Introduction
Psoriasis (Ps) is a chronic inflammatory
immune-mediated disease with skin and joint manifestations
(1). Histologically it is
characterized by abnormal and rapid proliferation of keratinocytes
and infiltration of psoriatic lesions with immune cells, especially
T cells and dendritic cells (DC) (2). Globally, Ps affects 2–4% of the adult
population, especially Caucasians, women and men equally, and
0.1–1% of children; prevalence is dependent on age, ethnicity,
geographic area, and environmental factors (3). It can start at any age, from childhood
to old age, but 75% of those affected developed the disease before
the age of 40 years. Psoriatic arthritis affects up to 30% of
patients diagnosed with Ps (children and adults) and approximately
50% of people who develop Ps observe changes in the nails (from
fingers and/or toes). Ps is associated with several co-morbidities
which include diabetes mellitus (4),
obesity (5), hypertension (6), and cardiovascular diseases (7). Due to visible lesions, Ps reduces the
self-esteem of patients and leads to a decrease in quality of life,
depression and suicidal ideations (8).
Ps has a multi-factorial aetiology with important
immunologic, genetic and environmental components (9). The main risk factors are ultraviolet
(UV) exposure (10), medications
(11,12), smoking (13), diet and obesity (14), alcohol intake (15), infections (16), and stress (17). It may occur in mild, moderate or
severe forms, with lesions varying in appearance depending on the
type of Ps: plaque (90%), guttate, pustular, inverse, and
erythrodermic. Ps is unique to each patient, the severity of Ps
lesions varies from person to person and the treatment depends on
the type of Ps, severity, the area where Ps is located, patient age
and medical history, and the effect that Ps has on the patient
(physically and emotionally). Besides classic Ps therapies, such as
topical treatment (for mild forms), UV light therapy (for moderate
to severe forms) and systemic treatments (for moderate to severe Ps
which has not successfully responded to topical treatments or UV
therapy) (18) biological therapies
(19) have entered the panel of
therapeutical approaches. Biologic treatments such as etanercept,
adalimumab, infliximab [anti-tumor necrosis factor (TNF)-α],
ustekinumab [anti-interleukin (IL)-12/-23], secukinumab
(anti-IL-17) are used for patients with severe Ps who have not
responded to systemic treatments such as methotrexate, ciclosporin
and acitretin. Recent studies suggest the role of complementary or
alternative medicine products on the psoriatic lesions (20,21).
Despite these various treatments, Ps remains incurable but
clinically manageable.
Extensive literature suggests that Ps is a T-cell
mediated disease and in its pathogenesis the innate and adaptative
immune cells are highly involved; an important role is played by
different T helper lymphocyte (Th) subsets accompanied by several
pro-inflammatory cytokines which maintain the chronic inflammatory
status (22). Although it is
considered a T cell mediated inflammatory disease, several cell
types from the adaptive and innate immunity arm, as well as
non-immune cells are highly involved. DC, natural killer (NK) cells
and macrophages from the innate immunity arm are involved in the
pathogenesis of Ps, establishing intense interactions with
keratinocytes and endothelial cells (9). The interactions between immune cells [T
cells, DC, NK cells, Langerhans cells (LC), macrophages] and
non-immune cells (hyperproliferative keratinocytes) are mediated by
immune-related molecules (cytokines, chemokines) and
non-immune-related molecules [vascular endothelial growth factor
(VEGF), keratinocyte growth factor (KGF)], all these interactions
lead to development of psoriatic lesions (23). Initiation of psoriatic events occurs
when, under the action of triggering factors (genetics,
environmental, skin injury, infections), non-specific immune cells
(NK cells, macrophage, plasmacytoid DC) and keratinocytes secrete
TNFα, IFNγ, IL-1β, IL-6 which will activate myeloid DC. The
activated myeloid DC are able to secrete IL-12 and IL-23, which
will further cause differentiation of resident T cells into Th1,
Th17 and Th22 cells. These effector Th subsets will release TNFα,
IFNγ, IL-17A/F and IL-22 therefore activating the keratinocytes
which will produce mainly pro-inflammatory cytokines (TNFα, IL-1β,
IL-6), chemokines (CXCL9, 10, 11) and LL-37 (an antimicrobial
peptide considered to be a possible autoantigen in Ps). Activated
keratinocytes, by releasing a panel of chemokines, will promote the
recruitment and activation of neutrophils and macrophages, thus
propagating and maintaining the skin inflammation (24).
In humans, NK cells are defined as cluster of
differentiation (CD)56+CD3− cells, and can be
divided into CD56bright NK cells (with predominantly
immunoregulatory properties) and CD56dim NK cells (with
marked cytotoxic function) (25). NK
cells were found in the inflammatory infiltrate in psoriatic skin
lesions and many cytokines known to be crucial of Ps are related to
NK biology and are either released by NK cells (IFNγ, TNF-α, and
IL-22) or are important in their activation (IL-15, IL-18, IL-12,
and IL-23). Although NK cells are involved in the inflammatory
process of Ps through these pro-inflammatory cytokines, their role
in this pathology is not yet fully elucidated (26). Especially known for their ability to
recognise and kill viral or cancer cells, NK cells are also
involved in some other cutaneous pathologies such as atopic
dermatitis (27), pemphigus vulgaris
(28) and alopecia areata
(29).
In order to aid information to the pathogenesis of
Ps and to identify potential therapeutic targets, numerous
experimental models performed in vitro and/or in vivo
were developed, each of them heaving advantages and disadvantages.
In vivo mouse models of Ps can be grouped into spontaneous
(chronic proliferative dermatitis cpdm/cpdm, flaky skin
Ttcfsn/Ttcfsn, homozygous
asebia Scd1ab/Scd1ab),
genetically engineered (Involucrin/IFNγ, K14-KGF, K14-VEGF, and
K5-Stat3C), xenotransplantation (human skin on SCID mice, on
athymic nude mice or on AGR129 mice), and directly induced
[intradermal injection of IL-23, 5% imiquimod (IMQ)] models
(30).
In recent years, one of the most used experimental
models of Ps, is IMQ-based mouse model of psoriasiform dermatitis.
This model has not only reduced the costs and high reproducibility
but also can furnish relevant results. IMQ has a nucleoside
[1-isobutyl-1H-imidazo(4,5-c)quinolin-4-amine] analogue of
imidazoquinoline family, is used in clinics for its anti-viral and
anti-neoplastic properties in the treatment of human papilloma
virus-derived genital warts (31),
squamous cell carcinoma (32) and
actinic keratosis (33).
Our prior published results using IMQ animal model
showed that there is a clear alteration of lymphocyte percentages
in peripheral blood (PB) and in secondary organs, pinpointing
towards NK lymphocyte deregulation (34). As NK cells are one of the main immune
populations that balance innate and adaptive immunity we enlarged
the evaluated subtypes of NK subpopulations identified in PB and in
secondary lymph organs seeking to establish the best pattern of NK
phenotype related to the evolution of psoriatic lesions.
