Introduction
Cerebral infarction is the most common
cerebrovascular disease and is associated with high morbidity and
mortality rates (1,2). In addition to damaging the site of
occurrence, a cerebral infarction also damages distantly located
regions of a tissue. For instance, cerebral infarctions may lead to
crossed cerebellar diaschisis (CCD) (3,4). CCD
following a cerebellar infarction is associated with depressed
cerebral metabolism and blood flow in the cerebellar hemisphere
located contralateral to the focal supratentorial lesion (5). A disruption of the
corticopontocerebellar pathway connecting the infarcted cerebellum
and contralateral hemisphere has been identified as the most likely
cause of CCD (6). Notably, the
diminished excitatory trans-synaptic neuronal input caused by
morphological degeneration is considered to account for the
subsequent reduction in metabolism and blood flow (7). Baron et al (8) were the first to report CCD in the
cerebral hemisphere contralateral to the central region of
supratentorial ischemic infarction. Since then, this phenomenon has
been observed in various clinical conditions, including
intracranial tumors (9),
arteriovenous malformations (10),
and hemorrhages (11). Previously,
positron emission tomography and single-photon emission computed
tomography were used to detect CCD (12,13).
However, these techniques are expensive and involve the use of
radiation. The use of magnetic resonance imaging (MRI) enables the
visualization of damage without an exposure to radiation. However,
conventional MRI is not sufficiently sensitive for the detection of
CCD in its early phase. A more recently developed form of MR,
diffusion tensor imaging (DTI), has allowed for the detection of
altered white matter fibers. Hence, it has enabled an accurate
assessment of various brain disorders (14). The superior spatial resolution of
this type of MRI is sensitive in detecting subtle morphologic
changes in affected cerebellar hemispheres (15).
Despite the mounting evidence of the association of
transneuronal depression with CCD, the mechanism of CCD
pathophysiology is still not completely understood (7). The repulsive guidance molecule a(RGMa)
has been demonstrated to impede neurite outgrowth in postnatal
cerebellar neurons (16,17). In rats, the induction of RGMa
expression following spinal cord injury at the site of the lesion
has been observed (18).
Neutralization of RGMa with local administration of an antibody was
identified to significantly facilitate axon regeneration following
spinal cord injury (15,19). In addition, RGMa has been indicated
to participate in the development of scar tissue following injury
and in the myelination of fiber tracts (18). Furthermore, RGMa has been indicated
to be one of the most potent inhibitors of axonal growth (17). In a previous study, the RGMb
expression levels in the brain tissue of rats with MCAO were
enhanced and this effect was suggested to be involved in the
regeneration and remodeling of axons and synapses after cerebral
ischemia injury (20). Furthermore,
RGMa suppressed angiogenesis following ischemia and reperfusion
injury in a rat MCAO model (21).
In the present study, CCD was induced in rats by
occluding the MCA and the relevant changes were detected using
MR-DTI. The changes were further quantified by determining the
fractional anisotropy (FA). Subsequently, in order to understand
the pathophysiology of CCD, the role of RGMa was investigated in
this disorder and the expression of RGMa in sections with
compromised fiber integrity was also determined using MR-DTI.
Materials and methods
Animals
A total of 70 adult male Specific Pathogen Free
Sprague Dawley rats (age, 10–12 weeks; weight, 270–320 g) were
purchased from the Laboratory Animal Center of Hennan Province
(Zhengzhou, China) and bred in the Experimental Animal Center of
Zheng Zhou University (Zhengzhou, China) with constant temperature
(22–25°C) and humidity (40–60%), a 12 h-light/dark cycle and free
access to standard chow and water prior to- and post-surgical
intervention. The experimental protocols were approved by the
Institutional Animal Care and Use Committee of Zhengzhou University
(Zhengzhou, China).
Establishment of the MCAO model
Rats were randomly divided into two groups: Sham
surgery (sham control, n=14) and MCAO (n=56). The MCAO rats were
randomly divided into a further seven groups (n=8) according to the
h assessed following surgery (at 1, 3 6, 9, 12, 24 and 72 h). MCAO
was induced as previously described by Longa et al (22). Briefly, the rats were anesthetized
intraperitoneally with ketamine (80 mg/kg) and xylazine (10 mg/kg).
Anesthesia was maintained with 1.5% isoflurane with 0.5–1.0 l/min
100% O2 through a face mask. The common carotid artery,
external carotid artery (ECA) and internal carotid artery (ICA)
were exposed and a 4-cm long 3-0 monofilament nylon suture (Harvard
Apparatus, Cambridge, MA, USA) was used to tie the proximal ECA,
ICA and the circle of Willis effectively to occlude the MCA.
Following an occlusion period of 60 min, the suture was withdrawn
to allow reperfusion. For sham surgery, all arteries were exposed
during the surgical period but the filament was not inserted into
the MCA. Following surgery, the rats were housed individually and
closely monitored for changes in behavior and vital signs. The MCAO
model was considered successfully established when the following
observations were indicated: i) Horner syndrome occurred in the
ipsilateral (left side) when the rat displayed wakefulness after
surgery; ii) the forelimbs did not completely stretch; and iii)
contralateral circling occurred when walking. Simultaneously, the
Zea-Longa neurological deficit scores were calculated. Scores of 2
and 3 were included in the MCAO model. The neurological scores were
blindly assessed independently by two pretrained technicians when
the rats awoke after MCAO surgery according to the Zea-Longa
neurological deficit scores (12).
