Introduction
Conjunctivitis associated systemic lupus
erythematosus (caSLE) is an autoimmune disease of connective tissue
with a large spectrum of clinical manifestations (1). Immune deregulation leads to
overproduction of autoantibody and immune complexes, complement
activation, and persistent tissue inflammation. This may lead to
uveitis, fundus changes or optic neuropathy. In severe forms it may
lead to sudden loss of vision; the exact mechanism not being fully
elucidated yet. To date, several SLE disease-causing antibodies
(ABs) have been identified, including anti-double-strand DNA ABs,
anti-nuclear ABs and anti-c1q antibodies. These ABs have been
associated with SLE and lupus nephritis activity. In clinical
practice Abs are currently widely used (2–5), while
caSLE research remains challenging.
Novel molecular genetic and genomic tools,
proteomics and transcriptomics are also used in order to study in
depth various diseases.
Vascular adhesion protein-1 (VAP-1) is an
endothelial adhesion molecule found in patients with synovitis
(6,7), being associated with white blood cells
(granulocytes) extravasation from blood into inflammatory tissue.
In the muscular stratum of the blood vessels and other tissues,
VAP-1 is either tissue-bound or is found in soluble isoforms. The
tissue-bound form seems to play a role in cellular differentiation,
lipid trafficking and deposition of extracellular matrix components
in the smooth muscle (8,9). The soluble isoform is both a
pre-inflammatory protein as well as an adhesion molecule, a primary
AOC3 amine oxidase and a regulator for leucocyte recruitment. This
form plays a role in the clinical manifestation of the disease
(9).
The aim of this study was to investigate the
expression of VAP-1 in caSLE patients and to study the relationship
between VAP-1 and other proteins in the onset of the disease.
Materials and methods
Sample collection
In the present study, 10 patients with caSLE (caSLE
group) and 10 healthy individuals (control group) were recruited.
The caSLE group were treated in the Jiamusi University, the First
Affiliated Hospital (Heilongjiang, China) between February 2015 and
February 2017. The study was approved by the Ethics Committee of
the First Affiliated Hospital of Jiamusi University (Heilongjiang,
China). All the patients signed an informed consent to participate
in the study.
Sample preparation
Blood serum samples were collected from each
volunteer. Depletion of albumin/IgG followed, using ProteoExtract
Albumin/IgG Removal kit (EMD Biosciences) according to the
manufacturer's instructions. All samples were concentrated and
desalted by ultrafiltration using a 3-kDa cutoff Centrifugal Filter
Device (EMD Millipore). 2-D Quant kit (GE Healthcare) was used for
determination of protein concentration. Depleted sera were stored
at −80°C for further analysis. Three CyDye DIGE fluors (GE
Healthcare), Cy2, Cy3 and Cy5 protocol previously described by
Hotte and Deyholos (10) was used
for labelling protein extracts.
Two-dimensional gel electrophoresis
image analysis
The method used for 2-D electrophoresis was
previously described by Liu et al (11). The reagents and equipment used for
first dimension were immobilized pH gradient (IPG) strips (24 cm;
pH 4.0–7.0); Ettan IPGphor 3 isoelectric focusing system; and
SDS-PAGE gels prepared on a low fluorescence borosilicate glass
plate (all from GE Healthcare). For the second dimension separation
Ettan DALT six electrophoresis system, and IPG strips (24 cm; pH
4.0–7.0) (both from GE Healthcare), Coomassie brilliant blue G-250
(Bio-Rad Laboratories, Inc.) were used. All images were collected
on a Typhoon Trio Variable Mode Imager (GE Healthcare). After the
protein spot analysis those with significant differences in
abundance (>1.5-fold) were selected for spot picking by mass
spectrometry.
MALDI mass spectrometer
The protein spots were measured using a 4800
MALDI-TOF/TOF™ Analyzer (Applied Biosystems). The method was
previously described by Junker et al (12). GPS Explorer software (version 3.6;
Applied Biosystems) was used for combined database search of MS and
MS/MS measurements. Peak lists were compared with the SwissProt
database v56.1 human taxonomy was used for comparison of peak
lists.
Enzyme-linked immunosorbent assay
(ELISA)
The expression of identified serum levels of VAP-1
proteins was confirmed using ELISA method as previously described
by Junker et al (12). ELISA
kit (no. 20201ES90; Micro BCA Protein Assay kit; Thermo Fisher
Scientific, Inc.) for VAP-1 was used according to the
manufacturer's specifications.
Western blotting
Western blotting was performed according to the
methodology of Lappas (13). RIPA
buffer was used (100 µl; Gibco; Thermo Fisher Scientific, Inc.).