Acknowledging the importance of NK lymphocyte
population in developing psoriatic lesions we used an IMQ-based
mouse model of psoriasiform dermatitis to study the NK cell
phenotype from PB and spleen cell suspensions detecting the
phenotypes described in Table I.
 | Table I.NK cell phenotype maturation,
activation and cytokine receptor markers. |
Table I.
NK cell phenotype maturation,
activation and cytokine receptor markers.
| Maturation
markers |
|---|
| Precursors |
|
Pre-NK | CD27+;
CD122− |
|
R-NK | CD27+;
CD122+ |
| Immature NK
cells |
| Stage
A | CD27+;
CD122+ |
| Stage
B | CD27+;
CD122+; NK1.1+; CD43+ |
| Stage
C | CD27+;
CD122+; NK1.1+; CD43+;
NKp46+ |
| Mature NK
cells |
|
| Stage
D | CD27+;
CD122+; NK1.1+; CD43+;
NKp46+; CD49b+ |
| Stage
E | CD27+;
CD122+; NK1.1+; CD43−;
NKp46+; CD49b+; CD11b+ |
| Stage
F | CD27−;
CD122+; NK1.1+; CD43−;
NKp46+; CD49b+; CD11b+;
KLRG1+ |
|
| Activation
markers |
|
| CD335 (NKp46,
NCR1); CD69; CD28; gp49R, CD45R (B220); CD11c |
|
| Markers for
cytokine receptors |
|
| CD25 (IL-2Rα);
CD122 (IL-2R/IL-15Rβ); CD132 (common γ chain) |
Materials and methods
IMQ-based mouse experimental model of
psoriatic dermatitis
C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME)
were provided by the Animal Husbandry from ‘Victor Babeș’ National
Institute of Pathology. They were accommodated in individual cages
in an open cage system, in a temperature-controlled,
air-conditioned animal house (20±4°C, 55±10% humidity) with a
12/12-light/dark cycle, and received food and water ad
libitum. The animals were monitored daily. The study design was
approved by the Ethics Committee from ‘Victor Babeș’ Institute, and
the experiments were done in accordance with recognized principles
of Laboratory Animal Care in the framework of EU Directive
2010/63/EU for animal experiments (35).
IMQ-based mouse model of psoriasiform dermatitis was
replicated according to the protocols described in literature
(36). Two groups of C57BL/6 mice
were considered (1:1 sex ratio, 8–11 weeks old): i) IMQ group:
received a daily topical dose of 62.5 mg IMQ cream (5% Aldara
Cream; MEDA AB Sweden) on the shaved back region for 5 consecutive
days (6 mice); and ii) Control group: no topical treatment (5
mice).
Erythema, skin scaling and thickening were monitored
daily on a 0–4 scale (0, none; 1, slight; 2, moderate; 3, marked;
and 4, very marked) and a modified PASI score (erythema + skin
scaling + thickening) was calculated daily in order to score the
inflammation due to IMQ treatment. Body weight was measured on the
first day of experiment and prior to sacrifice. At the end of IMQ
treatment (day 6) the mice were anesthetized (ketamine /
acepromazine, 100/5 mg/kg, Ketaset; Wyeth/Fort Dodge Animal Health,
Overland Park, KS, USA; Vedco, St. Joseph, MO, USA) and weighed.
Blood was collected in K2-EDTA coated tubes (Microvette, Sarstedt
AG & Co.) by retro-orbital veni-puncture and then the mice were
sacrificed for spleen and skin sampling. The spleens were removed
and weighed immediately (Balance AEP-1500 A; Adam Equipment Co.,
Ltd.) in order to assess the splenomegaly. Further spleens were
harvested in RPMI-1640 media with 5% FBS (Biochrom GmbH) and passed
through a 70 µm cell strainer (BD Falcon-BD Biosciences) to isolate
the entire spleen cell populations. The spleen cell suspensions
were centrifuged for 5 min at 350 × g (20°C), resuspended in RBC
Lysis Buffer (BioLegend), and incubated 5 min on ice. The lysis was
stopped by adding 10 ml Cell Staining Buffer (BioLegend). Cell
suspensions were centrifuged for 5 min at 350 × g (20°C) and the
cell pellet was resuspended twice in Cell Staining Buffer
(BioLegend). Viable cells were counted and resuspended in Cell
Staining Buffer (BioLegend) at 1×106 cells/ml. Skin
samples were processed for histopathological assessment (fixed in
10% buffered formalin, embedded in paraffin, sectioned in 5 µm
thick sections, stained with hematoxylin and eosin and examined by
pathologists).
Flow cytometry analysis
NK cell phenotype was performed by flow cytometry,
with an 8-color system setup, based on the expression of surface
markers. EDTA-anticoagulated whole blood samples and spleen cell
suspensions were incubated with TruStain fcX (anti-mouse CD16/32,
isotype Rat IgG2a, λ) antibody (BioLegend) in order to block
non-specific antibody binding. After blocking, all samples were
incubated for 20 min at room temperature in the dark with the
following monoclonal antibodies conjugated with fluorochromes (in
the quantities indicated by the producers): FITC anti-mouse CD3ε
(clone 145-2C11, isotype Armenian Hamster IgG), PerCP/Cy5.5
anti-mouse CD11c (clone N418, isotype Armenian Hamster IgG),
APC/Cy7 anti-mouse CD45R (B220) (clone RA3-6B2, isotype Rat IgG2a,
κ), PE/Cy7 anti-mouse CD69 (clone H1.2F3, isotype Armenian Hamster
IgG), PE anti-mouse KLRG1 (clone 2F1, isotype Syrian Hamster IgG),
PerCP/Cy5.5 anti-mouse/rat/human CD27 (clone LG.3A10, isotype
Armenian Hamster IgG), APC anti-mouse CD11b (clone M1/70, isotype
Rat IgG2b, κ), APC/Cy7 anti-mouse CD43 (clone RA3-6B2, isotype Rat
IgG2a, κ), PE/Cy7 anti-mouse CD335 (NKp46) (clone 29A1.4, isotype
Rat IgG2a, κ), PE anti-mouse CD132 (common γ chain) (clone TUGm2,
isotype Rat IgG2b, κ), PerCP/Cy5.5 anti-mouse CD122 (IL-2R/IL-15Rβ)
(clone TM-β1, isotype Rat IgG2b, κ), APC/Cy7 anti-mouse CD25
(IL-2Rα) (clone PC61, isotype Rat IgG1, λ), PE anti-mouse CD28
(clone 37.51, isotype Syrian Hamster IgG) (BioLegend), Brilliant
Violet 510 anti-mouse NK1.1 (clone PK136, isotype Mouse IgG2a, κ)
(BD Horizon, BD Biosciences), eFluor 450 anti-mouse CD49b (DX5)
(clone DX5, isotype Rat IgM, κ), eFluor 660 anti-mouse gp49R (clone
H1.1) (eBioscience Inc.). After surface staining, red blood cells
were lysed with RBC Lysis Buffer (BioLegend) for 10 min at room
temperature in the dark, followed by centrifugation for 5 min at
350 × g at 20°C. Cells were washed twice with Cell Staining Buffer
(BioLegend) and analysed by flow cytometry. Non-specific
fluorescence signals obtained due to spectral overlap were
automatically compensated (UltraComp eBeads, Invitrogen by Thermo
Fischer Scientific, Inc.) and unlabeled cells were used as negative
controls. Data acquisition and analysis were performed on a BD
FACSCanto II cytometer with BD FACSDiva v.6.1 software (BD
Biosciences). Cytometer performances were checked using CST beads
(BD Cytometer Setup & Tracking Beads kit; BD Biosciences).