The Zea-Longa assessment criteria were as follows: Score 0, normal,
no neurological sign; score 1, cannot completely stretch
contralateral forelimbs; score 2, contralateral circling when
walking; score 3, contralateral fall over when walking; and score
4, cannot walk and lowering of consciousness.
MR
Imaging of the experimental animals was performed at
1, 3, 6, 9, 12, 24 and 72 h and imaging of the sham control animals
was performed at 0 h following surgery using a Signa HDxt 3.0T MR
scanner (GE Healthcare, Chicago, IL, USA) employing the
multi-channel coil designed for rats (the Medical Science and
Technology Corporation of Chenguang, Shanghai, China). Imaging
consisted of axial T1-T2-weighted (T2W), sagittal T2W, DWI and DTI
sequence. The scanning parameters were as follows: Fast spin-echo
(FSE) sequence T1WI, time of repetition (TR)/echo time
(TE)=360/23.3 msec, field of view (FOV)=70×70 mm, number of
excitations (NEX)=4.00 and matrix 320×256; FSE sequence T2WI,
TR/TE=2,300/115.3 msec, FOV=110×110 mm, NEX=4 and matrix 256×256;
single-shot spin-echo/echo-planar imaging sequence DWI,
TR/TE=2350/78.9 msec, FOV=110×110 mm, NEX=4.00 and matrix 96×96;
and DTI, TR/TE=2500/92.2 msec, FOV=110×110 mm, NEX=4.00 and matrix
128×128.
Post-imaging processing
MR images were subjected to a series of processing
and statistical analyses by the workstation provided by the GE
company. Images obtained during DTI were processed using the
Workstation software version 5.4.07 (GE Healthcare). The threshold
was adjusted by covering the brain tissue with green lines.
Apparent diffusion coefficient (ADC) and FA maps were obtained and
images were batch saved. Regions of interest, including the infarct
core and bilateral cerebellar hemispheres, were measured on FA
maps. The measurements of each site were repeated three times and
data were recorded as an average.
Immunohistochemistry analysis
Following sacrifice, the cerebellar hemispheres were
excised and fixed in phosphate-buffered 4% formaldehyde for 24 h at
4°C, wax-embedded and cut into 5-µm sections prior to being
transferred to slides. Sections were treated with 3%
H2O2, blocked with 5% rabbit serum (Shanghai
Haoran Biological Technology Co., Ltd.) for 20 min at 25°C, and
incubated overnight at 4°C with RGMa antibody (1:100; cat. no.
ab216643; Abcam, Cambridge, MA, USA). Subsequently, samples were
treated with horseradish peroxidase-labeled secondary goat
anti-rabbit IgG antibody (1:10,000; cat. no. 7074; Cell Signaling
Technology, Inc., Danvers, MA, USA) for 30 min at 37°C, stained
with 3,3-′diaminobenzidine for 3 min at 25°C (Dako, Agilent
Technologies, Inc., Santa Clara, CA, USA) and observed under a
light microscope (Leica Microsystems, Ltd., Milton Keynes, UK).
