of proteins in human malignancy: A clinical
perspective. Cancer Manag Res. 5:367–375. 2013.PubMed/NCBI
|
|
10
|
Turksen K and Troy TC: Junctions gone bad:
Claudins and loss of the barrier in cancer. Biochim Biophys Acta.
1816:73–79. 2011.PubMed/NCBI
|
|
11
|
Singh AB, Sharma A and Dhawan P: Claudin
family of proteins and cancer: An overview. J Oncol.
2010:5419572010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon MJ: Emerging roles of claudins in
human cancer. Int J Mol Sci. 14:18148–18180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herschkowitz JI, Simin K, Weigman VJ,
Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S,
Chandrasekharan S, et al: Identification of conserved gene
expression features between murine mammary carcinoma models and
human breast tumors. Genome Biol. 8:R762007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buchholz TA, Hunt KK, Whitman GJ, Sahin AA
and Hortobagyi GN: Neoadjuvant chemotherapy for breast carcinoma:
Multidisciplinary considerations of benefits and risks. Cancer.
98:1150–1160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saeki R, Kondoh M, Kakutani H, Tsunoda S,
Mochizuki Y, Hamakubo T, Tsutsumi Y, Horiguchi Y and Yagi K: A
novel tumour-targeted therapy using a claudin-4-targeting molecule.
Mol Pharmacol. 76:918–926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walther W, Petkov S, Kuvardina ON, Aumann
J, Kobelt D, Fichtner I, Lemm M, Piontek J, Blasig IE, Stein U and
Schlag PM: Novel Clostridium perfringens enterotoxin suicide gene
therapy for selective treatment of claudin-3- and −4-overexpressing
tumors. Gene Ther. 19:494–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morin PJ: Claudin proteins in human
cancer: Promising new targets for diagnosis and therapy. Cancer
Res. 65:9603–9606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh AB and Dhawan P: Claudins and
cancer: Fall of the soldiers entrusted to protect the gate and keep
the barrier intact. Semin Cell Dev Biol. 42:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osanai M, Takasawa A, Murata M and Sawada
N: Claudins in cancer: Bench to bedside. Pflugers Arch. 469:55–67.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin X, Shang X, Manorek G and Howell SB:
Regulation of the epithelial-mesenchymal transition by claudin-3
and claudin-4. PLoS One. 8:e674962013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieman MT, Prudoff RS, Johnson KR and
Wheelock MJ: N-cadherin promotes motility in human breast cancer
cells regardless of their E-cadherin expression. J Cell Biol.
147:631–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rai H and Ahmed J: N-cadherin: A marker of
epithelial to mesenchymal transition in tumour progression.
Internet J Oncol. 10:1–8. 2014.
|
|
26
|
Zhou B, Moodie A, Blanchard AA, Leygue E
and Myal Y: Claudin 1 in breast cancer: New insights. J Clin Med.
4:1960–1976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baccelli I and Trumpp A: The evolving
concept of cancer and metastasis stem cells. J Cell Biol.
198:281–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chevallier B, Roche H, Olivier JP, Chollet
P and Hurteloup P: Inflammatory breast cancer. Pilot study of
intensive induction chemotherapy (FEC-HD) results in a high
histologic response rate. Am J Clin Oncol. 16:223–228. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lechpammer M, Resnick MB, Sabo E,
Yakirevich E, Greaves WO, Sciandra KT, Tavares R, Noble LC,
DeLellis RA and Wang LJ: The diagnostic and prognostic utility of
claudin expression in renal cell neoplasms. Mod Pathol.
21:1320–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu S, Singh K, Mangray S, Tavares R, Noble
L, Resnick MB and Yakirevich E: Claudin expression in high-grade
invasive ductal carcinoma of the breast: Correlation with the
molecular subtype. Mod Pathol. 26:485–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dias K, Dvorkin-Gheva A, Hallett RM, Wu Y,
Hassell J, Pond GR, Levine M, Whelan T and Bane AL: Claudin-low
breast cancer; Clinical & pathological characteristics. PLoS
One. 12:e01686692017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerhard R, Ricardo S, Albergaria A, Gomes
M, Silva AR, Logullo ÂF, Cameselle-Teijeiro JF, Paredes J and
Schmitt F: Immunohistochemical features of claudin-low intrinsic
subtype in metaplastic breast carcinomas. Breast. 21:354–360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kowalski PJ, Rubin MA and Kleer CG:
E-cadherin expression in primary carcinomas of the breast and its
distant metastases. Breast Cancer Res. 5:R217–R222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dewis R and Gribbin J: Breast cancer:
Diagnosis and treatment: An assessment of needNational
Collaborating Centre for Cancer (UK); Cardiff, UK: 2009
|
|
35
|
Ordóñez NG: Value of claudin-4
immunostaining in the diagnosis of mesothelioma. Am J Clin Pathol.