Statistical analysis
Statistical analysis was performed using Microsoft
Excel. The results for spleen weight and body weight are presented
as mean spleen weight ± SD, respectively mean ratio SW:BW ± SD. The
flow cytometry data were expressed as percentages of NK1.1+ cells
(mean values ± SD), gated from CD3ε− lymphocytes.
Student's t-test (two-tailed, assuming equal variance) was used to
assess the differences between the experimental groups, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Psoriasiform dermatitis mouse model
induced by IMQ
IMQ-based mouse model of psoriasiform dermatitis was
replicated according to the protocols described by us (34), by applying a daily topical dose of
Aldara cream on the shaved back skin of C57BL/6 mice for 5
consecutive days. Skin inflammation and the disease severity were
assessed using in vivo measurements (erythema, desquamation
and induration parameters, PASI modified score), splenomegaly
assessment and histopathological evaluation.
In vivo measurements of skin
inflammatory parameters
Erythema, desquamation and induration parameters
(EDI) were assessed to study the progress of skin inflammation and
hence the disease severity by daily monitoring. EDI were scored
daily on a scale from 0 to 4: 0, none; 1, slight; 2, moderate; 3,
marked; and 4, very marked. Fig. 1
presents a representative case of inflammation induced by IMQ and
the EDI scoring for all the mice in the IMQ group matched the
pattern previously published by us for this experimental model
(34). As previously shown, all EDI
parameters, erythema is the first parameter that can be scored
after one day of IMQ applications, followed after another day by
the subsequent registered parameters, thus starting from day 2, all
EDI parameters are registered in all the animals subjected to IMQ
(34).
As in psoriatic patients, in our animal model, the
severity of inflammation was estimated based on a modified PASI
score (0–12 scale), calculated daily by adding the independent
daily scores obtained for EDI (the affected area was not taken into
account). The PASI score had a progressive evolution during the
IMQ-treatment (Fig. 2) matching the
increased severity of the psoriatic lesions.
As the PASI score clearly depicted the evolution of
the psoriatic lesions we evaluated the histopathology of
psoriatic-like skin in our model.
Histopathological evaluation
After 5 days of treatment, IMQ-based cream induced
pathological alterations in the epidermis, by compromising its
integrity. Several histopathological features that are typical for
human Ps, such as hyperkeratosis, parakeratosis, acanthosis and
elongation of rete ridges were observed (Fig. 3B).
Splenomegaly assessment
At the end of the experiment (day 6) the mice were
weighed and sacrificed; spleens from all the animals were removed
and weighed in order to assess the splenomegaly, SW/BW ratio
(spleen weight/total body weight) was calculated. The IMQ-treated
mice SW was significantly higher compared to healthy mice (control
group) (0.215±0.03 vs. 0.09±0.02, P=4.9×10−4)
(representative measurement is presented in Figs. 4 and 5A). When assessing SW/BW ratio we found
that in IMQ-treated mice this ratio was 2.5 times greater compared
to control mice (0.010±0.002 vs. 0.004±0.0008,
P=2.6×10−3; Fig. 5B).
NK cell phenotype in PB and spleen
samples
To evaluate the immune populations that are
circulating in the PB and that are resident in the secondary lymph
organ such as the spleen, we have extensively characterized the NK
cell phenotype from IMQ-psoriatic mice compared to control animals.
Besides lineage markers such as CD161 (NK1.1) and CD3ε, a large
panel of surface markers was used as follows: i) maturation
markers: CD49b (DX5), CD11b, CD43, CD27, KLRG1; ii) activation
markers: CD335 (NKp46), CD69, CD28, gp49R, CD45R (B220), CD11c; and
iii) markers for cytokine receptors: CD25 (IL-2Rα), CD122 (IL-2R /
IL-15Rβ), CD132 (common γ chain).
Analysis of maturation markers (CD11b, CD43, CD27,
KLRG1) revealed a significant tendency to increase their expression
in NK cells, in both PB and spleen cell suspension, the only
exception being CD49b (Fig. 6A and
B). The percentages of CD49b+NK1.1+ cells
in IMQ-treated mice is lower compared to controls in both
compartments, PB and spleen, the difference being statistically
significant in the spleen (P=0.002; Table II).
 | Figure 6.Expression of CD49b, CD11b, CD43,
CD27 and KLRG1 levels on NK1.1+ cells. (A) PB.
CD49b+, CD11b+, CD43+,
CD27+ and KLRG1+ cells in IMQ-treated mice
(n=6) (57±5.8; 95±1.2, P=0.01; 96±1.6, P=0.04; 17±1.9 and 77±4.8,
P=0.007) compared to control group (n=5) (70±14.1, 92±1.8, 93±2.6,
15±3.7 and 63±7.6) in PB. (B) Spleen cell suspension.
CD49b+, CD11b+, CD43+,
CD27+ and KLRG1+ cells in IMQ-treated mice
(n=6) (47±10.2, P=0.002; 80±3.1, P=0.004; 81±3.4; 42±3.9, P=0.001
and 52±3.5, P=0.002) compared to control group (n=5) (69±4.2,
74±1.4, 82±3, 30±3.7 and 40±5.4) in spleen cell suspension. The
results are presented as a percentage from NK1.1+ cells
(mean ± SD); n, number of mice; IMQ, imiquimod; SD, standard
deviation; NK, natural killer cells; CD, cluster of
differentiation; KLRG1, killer cell lectin-like receptor G1. |
 | Table II.Distribution of maturation markers on
NK1.1+ cells in peripheral blood and spleen
suspension. |
Table II.