Images were captured at a ×200 magnification using Leica QWin Plus
v3 software (Leica Microsystems, Ltd.). A graphic analysis system
(Qianping Image Engineering Company, Wuhan, China) was used to
semi-quantitate the density of RGMa.
Statistical analysis
Data were analyzed using SAS software (SAS, 2002;
SAS Institute, Inc., Cary, NC, USA). Data were presented as the
mean ± standard deviation. Data were analyzed using one-way
analysis of variance followed by the least significant difference
post-hoc test for multiple pairwise comparisons. The correlation
between RMGa and FA was analyzed using Pearson's correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
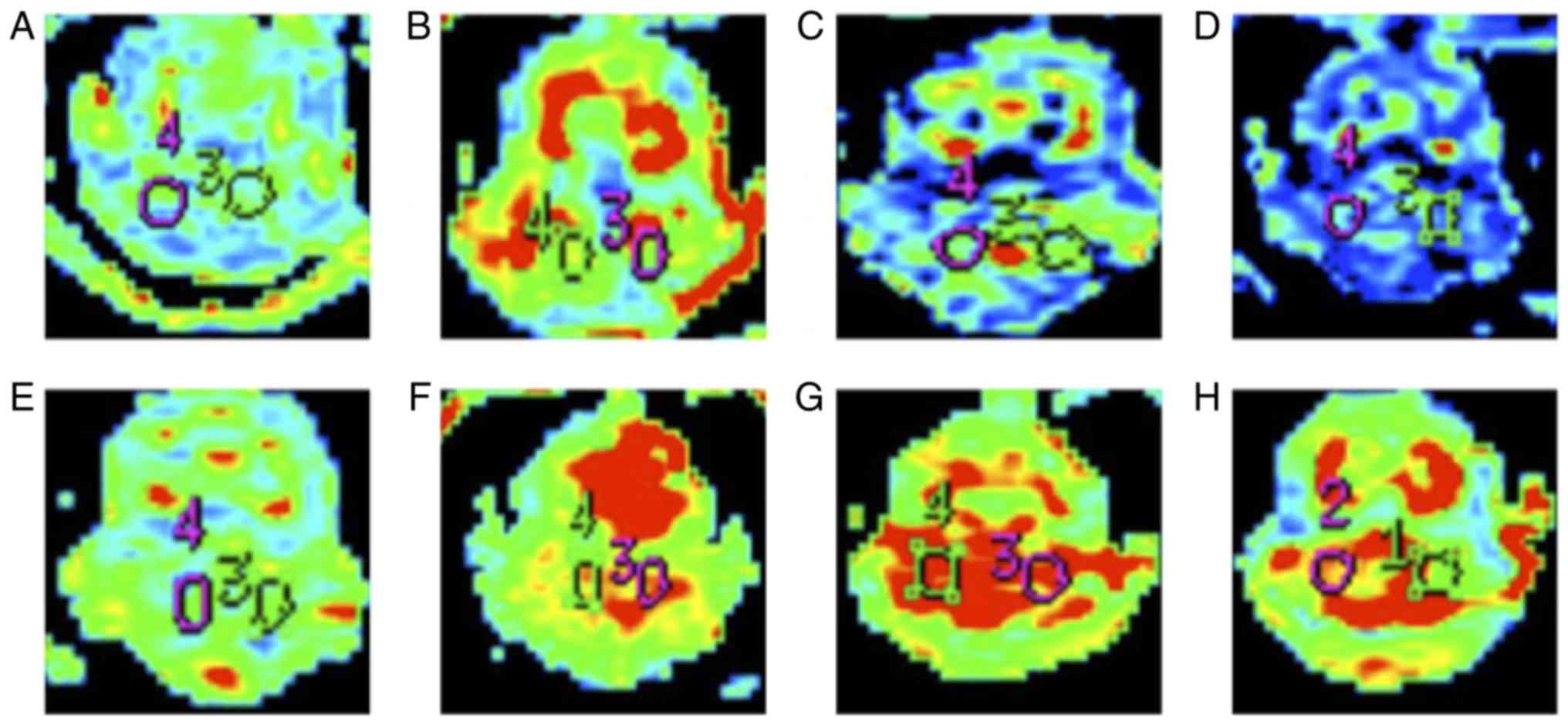
T2WI and DWI image analysis of MCAO
rats with bilateral cerebellar hemispheres
T2WI and DWI imaging of MCAO rats were obtained at
1, 3, 6, 9, 12, 24 and 72 h following surgery (Figs. 1 and 2). With the extension of infarct time, the
infarct size expanded from the left basal ganglia to the entire
left hemisphere and to the contralateral hemisphere. Notably, the
infarct size was the largest at 12 h and gradually decreased
thereafter.
FA values of MCAO rats with bilateral
cerebellar hemispheres
At each time point, the ADC and FA values of the
bilateral cerebellar hemispheres of MCAO rats were decreased
compared with those of sham control rats (Tables I and II; and Fig.
3). This difference was maximal at 12 h following MCAO.
Additionally, ADC was significantly decreased in contralateral
(right) cerebellar hemisphere and the ipsilateral (left) cerebellar
hemisphere at 9 and 12 h following MCAO compared with the sham
control group. FA values were significantly decreased in
contralateral (right) cerebellar hemisphere and the ipsilateral
(left) cerebellar hemisphere compared with the sham control at 1,
3, 6, 9, 12 and 24 h following MCAO. The results suggested that the
contralateral (right) cerebellar hemisphere indicated a large
degree of decline compared with the ipsilateral (left) cerebellar
hemisphere.
 | Table I.Apparent diffusion coefficient value
of the sham control and MCAO groups at different time points. |
Table I.
Apparent diffusion coefficient value
of the sham control and MCAO groups at different time points.
|
| Cerebellum |
|
|---|
|
|
|
|
|---|
| Group | Time (h) | Right side | Light side |
|---|
| Sham control | 0 | 6.95±0.83 | 6.96±0.85 |
| MCAO | 1 | 6.87±0.83 | 6.81±1.31 |
|
| 3 | 6.84±0.92 | 6.67±1.51 |
|
| 6 | 6.37±1.24 | 6.52±1.83 |
|
| 9 |
6.05±1.08a |
6.27±0.86a |
|
| 12 | 5.54±0.9
a |
5.49±0.41a |
|
| 24 | 6.36±0.32 | 6.47±1.24 |
|
| 72 | 6.96±0.62 | 6.57±1.24 |
 | Table II.FA value of the control and MCAO
group at different time points. |
Table II.