139:611–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szabó I, Kiss A, Schaff Z and Sobel G:
Claudins as diagnostic and prognostic markers in gynecological
cancer. Histol Histopathol. 24:1607–1615. 2009.PubMed/NCBI
|
|
37
|
Worsley CM, Mayne ES and Veale RB: Clone
war: The evolution of therapeutic resistance in cancer. Evol Med
Public Health. 2016:180–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun D, Dalin S, Hemann MT, Lauffenburger
DA and Zhao B: Differential selective pressure alters rate of drug
resistance acquisition in heterogeneous tumor populations. Sci Rep.
6:361982016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Asogan AB, Hong GS and Arni Prabhakaran
SK: Concordance between core needle biopsy and surgical specimen
for oestrogen receptor, progesterone receptor and human epidermal
growth factor receptor 2 status in breast cancer. Singapore Med J.
58:145–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dekker TJ, Smit VT, Hooijer GK, Van de
Vijver MJ, Mesker WE, Tollenaar RA, Nortier JW and Kroep JR:
Reliability of core needle biopsy for determining ER and HER2
status in breast cancer. Ann Oncol. 24:931–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
You K, Park S, Ryu JM, Kim I, Lee SK, Yu
J, Kim SW, Nam SJ and Lee JE: Comparison of core needle biopsy and
surgical specimens in determining intrinsic biological subtypes of
breast cancer with immunohistochemistry. J Breast Cancer.
20:297–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kinsella MD, Nassar A, Siddiqui MT and
Cohen C: Estrogen receptor (ER), progesterone receptor (PR), and
HER2 expression pre- and post-neoadjuvant chemotherapy in primary
breast carcinoma: A single institutional experience. Int J Clin Exp
Pathol. 5:530–536. 2012.PubMed/NCBI
|
|
43
|
Yin HF, Wang YH, Qin XQ, Zhang H, Li T, Ye
JM and Liu YH: Effect of neoadjuvant chemotherapy on histologic
grade and expression of biological markers in breast cancer.
Zhonghua Zhong Liu Za Zhi. 31:858–862. 2009.(In Chinese).
PubMed/NCBI
|
|
44
|
van de Ven S, Smit VT, Dekker TJ, Nortier
JW and Kroep JR: Discordances in ER, PR and HER2 receptors after
neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev.
37:422–430. 2011.PubMed/NCBI
|
|
45
|
Yoshida A, Hayashi N, Suzuki K, Takimoto
M, Nakamura S and Yamauchi H: Change in HER2 status after
neoadjuvant chemotherapy and the prognostic impact in patients with
primary breast cancer. J Surg Oncol. 116:1021–1028. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li P, Liu T, Wang Y, Shao S, Zhang W, Lv
Y, Yi J and Wang Z: Influence of neoadjuvant chemotherapy on
HER2/neu status in invasive breast cancer. Clin Breast Cancer.
13:53–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cabrera-Galeana P, Muñoz-Montaño W,
Lara-Medina F, Alvarado-Miranda A, Pérez-Sánchez V,
Villarreal-Garza C, Quintero RM, Porras-Reyes F, Bargallo-Rocha E,
Del Carmen I, et al: Ki67 Changes identify worse outcomes in
residual breast cancer tumors after neoadjuvant chemotherapy.
Oncologist. 23:670–678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moazed V, Jafari E, Kalantari Khandani B,
Nemati A, Roozdar A and Ben Razavi SA: Prognostic significance of
reduction in Ki67 index after neoadjuvant chemotherapy in patients
with breast cancer in kerman between 2009 And 2014. Iran J Pathol.
13:71–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dede DS, Gumuskaya B, Guler G, Onat D,
Altundag K and Ozisik Y: Evaluation of changes in biologic markers
ER, PR, HER 2 and Ki-67 index in breast cancer with administration
of neoadjuvant dose dense doxorubicin, cyclophosphamide followed by
paclitaxel chemotherapy. J BUON. 18:366–371. 2013.PubMed/NCBI
|
|
50
|
Lee HC, Ko H, Seol H, Noh DY, Han W, Kim
TY, Im SA and Park IA: Expression of immunohistochemical markers
before and after neoadjuvant chemotherapy in breast carcinoma, and
their use as predictors of response. J Breast Cancer. 16:395–403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou X, Zhang J, Yun H, Shi R, Wang Y,
Wang W, Lagercrantz SB and Mu K: Alterations of biomarker profiles
after neoadjuvant chemotherapy in breast cancer: Tumor
heterogeneity should be taken into consideration. Oncotarget.