Distribution of maturation markers on
NK1.1+ cells in peripheral blood and spleen
suspension.
|
| Peripheral
blood | Spleen
suspension |
|---|
|
|
|
|
|---|
| Markers | Control (mean ±
SD) | IMQ group (mean ±
SD) | P-value | Control (mean ±
SD) | IMQ group (mean ±
SD) | P-value |
|---|
| CD49b |
70±14.1 | 57±5.8 | NS |
69±4.2 |
47±10.2 | P=0.002 |
| CD11b | 92±1.8 | 95±1.2 | P=0.01 |
74±1.4 | 80±3.1 | P=0.004 |
| CD43 | 93±2.6 | 96±1.6 | P=0.04 | 82±3 | 81±3.4 | NS |
| CD27 | 15±3.7 | 17±1.9 | NS |
30±3.7 | 42±3.9 | P=0.001 |
| KLRG1 | 63±7.6 | 77±4.8 | P=0.007 |
40±5.4 | 52±3.5 | P=0.002 |
Depending on the presence or absence of CD11b and
CD27, four stages of maturation of NK cells can be distinguished:
immature NK cells (CD27−CD11b−), early mature
NK cells (CD27+CD11b−), mature NK cells
(CD27+CD11b+) and late mature cells
(CD27−CD11b+). In our model, in PB, we found
significantly decreased values for the immature stages and early
mature stages, while the percentages for mature subsets
(CD27+CD11b+ and
CD27−CD11b+) were higher in IMQ-treated mice
compared to the control group, but without statistical significance
(Table III). In spleen cells the
level of immature NK cells in IMQ-treated mice was significantly
decreased, while the values for early mature and completely matured
NK cells were increased. The late mature NK population had low
values (52±4.1 vs. 56±3) but not statistically significant
(Fig. 7A and B).
 | Figure 7.CD27CD11bNK1.1+
subpopulations in PB and spleen cell suspension. (A) PB.
Distribution of CD27−CD11b−,
CD27+CD11b−,
CD27+CD11b+ and
CD27−CD11b+ NK1.1+ cells in
IMQ-treated mice (n=6) (3±0.7, P=0.001; 2±0.5, P=0.004; 14±1.7 and
82±1.5) compared to control group (n=5) (6±1.6, 4±1.5, 11±2.4 and
78±5.3) in PB. (B) Spleen cell suspension. Distribution of
CD27−CD11b−,
CD27+CD11b−,
CD27+CD11b+ and
CD27−CD11b+ NK1.1+ cells in
IMQ-treated mice (n=6) (7±0.8, P=3×10−6; 13±2.4; 29±3.2,
P=0.002 and 52±4.1) compared to control group (n=5) (14±1.4,
10±1.5, 20±3.4 and 56±3) in spleen cell suspension. The results are
presented as a percentage from NK1.1+ cells (mean ± SD).
n, number of mice; PB, peripheral blood; IMQ, imiquimod; SD,
standard deviation; NK, natural killer cells; CD, cluster of
differentiation. |
 | Table III.Distribution of NK1.1+
subsets in peripheral blood and spleen suspension. |
Table III.
Distribution of NK1.1+
subsets in peripheral blood and spleen suspension.
|
| Peripheral
blood | Spleen
suspension |
|---|
|
|
|
|
|---|
|
| Control (mean ±
SD) | IMQ (mean ±
SD) | P-value | Control (mean ±
SD) | IMQ (mean ±
SD) | P-value |
|---|
| Immature
(CD27−CD11b−) |
6±1.6 |
3±0.7 | P=0.001 |
14±1.4 |
7±0.8 |
P=3×10−6 |
| Early mature
(CD27+CD11b−) |
4±1.5 |
2±0.5 | P=0.004 |
10±1.5 | 13±2.4 | NS |
| Mature
(CD27+CD11b+) | 11±2.4 | 14±1.7 | NS |
20±3.4 | 29±3.2 | P=0.002 |
| Late mature
(CD27−CD11b+) | 78±5.3 | 82±1.5 | NS | 56±3 | 52±4.1 | NS |
NKp46 expression on NK cells showed decreased values
for IMQ-treated mice as compared to the control group, in both PB
(91±13.1 vs. 98±0.2) and spleen cells (64±2.7 vs. 74±6.9).
Significant differences for this activating receptor were observed
in spleen cells (P=0.008; Table IV,
Fig. 8).
 | Table IV.Distribution of activation markers on
NK1.1+ cells in peripheral blood and spleen
suspension. |
Table IV.
Distribution of activation markers on
NK1.1+ cells in peripheral blood and spleen
suspension.
|
| Peripheral
blood | Spleen
suspension |
|---|
|
|
|
|
|---|
|
| Control (mean ±
SD) | IMQ (mean ±
SD) | P-value | Control (mean ±
SD) | IMQ (mean ±
SD) | P-value |
|---|
| NKp46 |
98±0.2 |
91±13.1 | NS |
74±6.9 |
64±2.7 | P=0.008 |
| CD69 |
12±1.4 |
60±13 |
P=0.005 |
2±0.2 | 62±3 |
P=1×10−10 |
| B220 |
7±2.6 |
6±2 | NS |
9±0.8 |
14±2.2 | P=0.003 |
| CD11c |
25±2.4 |
66±11 |
P=6×10−5 |
21±0.6 |
76±6.2 |
P=1×10−7 |
| gp49R |
1±0.3 | 11±7 | P=0.02 |
1±0.5 |
23±5.7 |
P=5×10−5 |
| CD28 | 0.4±0 |
3±2 | NS | 0.5±0.2 |
33±6.3 |
P=8×10−6 |
Analysis of NK cell activation markers (CD69, B220,
CD11c, gp49R, CD28) revealed significantly increased values in
spleen cells in IMQ-treated mice as compared to control group
(Table IV, Fig. 9B). In PB, of all the tested
activation markers, only CD69, CD11c and gp49R showed significantly
increased values as compared to the control group (Fig. 9A).
 | Figure 9.Expression of CD69, B220, CD11c,
gp49R and CD28 levels on NK1.1+ cells; (A) PB.
CD69+, B220+, CD11c+,
gp49R+ and CD28+ cells in IMQ-treated mice
(n=6) (60±13, P=0.005; 6±2; 66±11, P=6×10−5; 11±7,
P=0.02 and 3±2) compared to control group (n=5) (12±1.4; 7±2.6;
25±2.4; 1±0.3 and 0.4±0) in PB. (B) Spleen cell suspension.