FA value of the control and MCAO
group at different time points.
|
| Cerebellum |
|
|---|
|
|
|
|
|---|
| Group | Time (h) | Right side | Light side |
|---|
| Sham control | 0 | 0.299±0.033 | 0.299±0.033 |
| MCAO | 1 |
0.245±0.041a | 0.256±0.060 |
|
| 3 |
0.244±0.043a |
0.253±0.052a |
|
| 6 |
0.237±0.064a |
0.251±0.021a |
|
| 9 |
0.236±0.045a |
0.246±0.049a |
|
| 12 |
0.228±0.042a |
0.244±0.033a |
|
| 24 |
0.250±0.014a | 0.265±0.033 |
|
| 72 | 0.265±0.024 | 0.269±0.034 |
Protein expression of RMGa in
bilateral cerebellar hemispheres of MCAO rats
RGMa protein expression level was increased over
time, with maximal expression indicated at 24 h following
infarction. RGMa protein expression was significantly increased in
contralateral (right) cerebellar hemisphere and the ipsilateral
(left) cerebellar hemisphere compared with sham control at 1, 3, 6,
9, 12, and 24 h following MCAO. Furthermore, the RGMa protein
expression level in the contralateral (right) cerebellar hemisphere
was higher compared with that in the ipsilateral (left) cerebellar
hemisphere (Table III and Fig. 4). The results suggested that RGMa
expression was the largest at 12 h and gradually decreased
thereafter following MCAO in bilateral cerebellar hemispheres of
rats, and RGMa expression in contralateral (right) cerebellar
hemisphere was higher than that in in the ipsilateral (left)
cerebellar hemisphere.
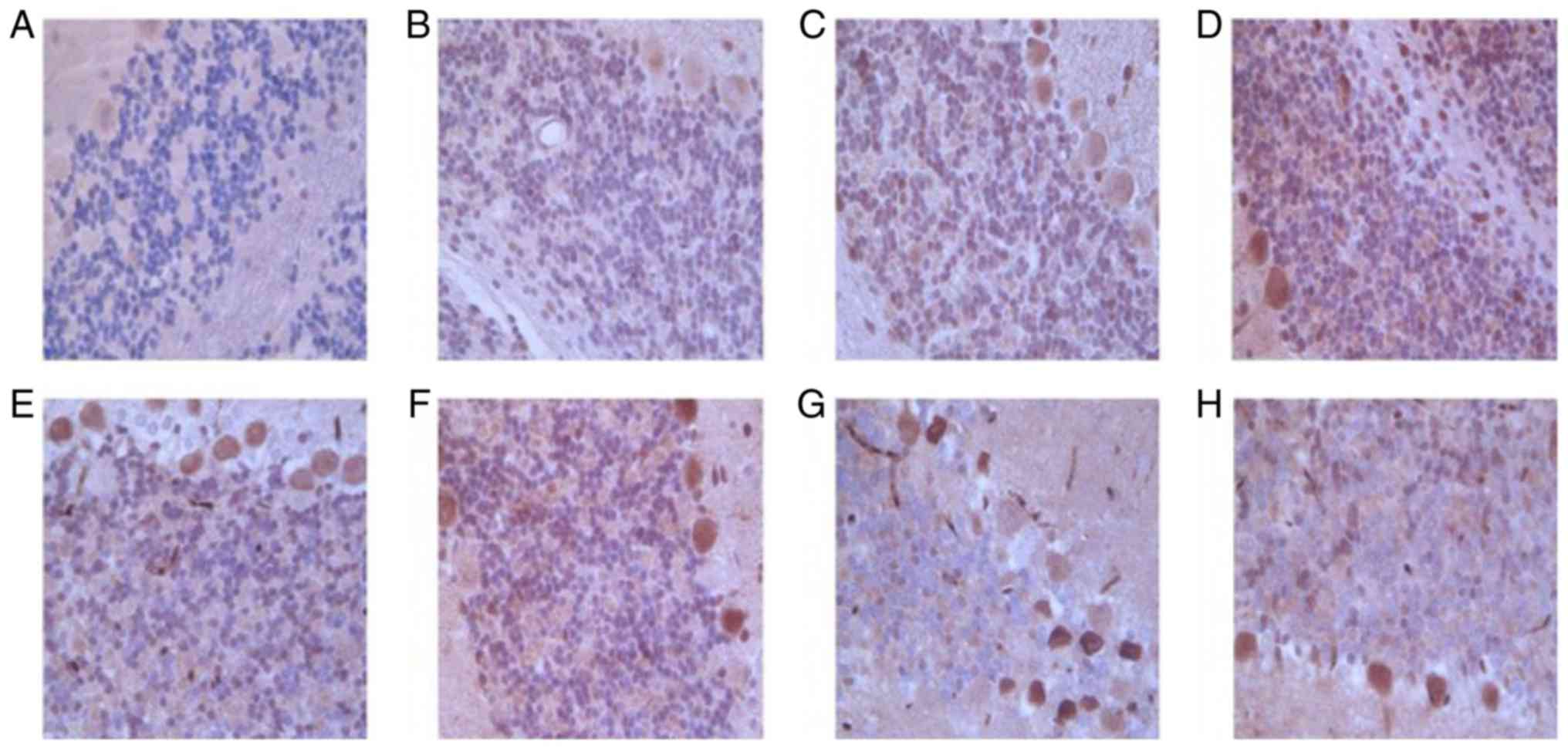 | Figure 4.Representative images of
immunohistochemical staining of RGMa in bilateral cerebellar
hemispheres of the (A) sham control and MCAO rats at (B) 1, (C) 3,
(D) 6, (E) 9, (F) 12, (G) 24 and (H) 72 h following infarction
(magnification, ×200). Integrated optical density value of the RGMa
positive zone was used to semi-quantitatively measure the protein
expression. (A) Representative image observed at 1–72 h of the
infarction, whereas, each of the MCAO rat images were
representative of three images from varied zones of infarction.