BCA Protein Assay (Pierce) was used to determine the protein
concentration and 20 µg of protein/lane was separated via SDS-PAGE
on a 12% gel. The separated proteins were subsequently transferred
onto a polyvinylidene difluoride membrane (EMD Millipore) and
blocked for 1.5–2 h at 34°C with 1% bovine serum albumin (BSA).
Following antibodies were utilized: primary antibodies, RhoB
(dilution 1:1,000; cat. no. ab53743, Abcam), and β-actin (dilution
1:20,000, cat. no. ab8227, Abcam), secondary antibodies conjugated
with horseradish peroxidase (HRP) (dilution 1:10,000, cat. no.
ENZ-321; GE Healthcare). Protein bands were visualized using the
Pierce™ ECL Plus Western Blotting Substrate (Pierce; Thermo Fisher
Scientific, Inc.). Quantity One 4.2.1 image analysis software
(Bio-Rad Laboratories, Inc.) was used for quantitative analysis of
the bands obtained in western blot analyses.
Statistical analysis
All the samples were worked in triplicates.
Numerical continuous variables were expressed as the mean ± the
standard deviation (mean ± SD). Data were analysed using Stata
software (Stata 15.1; StataCorp). Comparison between the two groups
was performed by Students t-test. The bioinformatic analysis was
performed using STRING database (version 8.3; http://string.embl.de/) in order to identify the
proteins for further study.
Results
Validation of altered VAP-1 by
ELISA
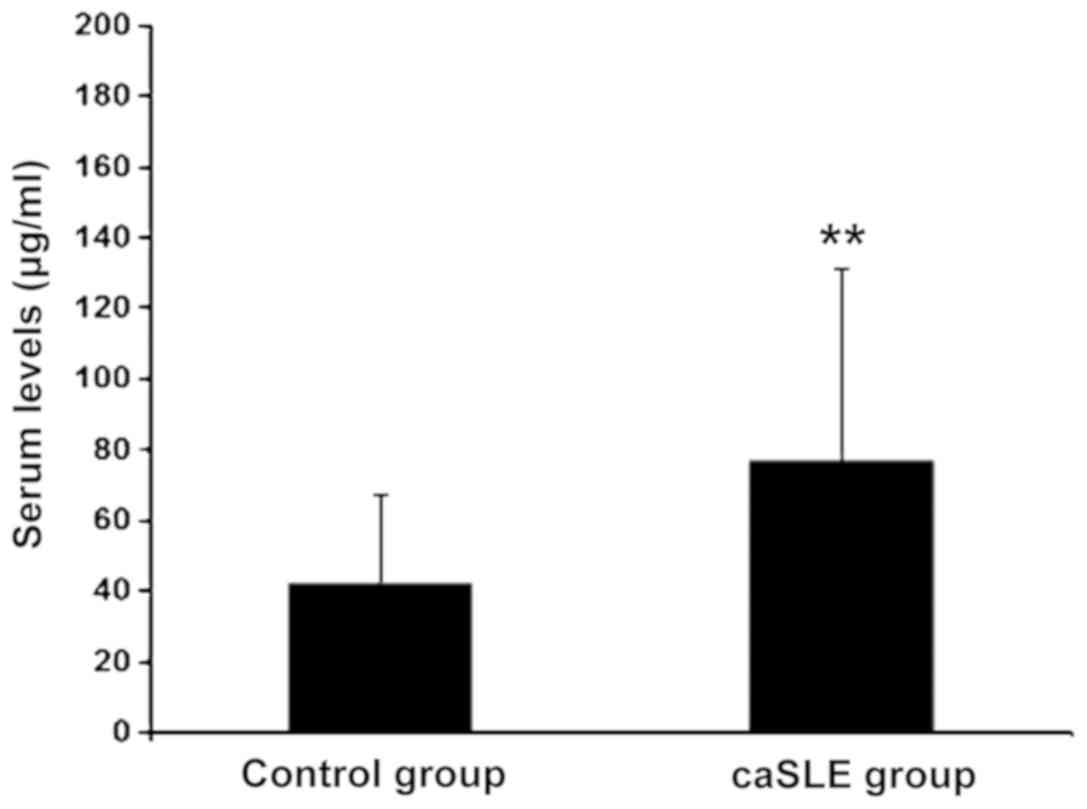
The caSLE group VAP-1 levels were increased as
compared to the control group, (risk-ratio = 2.77; P<0.01)
(Fig. 1).
Electrophoresis of fluorescein
markers
In Fig. 2 the
distribution of protein expression assessed by immune fluorescence
in patients with caSLE and conjunctivitis is presented. Among the
caSLE patient samples and controls we identified different
expression in 8 proteins, 2 of which were downregulated while 6
were upregulated (Table I and
Fig. 2).
 | Table I.Differential protein. |
Table I.