CD69+, B220+, CD11c+,
gp49R+ and CD28+ cells in IMQ-treated mice
(n=6) (62±3, P=1×10−10; 14±2.2, P=0.003; 76±6.2,
P=1×10−7; 23±5.7, P=5×10−5 and 33±6.3,
P=8×10−6) compared to control group (n=5) (2±0.2; 9±0.8;
21±0.6; 1±0.5 and 0.5±0.2) in spleen cell suspension. The results
are presented as a percentage from NK1.1+ cells (mean ±
SD); n, number of mice; PB, peripheral blood; IMQ, imiquimod; SD,
standard deviation; NK, natural killer cells; CD, cluster of
differentiation. |
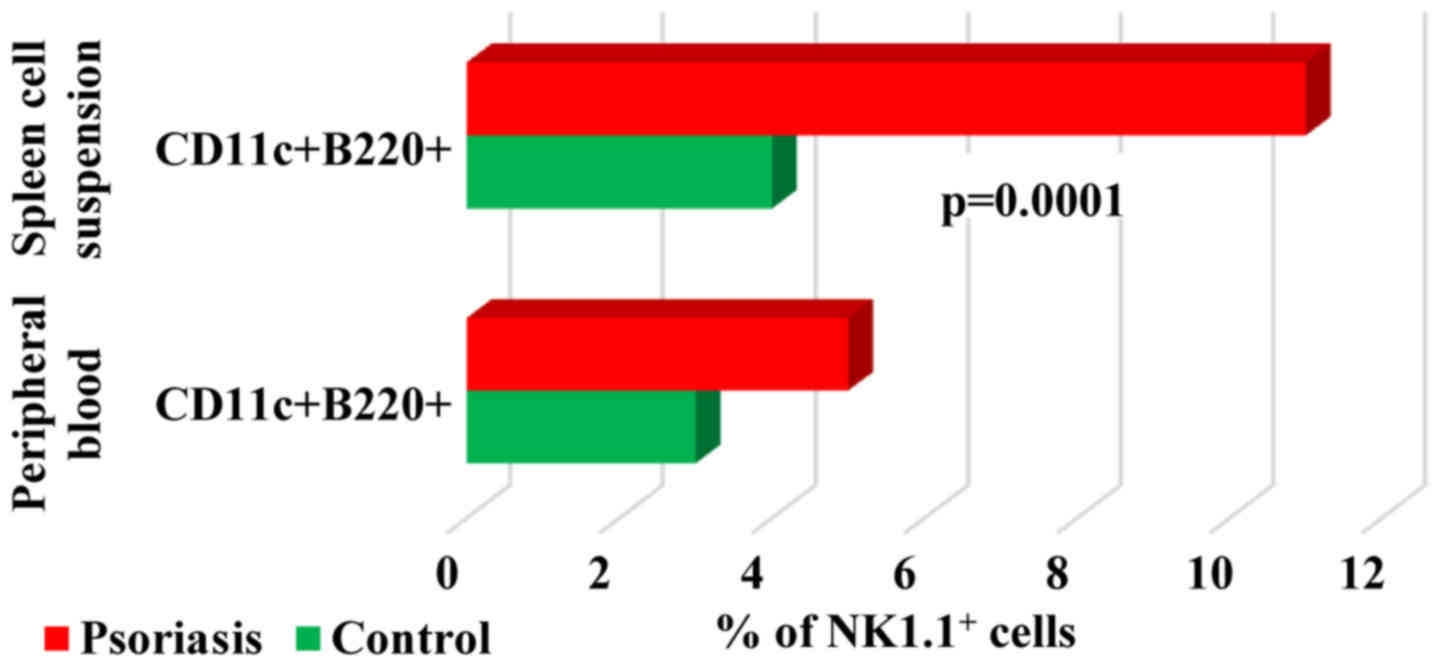
When studying
B220+CD11c+NK1.1+ cell population,
a DC subset overlapping functionally with NK cells (37) we found in both PB and spleen cells
that their percentage was higher in IMQ-treated mice (5±2 and
11±2.2 vs. 3±1.3 and 4±0.5). Significant differences between the
experimental groups were observed only in spleen cell suspension
(P=0.0001; Fig. 10).
Regarding the distribution of cytokine receptors,
the main changes observed in our experimental data for IMQ-treated
mice was the significant increase of the receptor for IL-2. This
finding was consistent for both of the receptor units (CD132 and
CD122) (73±8.3, P=5×10−7 vs. 7±4.3 and 95±1,
P=2×10−5 vs. 89±1.3) expressed on NK cells from PB. This
finding is somewhat to be expected as it comprises the functional
units of the same cytokine receptor. CD25 is the α chain of the
high-affinity IL-2 receptor and its expression was significantly
low in IMQ-treated mice (0.5±0.2, P=5×10−9) as compared
with the control mice (8±0.6) (Fig.
11A). Contrary to PB, in spleen cells, the levels of CD132 and
CD122 are lower in IMQ-treated mice (16±4.4; 75±8, P=0.004) than in
the control group (18±3.6; 91±2.6), as well as in PB. The level of
CD25 was found increased (6±1.5 vs. 2±0.6) but not statistically
significant (Fig. 11B).
 | Figure 11.Expression of CD25, CD122 and CD132
levels for NK1.1+ cells. (A) PB. CD25+,
CD122+ and CD132+ NK1.1+ cells in
IMQ-treated mice (n=6) (0.5±0.2, P=5×10−9; 95±1,
P=2×10−5 and 73±8.3, P=5×10−7) compared to
control group (n=5) (8±0.6, 89±1.3 and 7±4.3) in PB. (B) Spleen
cell suspension. CD25+, CD122+ and
CD132+ NK1.1+ cells in IMQ-treated mice (n=6)
(6±1.5; 75±8, P=0.004 and 16±4.4) compared to control group (n=5)
(2±0.6, 91±2.6 and 18±3.6) in spleen cell suspension. The results
are presented as a percentage from NK1.1+ cells (mean ±
SD); n, number of mice; PB, peripheral blood; IMQ, imiquimod; SD,
standard deviation; NK, natural killer cells; CD, cluster of
differentiation. |
Discussion
IMQ-based mice model of psoriasiform dermatitis
replicates human Ps in terms of skin inflammation and disease
severity assessed by erythema, desquamation, induration parameters,
PASI modified score and histopathological evaluation
(hyperkeratosis, parakeratosis, acanthosis and elongation of rete
ridges). Moreover, the mouse model displays splenomegaly and
altered NK populations related to the severity of the disease.
In our previous study regarding psoriasiform
dermatitis in a mouse model, we investigated immunological changes
induced by IMQ topical application in lymphocyte populations from
PB and spleen (34). One of the main
observed changes was the significant decrease of NK1.1+
cell percentages, which led to the present study to investigate
complex phenotypic pattern of circulating and spleen resident NK.
Using flow-cytometry, we investigated a large panel of surface
markers: maturation and activation markers (CD49b, CD11b, CD43,
CD27, KLRG1, NKp46, CD69, CD28, gp49R, CD45R, CD11c) and markers
for cytokine receptors (CD25, CD122, CD132). To our knowledge there
is no similar study regarding NK cells in blood and spleen cells in
an IMQ-based mouse model. We have previously used this panel to
characterize NK cells in another mouse model of skin cancer, namely
in cutaneous melanoma bearing mice (38).