MCAO, middle cerebral artery occlusion; RCMa, repulsive guidance
molecule a. |
 | Table III.Expression of RGMa protein at
different time points following MCAO. |
Table III.
Expression of RGMa protein at
different time points following MCAO.
|
| Cerebellum |
|
|---|
|
|
|
|
|---|
| Group | Time (h) | Right side | Light side |
|---|
| Sham control | 0 | 83.06±2.87 | 83.06±2.87 |
| MCAO | 1 |
101.5±4.42a |
95.38±2.56a |
|
| 3 |
111.05±5.57a |
100.98±4.32a |
|
| 6 |
113.8±7.56a |
103.28±9.71a |
|
| 9 |
118.52±9.59a |
103.71±6.73a |
|
| 12 |
120.23±7.68a |
105.27±2.5a |
|
| 24 |
136.44±5.10a |
120.74±5.06a |
|
| 72 |
122.96±9.8a | 109.46±
5.27a |
Correlation between FA changes and
expression of RGMa protein
To determine the association between the changes in
FA and the expression of RGMa protein in the bilateral cerebellar
hemispheres measured at 1, 3, 6, 9, 12, 24 and 72 h following MCAO,
correlation analysis was performed. The results indicated that FA
values and the expression of RGMa protein were negatively
correlated (Spearman correlation coefficient r=−0.641,
P<0.05; Fig. 5).
Discussion
The objective of the present study was to use MR-DTI
to detect the changes of distant regional diffusion parameters
after supratentorial cerebral infarction in rats. A limited number
of studies have assessed supratentorial cerebral infarction with
MR-DTI. Notably, DTI based on DWI is a relatively novel technique
that can reveal the direction of molecular diffusion (23–26). In
the present study, DWI allowed for the examination of changes in
the cerebellum located at a distance from the infracted site.
Subsequently, RGMa, an inhibitor of axon growth, was used to
investigate its role in CCD following supratentorial cerebral
infarction.
Previously, DTI was mostly used to evaluate
diffusion changes in the core area of acute infarction (27,28).
Only a few reports have evaluated changes in the regions remote
from the supratentorial infarction, including the thalamus
(29), pons (30) and cerebellum (9). The calculated FA values based on DTI
reflect the extent of directional sensitivity of water diffusion, a
measure of fiber connectivity within a voxel. Therefore, DWI serves
as an appropriate measure of the possible alterations in
microstructures of white matter tract (31). In the present study, the Longa's
suture method with some modifications was used to establish the rat
cerebral ischemia model of MCAO. Results indicated that a reduction
in the FA values of the core region of the infarction and in the
bilateral cerebellum was detectable at 1 h of cerebral infarction
and continued to decline until 12 h. Following the 12-h time point,
the FA values began to recover; however, the FA values were still
lower in the MCAO group compared with the sham control group.
Perhaps due to the modified MCAO technique, a pre-eminent
infarction was induced and therefore it was not completely
recovered within 72 h. The results confirmed the change in
diffusion parameters in the regions remote from the infracted
cerebellum. Furthermore, in the hyperacute and acute phases after
cerebral ischemia in rats, a significant decrease in ADC values in
the infarct core region was detected. It was previously proposed
that, following a hyperacute supratentorial infarction, the blood
flow volume in the brain microcirculation was reduced and caused
dysfunctioning of the Na+-K+ pump (32). As a result, cellular ionic
homeostasis was disrupted, which accounted for movement of
extracellular water into cells, thereby resulting in edema and cell
death (33). These physiological
changes affect the integrity of nerve fibers connecting the
cerebral cortex to both sides of the brain, thereby impeding the
transmission of neural signals sent by the cerebral cortex to both
sides of the brain. Previous findings have suggested that the
diffusion of water into the intracellular compartment is impeded by
the presence of organelles in acute cerebellar infarction.
Subsequently, the narrowing of the extracellular space due to the
swelling of cells decreased the ADC values (16,34). The
FA measures in the white matter reflect fiber density, axonal
diameter and myelination (27,35). In
the present study, the changes in FA in the bilateral cerebral
hemispheres of the MCAO rats were significantly decreased compared
with the sham control group. This observation may account for
changes observed following supra-tentorial infarction, including
changes the normal conduction of the corpus callosum combined with
bilateral cerebral hemispheres and the cortical-pons-cerebellum
path, ultimately resulting in limited function in distant regions,
which may cause corresponding changes in blood flow and metabolic
activity of coupled cerebellar regions (36–38).
Thus, reduced FA values reflecting the atrophy of nerve fiber
bundles affect the degree and direction of the diffusion of water
molecules within the CCD-bridge-cerebellar cortex network.
In the present study, the reduced FA values of
bilateral cerebellar hemispheres following the core area infarct
suggested the occurrence of CCD following supratentorial cerebral
infarction. The infarction-induced change in the contralateral
cerebellar hemisphere was significantly greater than that of the
ipsilateral cerebellar hemisphere. The possible cause of this
phenomenon is that most nerve fibers connect the cerebral cortex to
the contralateral cerebellum, whereas only a small number of these
tracts relate to the ipsilateral cerebellum (39). Therefore, following infarction, the
right cerebellar hemisphere was affected more predominantly
compared with the left cerebellar hemisphere.