6:36894–36902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yoshioka T, Hosoda M, Yamamoto M, Taguchi
K, Hatanaka KC, Takakuwa E, Hatanaka Y, Matsuno Y and Yamashita H:
Prognostic significance of pathologic complete response and Ki67
expression after neoadjuvant chemotherapy in breast cancer. Breast
Cancer. 22:185–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Adams AL, Eltoum I, Krontiras H, Wang W
and Chhieng DC: The effect of neoadjuvant chemotherapy on
histologic grade, hormone receptor status, and HER2/neu status in
breast carcinoma. Breast J. 14:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, McShane LM and Dowsett M: HER2 testing in breast cancer:
American society of clinical oncology/college of American
pathologists clinical practice guideline focused update summary. J
Oncol Pract. 14:437–441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Davila E and Amazon K: The clinical
importance of the heterogeneity of HER2 neu. Case Rep Oncol.
3:268–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dhawan P, Singh AB, Deane NG, No Y, Shiou
SR, Schmidt C, Neff J, Washington MK and Beauchamp RD: Claudin-1
regulates cellular transformation and metastatic behavior in colon
cancer. J Clin Invest. 115:1765–1776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fortier AM, Asselin E and Cadrin M:
Keratin 8 and 18 loss in epithelial cancer cells increases
collective cell migration and cisplatin sensitivity through
claudin1 up-regulation. J Biol Chem. 288:11555–11571. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li M, Balch C, Montgomery JS, Jeong M,
Chung JH, Yan P, Huang TH, Kim S and Nephew KP: Integrated analysis
of DNA methylation and gene expression reveals specific signaling
pathways associated with platinum resistance in ovarian cancer. BMC
Med Genomics. 2:342009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Blanchard AA, Ma X, Dueck KJ, Penner C,
Cooper SC, Mulhall D, Murphy LC, Leygue E and Myal Y: Claudin 1
expression in basal-like breast cancer is related to patient age.
BMC Cancer. 13:2682013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Blanchard AA, Skliris GP, Watson PH,
Murphy LC, Penner C, Tomes L, Young TL, Leygue E and Myal Y:
Claudins 1, 3, and 4 protein expression in ER negative breast
cancer correlates with markers of the basal phenotype. Virchows
Arch. 454:647–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Qian X, Anzovino A, Kim S, Suyama K, Yao
J, Hulit J, Agiostratidou G, Chandiramani N, McDaid HM, Nagi C, et
al: N-cadherin/FGFR promotes metastasis through
epithelial-to-mesenchymal transition and stem/progenitor cell-like
properties. Oncogene. 33:3411–3421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
ElMoneim HM and Zaghloul NM: Expression of
E-cadherin, N-cadherin and snail and their correlation with
clinicopathological variants: An immunohistochemical study of 132
invasive ductal breast carcinomas in Egypt. Clinics (Sao Paulo).
66:1765–1771. 2011.PubMed/NCBI
|
|
63
|
Shang X, Lin X, Manorek G and Howell SB:
Claudin-3 and claudin-4 regulate sensitivity to cisplatin by
controlling expression of the copper and cisplatin influx
transporter CTR1. Mol Pharmacol. 83:85–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kulka J, Szász AM, Németh Z, Madaras L,
Schaff Z, Molnár IA and Tokés AM: Expression of tight junction
protein claudin-4 in basal-like breast carcinomas. Pathol Oncol
Res. 15:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Soini Y: Claudins 2, 3, 4, and 5 in
Paget's disease and breast carcinoma. Hum Pathol. 35:1531–1536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sabatier R, Finetti P, Guille A, Adelaide
J, Chaffanet M, Viens P, Birnbaum D and Bertucci F: Claudin-low
breast cancers: Clinical, pathological, molecular and prognostic
characterization. Mol Cancer. 13:2282014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Singhai R, Patil VW, Jaiswal SR, Patil SD,
Tayade MB and Patil AV: E-Cadherin as a diagnostic biomarker in
breast cancer. N Am J Med Sci. 3:227–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang W, Wang L, Mizokami A, Shi J, Zou C,
Dai J, Keller ET, Lu Y and Zhang J: Down-regulation of E-cadherin
enhances prostate cancer chemoresistance via Notch signaling. Chin
J Cancer. 36:352017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nakamura T, Kato Y, Fuji H, Horiuchi T,
Chiba Y and Tanaka K: E-cadherin-dependent intercellular adhesion
enhances chemoresistance. Int J Mol Med. 12:693–700.
2003.PubMed/NCBI
|