Murine NK cells develop in specialized bone marrow
niches and derive from common lymphoid progenitor, going through
three important stages: NK cell progenitors (pre-NK cell precursors
and refined-NK cell precursors), immature NK cells (stage A-C) and
mature NK cells (stage D-F). Early stages are characterized by the
expression of IL-7Rα (CD127), CD27, CD244 and CD122, while the
acquisition of NKG2D, CD27 (stage A), NK1.1, CD43, CD62L, CD226
(stage B) and NCR1 (stage C) marks the conversion to immature NK
cells. Mature NK cells further express CD49b, Ly49 (stage D), loose
CD43 and acquire CD11b (stage E) and in the final stage of
maturation (stage F) downregulate CD27 and acquire KLRG1 (39). KLRG1 is a C-type lectin inhibitory
receptor (also expressed on subsets of T cells) allowing
identification of NK terminal development stages and is associated
with diminished proliferation and effector functions (40).
In our model, analysis of maturation markers (CD11b,
CD43, CD27, KLRG1) revealed a significant tendency to increase
their expression on NK cells, in both PB and spleen cell suspension
with the exception of CD49b. The percentage of
CD49b+NK1.1+ cells in IMQ-treated mice is
significantly lower than in the controls in spleen cell suspension.
This finding agrees with another recently reported study where
CD49b+ was found low on T cells in psoriatic patients,
this decrease being correlated with the severity of the disease
(41).
Depending on the presence or absence of CD11b and
CD27, there are several maturation stages of NK cells.
CD27+ NK cell subsets display a greater effector
function and responsiveness to chemokines then CD27− NK
cell subsets. CD27−CD11b+NK cell subsets
represent the final stage of maturation (42). In PB, our experimental data revealed
significant decreased values for the immature stages, while the
percentages for mature subsets were higher in IMQ-treated mice than
in control group, but without statistical significance. In spleen
cell suspension the level of immature NK cells in IMQ-treated mice
was significantly decreased (P=3×10−6), while the values
increased for early mature and mature (P=0.002) NK cells, and in
the final stage of maturation, the values were low but not
statistically significant. This finding reflects the immune
engagement toward activatory profile of NK cells and draws
attention to evaluate Ps intensity correlated with the mature
profile of circulating NK cells.
NKp46 (similar NCR1 or CD335), a major activating
receptor, is an NK cell specific surface marker and is found on all
mature NK cells. It is part of natural cytotoxicity receptor group
together with NKp44 and NKp30 (43).
It is involved in human NK cell activation, in tumor cell
recognition and plays an important role in natural cytotoxicity
against different tumor target cells (44). Our data showed a decreased NKp46
expression on NK cells for IMQ-treated mice as compared to the
control group, in both PB and spleen cell suspensions. The finding
is not surprising because, as already mentioned, the maturation and
activation of NK cells are characteristics of our psoriatic mouse
model.
In addition to NKp46, our study also included other
relevant markers to NK cell activation such as CD69, B220, CD11c,
gp49R and CD28. Leukocyte activation receptor CD69, also called
very early antigen, was found on human NK cells from psoriatic
lesions inflammatory infiltrate. Most of these NK cells had a
CD56bright phenotype and are known to produce in
vitro large quantities of IFNγ as a response to IL-2
stimulation (45). Murine NK cells
also express inhibitory receptors belonging to Ig
superfamily-related (gp49) receptors. Activated NK cells express
gp49B receptor which displays structural homology with human killer
inhibitory receptors (46). CD28, a
cell surface molecule with a critical role in T cell activation is
also expressed by mouse NK cells, and its triggering NK cell
proliferation, cytotoxicity, and cytokine secretion (47). Analysis of activation markers of NK
cells (CD69, B220, CD11c, gp49R, CD28) revealed significant
increased values in spleen cell suspension in IMQ-treated mice as
compared to control group. In PB, only CD69, CD11c and gp49R showed
significantly increased values as compared to the control group.
The finding reveals that psoriatic lesions can induce high
activation in secondary lymph organs. When evaluated in periphery,
only CD69, CD11c and gp49R showed significant increased values as
compared to control group. In an attempt to obtain a panel of
peripheral immune cells that can be applied further to Ps patients
a thorough selection of significant activation should be done
because although lymphoid organs display a high activation pattern,
in periphery only selected activated populations can be
identified.
B220+CD11c+NK1.1+
cells appear to be the equivalent of human CD56bright NK
cells due to their ability to produce higher levels of IFNγ
(37). The percentages of
B220+CD11c+NK1.1+ subset in both
PB and spleen cell suspension was higher in IMQ-treated mice than
in control mice, finding that emphasizes once more the activation
of this lymphocyte subset. Moreover the cytokine network that is
triggered by NK activation can act on the cells by themselves in an
autocrine manner and/or can influence other important players in
the immune response. Thus, the cytokine network is particularly
important for the proliferation, activation and functional capacity
of NK cells. In our study, we investigated the distribution of
three cytokine receptors: CD25 (IL-2Rα), CD122 (IL-2R/IL-15Rβ),
CD132 (common γ chain-IL-2, IL-4, IL-7, IL-9, IL-15, IL-21). The
main changes observed in our experimental data for IMQ-treated mice
were the significant increase of CD132 (P=5×10−7) and
CD122 (P=2×10−5) expression on NK cells in PB. The
expression of CD25 is significantly low in IMQ-treated mice as
compared with the control mice. In spleen cells, the levels of
CD132 and CD122 are lower in IMQ-treated mice than in the control
group, as well as in PB. The level of CD25 increased but was not
statistically significant. As we have an inverse correlation of
these cytokine receptors we can postulate that there is a flux of
activated cells toward periphery from the secondary lymphoid organs
that are drawn toward the induced psoriatic lesions. IL-17 plays a
leading role in the pathogenesis of Ps and in the concert of cells
that secrete this regulatory cytokine, NK cells are one of the main
cells (48). IL-17 has a
heterodimeric receptor IL-17RA/IL-17RC located on non-immune cells
such as keratinocytes, endothelial cells and fibroblasts and if
IL-17 increases the Ps prognosis is not favorable as it is
sustaining the development of psoriatic lesions. This finding
correlates with the decrease of IL-2 circulatory level, recently
reported in psoriatic patients (49). We can again postulate that there is a
negative regulation in psoriatic disease where NK cells balance
this cytokine axes IL-17-IL-2 and if this balance is deregulated
activation of non-immune cells, e.g., keratinocytes, is induced and
the psoriatic lesions appear.
Taking into account that there is a continuous
search for developing new therapy targets in Ps (50) and that NK cells can be future
modulatory targets (26,27) finding new markers that can aid the
dermatologists in clinical management (51–53)
would lead to novel cellular pattern for monitoring this
auto-immune disease.
In conclusion, imiquimod-based murine model of
psoriasiform dermatitis was analysed in order to evaluate the
involvement of NK cells in the pathogenesis of this autoimmune
disease. Evaluating a large panel of NK surface markers from the
maturation and activation sets along with markers for cytokine
receptors we obtained important differences in experimentally
induced mouse NK cell phenotypes as compared to the control group,
reflecting high activation in correlation with the degree of the
psoriatic lesions. Our evaluation was intended to shed light on the
involvement of NK functionality in Ps development and draw some
outlines regarding NK as disease evolution cellular marker.