Supratentorial infarction caused atrophy of the
neuronal fibers and functional depression in the local region of
the lesion as well as in the distal site (38,40). The
severity and rate of necrosis of neuronal cells were affected by
several factors (16,41). Among them, RGMa was identified to
inhibit axonal growth and neural tube closure, as well as sham
control the proliferation and differentiation of neuronal cells via
repulsive guidance signals that exist in the choroid plexus of
normal rats, cerebellar Purkinje fibers and perivascular and
brainstem neurons (42). Schwab
et al (17) identified that
RGMa was accumulated in scar tissue following spinal cord injury.
Furthermore, the use of RGMa antibody reversed the axonal growth
inhibitory effect of RGMa. As a result, neurological degeneration
was blocked, thereby suggesting that RGMa participated in axonal
growth inhibition after central nervous system injury. Recent in
vitro functional studies using loss of function models have
evidenced the direct involvement of specific guidance molecules in
determining the growth and targeting of axonal fibers (15,43).
Thus, in the absence of these guidance molecules, the growing axons
lose their directional sense and result in disturbances in the
well-defined anatomical construction of axonal networks in the
hippocampus (44). The inhibiting
role of RGMa in neurite outgrowth and spinal cord injury has been
demonstrated in vitro and in vivo (45–47);
however, little is known regarding its contribution in cerebral
ischemia. The present study indicated that RGMa protein expression
was elevated after 1 h of the cerebral infarction and was maximal
at 24 h. However, a slight decrease in RGMa protein levels was
revealed at 72 h. Nevertheless, this level was higher in the tissue
from the MCAO group compared with that in sham control group. These
findings suggest that the elevated expression of RGMa protein in
the core area of the supratentorial infarction and the remotely
affected area of the cerebellum may be associated with axonal
atrophy in these regions. Notably, the gain- and loss-of-function
analysis of RGMa has demonstrated a role of this protein in
controlling the proliferation and differentiation of neuronal cells
and axon guidance (16).
In previous studies, in the early phase of acute
cerebral ischemia, RGMa protein overexpression was demonstrated to
inhibit the regeneration of nerve fibers and hindered the recovery
of neurological function. The RGMa-mediated inhibition of axonal
growth manifested as demyelination and atrophy of nerve fibers.
These changes led to the defective diffusion of water molecules in
brain tissue, which subsequently resulted in decreased FA values
(48,49). The decrease in FA values in the
supratentorial infarction area and the affected remote region of
bilateral cerebellar hemispheres was accompanied with an increased
expression of RGMa protein in these regions, thereby indicating a
negative correlation.
The present study had certain limitations. The
mechanisms underlying RGMa-mediated regulation of FA and the
development of CCD and MCAO have not been elucidated.
In conclusion, an established rat MCAO model of
ischemia-induced neuronal injury indicated that reduced FA values
were associated with increased expression of RGMa protein. These
results also confirmed that MR-DTI is reasonably sensitive for
detecting CCD, even as early as 1 h following the occurrence of a
supratentorial infarction. This non-invasive technique is useful in
measuring secondary changes in the distant regions after
supratentorial infarction. Furthermore, the measure of RGMa protein
expression reflected the extent of CCD in the present study and may
be used in understanding the mechanisms of neuronal atrophy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and YY conceived and designed the present study.
YZ, XW and JC developed the methodology. YZ, XW, JC, YL and LY
completed the experiments and collected the data. YZ, LY and ZC
analyzed and interpreted the data. YZ and YY drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Institutional Animal Care and Use Committee of Zhengzhou University
(Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Mendis S: Stroke disability and
rehabilitation of stroke: World Health Organization perspective.
Int J Stroke. 8:3–4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savitz SI and Caplan LR: Vertebrobasilar
disease. N Engl J Med. 352:2618–2626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta R, Joshi S, Mittal A, Luthra I,
Mittal P and Verma V: Magnetic resonance imaging depiction of
acquired Dyke-Davidoff-Masson syndrome with crossed
cerebro-cerebellar diaschisis: Report of two cases. J Pediatr
Neurosci. 10:294–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang KM, Sohn CH, Kim BS, Kim YI, Choi SH,
Yun TJ, Kim JH, Park SW, Cheon GJ and Han MH: Correlation of
asymmetry indices measured by arterial Spin-Labeling MR Imaging and
SPECT in patients with crossed cerebellar diaschisis. AJNR Am J
Neuroradiol. 36:1662–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Komaba Y, Mishina M, Utsumi K, Katayama Y,
Kobayashi S and Mori O: Crossed cerebellar diaschisis in patients
with cortical infarction: Logistic regression analysis to control
for confounding effects. Stroke. 35:472–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Lee SK, Lee JD, Kim YW and Kim DI:
Decreased fractional anisotropy of middle cerebellar peduncle in
crossed cerebellar diaschisis: Diffusion-tensor
imaging-positron-emission tomography correlation study. AJNR Am J
Neuroradiol. 26:2224–2228. 2005.PubMed/NCBI
|
|
7
|
Dani KA, Santosh C, Brennan D, Hadley DM
and Muir KW: Crossed cerebellar diaschisis: Insights into oxygen
challenge MRI. J Cereb Blood Flow Metab. 32:2114–2117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baron JC, Bousser MG, Comar D and
Castaigne P: ‘Crossed cerebellar diaschisis’ in human
supratentorial brain infarction. Trans Am Neurol Assoc.
105:459–461. 1981.PubMed/NCBI
|
|
9
|
Patay Z, Parra C, Hawk H, George A, Li Y,
Scoggins M, Broniscer A and Ogg RJ: Quantitative longitudinal
evaluation of diaschisis-related cerebellar perfusion and diffusion
parameters in patients with supratentorial hemispheric high-grade
gliomas after surgery. Cerebellum. 13:580–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takasawa M, Hashikawa K, Ohtsuki T,
Imaizumi M, Oku N, Kitagawa K, Hori M and Matsumoto M: Transient
crossed cerebellar diaschisis following thalamic hemorrhage. J
Neuroimaging. 11:438–440. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strother MK, Buckingham C, Faraco CC,
Arteaga DF, Lu P, Xu Y and Donahue MJ: Crossed cerebellar
diaschisis after stroke identified noninvasively with cerebral
blood flow-weighted arterial spin labeling MRI. Eur J Radiol.
85:136–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Karonen JO, Nuutinen J, Vanninen E,
Kuikka JT and Vanninen RL: Crossed cerebellar diaschisis in acute
ischemic stroke: A study with serial SPECT and MRI. J Cereb Blood
Flow Metab. 27:1724–1732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peled S and Yeshurun Y: Superresolution in
MRI: Application to human white matter fiber tract visualization by
diffusion tensor imaging. Magn Reson Med. 45:29–35. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon B, Kim JS, Lee KS, Kim BS, Chung SR
and Kim YI: Early pathological changes in the cerebellum of
patients with pure cerebellar syndrome demonstrated by
diffusion-tensor imaging. Eur Neurol. 56:166–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hata K, Fujitani M, Yasuda Y, Doya H,
Saito T, Yamagishi S, Mueller BK and Yamashita T: RGMa inhibition
promotes axonal growth and recovery after spinal cord injury. J
Cell Biol. 173:47–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsunaga E, Nakamura H and Chédotal A:
Repulsive guidance molecule plays multiple roles in neuronal
differentiation and axon guidance. J Neurosci. 26:6082–6088. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwab JM, Conrad S, Monnier PP, Julien S,
Mueller BK and Schluesener HJ: Spinal cord injury-induced lesional
expression of the repulsive guidance molecule (RGM). Eur J
Neurosci. 21:1569–1576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Key B and Lah GJ: Repulsive guidance
molecule A (RGMa) A molecule for all seasons. Cell Adh Migr.
6:85–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Severyn CJ, Shinde U and Rotwein P:
Molecular biology, genetics and biochemistry of the repulsive
guidance molecule family. Biochem J. 422:393–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Cheng JL, Ran YC, Zhang Y, Yang L
and Lin YN: Expression of RGMb in brain tissue of MCAO rats and its
relationship with axonal regeneration. J Neurol Sci. 383:79–86.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Zhang R, Xing X, Guo J, Xie F,
Zhang G and Qin X: Repulsive guidance molecule asuppresses
angiogenesis after ischemia/reperfusion injury of middle cerebral
artery occlusion in rats. Neurosci Lett. 662:318–323. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng G, Chen X, Xu B, Zhang J, Lv X, Li
J, Li F, Hu S, Zhang T and Li Y: Plasticity of language pathways in
patients with low-grade glioma: A diffusion tensor imaging study.
Neural Regen Res. 8:647–654. 2013.PubMed/NCBI
|
|
24
|
Kwon YH, Jang SH and Yeo SS: Age-related
changes of lateral ventricular width and periventricular white
matter in the human brain: A diffusion tensor imaging study. Neural
Regen Res. 9:986–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng X, Wang Q, Hou J, Zhang X, Wang E, Li
Q, Zeng Q, Wang Q, Li C and Ma X: Diffusion tensor imaging of
normal-appearing white matter in unilateral cerebral arterial
occlusive disease. J Magn Reson Imaging. 38:650–654. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Wan S and Zhang X:
Geniculocalcarine tract disintegration after ischemic stroke: A
diffusion tensor imaging study. AJNR Am J Neuroradiol.
34:1890–1894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Bihan D, Mangin JF, Poupon C, Clark CA,
Pappata S, Molko N and Chabriat H: Diffusion tensor imaging:
Concepts and applications. J Magn Reson Imaging. 13:534–546. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Chang W and Liu Y: Clinical
application of diffusion tensor imaging in acute stroke. Clin Med
China. 30:1166–1168. 2014.
|
|
29
|
Erbetta A, Mandelli M, Savoiardo M,
Grisoli M, Bizzi A, Soliveri P, Chiapparini L, Prioni S, Bruzzone
MG and Girotti F: Diffusion tensor imaging shows different
topographic involvement of the thalamus in progressive supranuclear
palsy and corticobasal degeneration. AJNR Am J Neuroradiol.