Acknowledgements
The presented study will be integrated into the
original part of PhD thesis of author Mihaela Surcel.
Funding
This study was supported by Grants PN 19.29.01.01,
PN 19.29.02.03, PN-III-P1-1.2-PCCDI-2017-0341/2018, Grant COP A
1.2.3., ID: P_40_197/2016, Ctr. 52/2016 and by Ministry of Research
and Innovation in Romania, under Program 1-The Improvement of the
National System of Research and Development, Subprogram
1.2-Institutional Excellence-Projects of Excellence Funding in RDI,
Contract no. 7PFE/16.10.2018.
Availability of data and materials
The data sets used and/or analysed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
MS, RIH, GI, MN and MC: research creation and
design, data acquisition, analysis and interpretation of data,
statistical analysis, manuscript drafting, critical revision of the
manuscript for important intellectual content. ANM, IRP, OB, CarC,
LS, IZ and ConC: interpretation of data, manuscript drafting,
critical revision of the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of ‘Victor Babeș’ National Institute of Pathology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APC
|
allophycocyanin
|
|
BD
|
Becton Dickinson
|
|
CD
|
cluster of differentiation
|
|
DC
|
dendritic cell
|
|
EDI
|
erythema, desquamation and
induration
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FBS
|
fetal bovine serum
|
|
H&E
|
hematoxylin and eosin
|
|
FITC
|
fluorescein isothiocyanate
|
|
IFN
|
interferon
|
|
Ig
|
immunoglobulin
|
|
IL
|
interleukin
|
|
IMQ
|
imiquimod
|
|
K2-EDTA
|
kalium 2
ethylenediaminetetraacetate
|
|
KGF
|
keratinocyte growth factor
|
|
KLRG1
|
killer cell lectin-like receptor
G1
|
|
LC
|
langerhans cell
|
|
NK
|
natural killer cells
|
|
PASI
|
psoriasis area severity index
|
|
PB
|
peripheral blood
|
|
PE
|
phycoerythrin
|
|
PE/Cy
|
phycoerythrin complex with cyanine
|
|
PerCP/Cy
|
peridinin chlorophyll protein complex
with cyanine
|
|
R
|
receptor
|
|
RBC
|
red blood cell
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
SD
|
standard deviation
|
|
SW/BW
|
spleen weight/body weight
|
|
Th
|
helper T cells
|
|
TLR
|
Toll-like receptor
|
|
TNF
|
tumor necrosis factor
|
|
UV
|
ultraviolet
|
|
VEGF
|
vascular endothelial growth
factor
|
References
|
1
|
Boehncke WH and Schön MP: Psoriasis.
Lancet. 386:983–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dowlatshahi EA, van der Voort EAM, Arends
LR and Nijsten T: Markers of systemic inflammation in psoriasis: A
systematic review and meta-analysis. Br J Dermatol. 169:266–282.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parisi R, Symmons DPM, Griffiths CEM and
Ashcroft DM; Identification and Management of Psoriasis and
Associated ComorbidiTy (IMPACT) project team, : Global epidemiology
of psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen AD, Dreiher J, Shapiro Y, Vidavsky
L, Vardy DA, Davidovici B and Meyerovitch J: Psoriasis and
diabetes: A population-based cross-sectional study. J Eur Acad
Dermatol Venereol. 22:585–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armstrong AW, Harskamp CT and Armstrong
EJ: The association between psoriasis and obesity: A systematic
review and meta-analysis of observational studies. Nutr Diabetes.
2:e542012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah K, Mellars L, Changolkar A and
Feldman SR: Real-world burden of comorbidities in US patients with
psoriasis. J Am Acad Dermatol. 77:287–292.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kölliker Frers RA, Bisoendial RJ, Montoya
SF, Kerzkerg E, Castilla R, Tak PP, Milei J and Capani F: Psoriasis
and cardiovascular risk: Immune-mediated crosstalk between
metabolic, vascular and autoimmune inflammation. IJC Metab Endocr.
6:43–54. 2015. View Article : Google Scholar
|
|
8
|
Møller AH, Erntoft S, Vinding GR and Jemec
GB: A systematic literature review to compare quality of life in
psoriasis with other chronic diseases using EQ-5D-derived utility
values. Patient Relat Outcome Meas. 6:167–177. 2015.PubMed/NCBI
|
|
9
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
10
|
Wolf P, Weger W, Patra V,
Gruber-Wackernagel A and Byrne SN: Desired response to phototherapy
vs photoaggravation in psoriasis: What makes the difference? Exp
Dermatol. 25:937–944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dalkilic E, Bulbul Baskan E, Alkis N,
Gullulu M, Yavuz M, Dilek K, Ersoy A and Yurtkuran M: Tumor
necrosis factor-alpha antagonist therapy-induced psoriasis in
Turkey: Analysis of 514 patients. Mod Rheumatol. 22:738–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tatu AL and Nwabudike LC:
Metoprolol-associated onset of psoriatic arthropathy. Am J Ther.
24:e370–e371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moriwaki Y, Takada K, Tsuji S, Kawashima K
and Misawa H: Transcriptional regulation of SLURP2, a
psoriasis-associated gene, is under control of IL-22 in the skin: A
special reference to the nested gene LYNX1. Int Immunopharmacol.
29:71–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wheatley R, Brooks J, Stumpf B and Boh E:
Obesity, diet, and inflammation in psoriasis. J Psoriasis Psoriatic
Arthritis. 2:97–101. 2017. View Article : Google Scholar
|
|
15
|
Farkas A and Kemény L: Alcohol, liver,
systemic inflammation and skin: A focus on patients with psoriasis.
Skin Pharmacol Physiol. 26:119–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fry L and Baker BS: Triggering psoriasis:
The role of infections and medications. Clin Dermatol. 25:606–615.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng J, Luo S, Huang Y and Lu Q: Critical
role of environmental factors in the pathogenesis of psoriasis. J
Dermatol. 44:863–872. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mrowietz U and Reich K: Psoriasis - new
insights into pathogenesis and treatment. Dtsch Arztebl Int.
106:11–18, quiz 19. 2009.PubMed/NCBI
|
|
19
|
Carrascosa JM, Jacobs I, Petersel D and
Strohal R: Biosimilar drugs for psoriasis: Principles, present, and
near future. Dermatol Ther (Heidelb). 8:173–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niculet E, Neculia GV, Tatu AL and Buzia
OD: Curcumin-extraction, physical and chemical analysis, formulas
and control. basic methods for further research. Mater Plast.
55:672–675. 2018.
|
|
21
|
Nwabudike LC and Tatu AL: Using
complementary and alternative medicine for the treatment of
psoriasis. A step in the right direction. JAMA Dermatol. Mar
13–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karczewski J, Dobrowolska A,
Rychlewska-Hańczewska A and Adamski Z: New insights into the role
of T cells in pathogenesis of psoriasis and psoriatic arthritis.