30:1482–1487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karampinos DC, Van AT, Olivero WC,
Georgiadis JG and Sutton BP: High-resolution diffusion tensor
imaging of the human pons with a reduced field-of-view, multishot,
variable-density, spiral acquisition at 3 T. Magn Reson Med.
62:1007–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lao Y, Kang Y, Collignon O, Brun C,
Kheibai SB, Alary F, Gee J, Nelson MD, Lepore F and Lepore N: A
study of brain white matter plasticity in early blinds using
tract-based spatial statistics and tract statistical analysis.
Neuroreport. 26:1151–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brillault J, Lam TI, Rutkowsky JM,
Foroutan S and O'Donnell ME: Hypoxia effects on cell volume and ion
uptake of cerebral microvascular endothelial cells. Am J Physiol
Cell Physiol. 294:C88–C96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang GY, Chen SF, Kinouchi H, Chan PH and
Weinstein PR: Edema, cation content, and ATPase activity after
middle cerebral artery occlusion in rats. Stroke. 23:1331–1336.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sorensen AG, Wu O, Copen WA, Davis TL,
Gonzalez RG, Koroshetz WJ, Reese TG, Rosen BR, Wedeen VJ and
Weisskoff RM: Human acute cerebral ischemia: Detection of changes
in water diffusion anisotropy by using MR imaging. Radiology.
212:785–792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Foong J, Maier M, Clark CA, Barker GJ,
Miller DH and Ron MA: Neuropathological abnormalities of the corpus
callosum in schizophrenia: A diffusion tensor imaging study. J
Neurol Neurosurg Psychiatry. 68:242–244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ito H, Takahashi K, Hatazawa J, Kim SG and
Kanno I: Changes in human regional cerebral blood flow and cerebral
blood volume during visual stimulation measured by positron
emission tomography. J Cereb Blood Flow Metab. 21:608–612. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Devor A, Ulbert I, Dunn AK, Narayanan SN,
Jones SR, Andermann ML, Boas DA and Dale AM: Coupling of the
cortical hemodynamic response to cortical and thalamic neuronal
activity. Proc Natl Acad Sci USA. 102:3822–3827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishihara M, Kumita S, Mizumura S and
Kumazaki T: Crossed cerebellar diaschisis: The role of motor and
premotor areas in functional connections. J Neuroimaging. 9:30–33.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeon YW, Kim SH, Lee JY, Whang K, Kim MS,
Kim YJ and Lee MS: Dynamic CT perfusion imaging for the detection
of crossed cerebellar diaschisis in acute ischemic stroke. Korean J
Radiol. 13:12–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SE and Lee MC: Cerebellar
vasoreactivity in stroke patients with crossed cerebellar
diaschisis assessRed by acetazolamide and 99mTc-HMPAO SPECT. J Nucl
Med. 41:416–420. 2000.PubMed/NCBI
|
|
41
|
Matsunaga E, Tauszig-Delamasure S, Monnier
PP, Mueller BK, Strittmatter SM, Mehlen P and Chédotal A: RGM and
its receptor neogenin regulate neuronal survival. Nat Cell Biol.
6:749–755. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian C and Liu J: Repulsive guidance
molecules (RGMs) and neogenin in bone morphogenetic protein (BMP)
signaling. Mol Reprod Dev. 80:700–717. 2013.PubMed/NCBI
|
|
43
|
Wang T, Wu X, Yin C, Klebe D, Zhang JH and
Qin X: CRMP-2 is involved in axon growth inhibition induced by RGMa
in vitro and in vivo. Mol Neurobiol. 47:903–913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brinks H, Conrad S, Vogt J, Oldecamp J,
Sierra A, Deitinghoff L, Bechmann I, Alvarez-Bolado G, Heimrich B,
Monnier PP, et al: The repulsive guidance molecule RGMa is involved
in the formation of afferent connections in the dentate gyrus. J
Neurosci. 24:3862–3869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Metzger M, Conrad S, Skutella T and Just
L: RGMa inhibits neurite outgrowth of neuronal progenitors from
murine enteric nervous system via the neogenin receptor in vitro. J
Neurochem. 103:2665–2678. 2007.PubMed/NCBI
|
|
46
|
Tassew NG, Charish J, Seidah NG and
Monnier PP: SKI-1 and Furin generate multiple RGMa fragments that
regulate axonal growth. Dev Cell. 22:391–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tao T, Xu G, Si Chen C, Feng J, Kong Y and
Qin X: Minocycline promotes axonal regeneration through suppression
of RGMa in rat MCAO/reperfusion model. Synapse. 67:189–198. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng J, Wang T, Li Q, Wu X and Qin X: RNA
interference against repulsive guidance molecule A improves axon
sprout and neural function recovery of rats after MCAO/reperfusion.
Exp Neurol. 238:235–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nimsky C, Ganslandt O, Hastreiter P, Wang
R, Benner T, Sorensen AG and Fahlbusch R: Intraoperative
diffusion-tensor MR imaging: Shifting of white matter tracts during
neurosurgical procedures-initial experience. Radiology.
234:218–225. 2005. View Article : Google Scholar : PubMed/NCBI
|