Autoimmunity. 49:435–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Surcel M, Huica R, Constantin C, Ursaciuc
C and Neagu M: Biomarkers insights in psoriasis - Regulatory
cytokines. Curr Biomark. 7:3–11. 2017. View Article : Google Scholar
|
|
24
|
Mahil SK, Capon F and Barker JN: Update on
psoriasis immunopathogenesis and targeted immunotherapy. Semin
Immunopathol. 38:11–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caligiuri MA: Human natural killer cells.
Blood. 112:461–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunphy SE, Sweeney CM, Kelly G, Tobin AM,
Kirby B and Gardiner CM: Natural killer cells from psoriasis
vulgaris patients have reduced levels of cytotoxicity associated
degranulation and cytokine production. Clin Immunol. 177:43–49.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Bubnoff D, Andrès E, Hentges F, Bieber
T, Michel T and Zimmer J: Natural killer cells in atopic and
autoimmune diseases of the skin. J Allergy Clin Immunol. 125:60–68.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi H, Amagai M, Tanikawa A, Suzuki
S, Ikeda Y, Nishikawa T, Kawakami Y and Kuwana M: T helper type
2-biased natural killer cell phenotype in patients with pemphigus
vulgaris. J Invest Dermatol. 127:324–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zakka LR, Fradkov E, Keskin DB, Tabansky
I, Stern JNH and Ahmed AR: The role of natural killer cells in
autoimmune blistering diseases. Autoimmunity. 45:44–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bocheńska K, Smolińska E, Moskot M,
Jakóbkiewicz-Banecka J and Gabig-Cimińska M: Models in the research
process of psoriasis. Int J Mol Sci. 18:E25142017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banerjee S and Kaunelis D: Imiquimod for
the treatment of genital warts: A review of clinical effectiveness
and cost-effectivenessCADTH Rapid Response Report: Summary with
critical appraisal. Canadian Agency for Drugs and Technologies in
Health; Ottawa, ON: 2017
|
|
32
|
Bhatta AK, Wang P, Keyal U, Zhao Z, Ji J,
Zhu L, Wang X and Zhang G: Therapeutic effect of Imiquimod enhanced
ALA-PDT on cutaneous squamous cell carcinoma. Photodiagnosis
Photodyn Ther. 23:273–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Banerjee S and Kaunelis D: Imiquimod for
the treatment of actinic keratosis: A review of clinical
effectiveness and cost-effectivenessCADTH Rapid Response Report:
Summary with critical appraisal. Canadian Agency for Drugs and
Technologies in Health; Ottawa, ON: 2017
|
|
34
|
Surcel M, Huică R-I, Munteanu AN, Isvoranu
G, Pîrvu IR, Ciotaru D, Constantin C, Bratu O, Căruntu C, Neagu M,
et al: Phenotypic changes of lymphocyte populations in psoriasiform
dermatitis animal model. Exp Ther Med. 17:1030–1038.
2019.PubMed/NCBI
|
|
35
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals8th.
National Academies Press (US); Washington, DC: 2011
|
|
36
|
van der Fits L, Mourits S, Voerman JSA,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP, et al: Imiquimod-induced psoriasis-like skin inflammation in
mice is mediated via the IL-23/IL-17 axis. J Immunol.
182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blasius AL, Barchet W, Cella M and Colonna
M: Development and function of murine B220+CD11c+NK1.1+ cells
identify them as a subset of NK cells. J Exp Med. 204:2561–2568.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Isvoranu G, Surcel M, Huică R-I, Munteanu
AN, Pîrvu IR, Ciotaru D, Constantin C, Bratu O, Neagu M and
Ursaciuc C: Natural killer cell monitoring in cutaneous melanoma -
new dynamic biomarker. Oncol Lett. 17:4197–4206. 2019.PubMed/NCBI
|
|
39
|
Abel AM, Yang C, Thakar MS and Malarkannan
S: Natural killer cells: Development, maturation, and clinical
utilization. Front Immunol. 9:18692018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huntington ND, Tabarias H, Fairfax K,
Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM and
Nutt SL: NK cell maturation and peripheral homeostasis is
associated with KLRG1 up-regulation. J Immunol. 178:4764–4770.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim J, Lee J, Gonzalez J, Fuentes-Duculan
J, Garcet S and Krueger JG: Proportion of CD4+CD49b+LAG-3+ Type 1
regulatory T cells in the blood of psoriasis patients inversely
correlates with psoriasis area and severity index. J Invest
Dermatol. 138:2669–2672. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chiossone L, Chaix J, Fuseri N, Roth C,
Vivier E and Walzer T: Maturation of mouse NK cells is a 4-stage
developmental program. Blood. 113:5488–5496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hadad U, Thauland TJ, Martinez OM, Butte
MJ, Porgador A and Krams SM: NKp46 clusters at the immune synapse
and regulates NK cell polarization. Front Immunol. 6:4952015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pessino A, Sivori S, Bottino C, Malaspina
A, Morelli L, Moretta L, Biassoni R and Moretta A: Molecular
cloning of NKp46: A novel member of the immunoglobulin superfamily
involved in triggering of natural cytotoxicity. J Exp Med.
188:953–960. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dunphy S and Gardiner CM: NK cells and
psoriasis. J Biomed Biotechnol. 2011:2483172011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang LL, Chu DT, Dokun AO and Yokoyama WM:
Inducible expression of the gp49B inhibitory receptor on NK cells.
J Immunol. 164:5215–5220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nandi D, Gross JA and Allison JP:
CD28-mediated costimulation is necessary for optimal proliferation
of murine NK cells. J Immunol. 152:3361–3369. 1994.PubMed/NCBI
|
|
48
|
Georgescu SR, Tampa M, Caruntu C, Sarbu
MI, Mitran CI, Mitran MI, Matei C, Constantin C and Neagu M:
Advances in understanding the immunological pathways in psoriasis.
Int J Mol Sci. 20:E7392019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Solberg SM, Sandvik LF, Eidsheim M,
Jonsson R, Bryceson YT and Appel S: Serum cytokine measurements and
biological therapy of psoriasis - Prospects for personalized
treatment? Scand J Immunol. 88:e127252018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guarene M, Pasi A, Bolcato V, Cananzi R,
Piccolo A, Sbarsi I, Klersy C, Cacciatore R and Brazzelli V: The
presence of HLA-A Bw4-80I KIR ligands could predict
‘Difficult-to-Treat’ psoriasis and poor response to Etanercept. Mol
Diagn Ther. 22:471–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in
vivo reflectance confocal microscopy. Exp Ther Med.
15:1241–1246. 2018.PubMed/NCBI
|
|
52
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl):1191–1196.
2014.PubMed/NCBI
|
|
53
|
Negrei C, Căruntu C, Ginghină O, Burcea
Dragomiroiu GTA, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim
Buchar. 66:607–610. 2015.
|