Differential protein.
| Uniprot accession
no. | Name | Symbol | Average ratio | P-value (t-test) |
|---|
| P02741 | CRP_HUMAN C-reactive
protein | CRP_HUMAN | +2.79 | <0.001 |
| P68871 | Hemoglobin subunit β
(β-globin) HBB | HBB_HUMAN | +2.34 | <0.001 |
| Q16853 | Vascular adhesion
protein-1, VAP-1 | VAP-1_HUMAN | +11.72 | <0.001 |
| P43652 | Afamin (A-albumin,
A-Alb) AFM | AFAM HUMAN | +2.02 | <0.001 |
| P06733 | Plasminogen-binding
protein, ENOA | ENOA_HUMAN | +1.79 | <0.001 |
| P01871 | Immunoglobulin heavy
constant mu, IGHM | IGHM_HUMAN | +2.54 | <0.001 |
| P10914 | Interferon regulatory
factor-1, IRF-1 | IRF1_HUMAN | −1.71 | <0.001 |
| P0DJI9 | Serum amyloid A-2
protein, SAA2 | SAA2_HUMAN | −1.73 | <0.001 |
Mass spectrometry analysis, revealed six upregulated
proteins including C-reactive protein (CRP), hemoglobin subunit β
(HBB), VAP-1, A-albumin (AFAM), enolase (ENOA), immunoglobulin
heavy constant mu (IGHM), and two downregulated proteins:
interferon regulatory factor-1 (IRF-1) and serum amyloid A2 protein
(SAA2) (Fig. 2 and Table I).
Bioinformatics analysis
Using an online tool for identifying and analyzing
the differentially expressed proteins Metacore software (GeneGo
Inc.) it was demonstrated that these differences were involved in
various biological processes of protein such as growth, secretion,
pathogenesis, translation, cell apoptosis, death, digestion and
signal transduction (Table I).
Interestingly, it was shown that the VAP-1 protein participates in
thirteen important biological processes.
Western blotting
Plasma proteins were extracted from the caSLE and
the control group respectively. VAP-1 protein expressed in the
control group has a significantly lower expression level than the
caSLE patient group, which was consistent with the results of
two-dimensional electrophoresis. A significant difference between
the two groups was observed (Fig.
3).
Expression of VAP-1 in database
We used String database to search protein VAP-1.
According to the network structure, both control and caSLE groups
involved multiple biological processes; positive regulation of
T-cell mediated (12.41%); oxygen transport (5.21%); peroxidase
activity binding (28.71%); and catalytic activity (18.68%)
(Fig. 4).
Discussion
SLE is a common autoimmune disease. Promotion of
inflammatory cytokines and anti-inflammatory cytokines in humans
play an important role in the occurrence and development of SLE as
well as in the disease associated multiple organs involvement
(8,14). This comparative proteomics study
found a number of proteins that are differentially expressed in
caSLE affected individuals.
The wide application of proteomic techniques has led
to many studies in the field of caSLE (8). Some results in this study were achieved
using proteomics, however application of proteomics in the present
study was methodologically different.
Proteomics research based on fluorescence
differential bidirectional gel electrophoresis (2-DIGE) is a new
trend. 2-DIGE has many advantages over conventional 2-DE: The use
of 3 different fluorescent dyes for marking can separate multiple
samples with different fluorescent tags on the same glue. Different
fluorescence labeling samples have different excitation
wavelengths, which can record the results of each non-interference
with different filters. Avoiding the error of glue, to reflect the
real degree of protein change, thus improving accuracy; using an
internal standard, the application software can automatically
calibrate expression according to the internal standard of each
protein point; reduce the error; there are fewer human factors to
ensure higher throughput and experimental accuracy (12,15–19). In
a mouse model study with late-onset systemic lupus erythematosus
E2F2, Azkargorta et al (20)
showed using two-dimensional fluorescence difference gel
electrophoresis (2D-DIGE) and mass spectroscopy, that when
comparing T-lymphocytes in the study group and in the control
group, a significant difference in T-cells and protein expression
was implicated in receptor mediated stress response, signal
transduction and cell survival.
This study used coremine database (http://www.coremine.com/) to identify differentially
expressed proteins in 16 different proteins. These proteins are
involved in 1,446 different biological processes, former eight
biological processes, growth, secretion, pathogenesis, translation,
cell apoptosis, death, digestive and signal transduction. The newly
found biological processes include cell stimulation,
single-organism process, multiceller organismal process, binding,
immune system process. It is worth noting that the VAP-1 protein is
involved in the 13 most important biological processes. Using the
online tool STRING to VAP-1 protein separation study found that:
VAP-1 protein can participate in important functions such as
complex inflammatory signaling pathways and receptor activities
play an important role in biological processes. Using a single
protein as a target, Dunkel et al (21) suggested that VAP-1 protein had a
close relationship with other identification proteins (Fig. 4).
Amine oxidase, copper containing 3 (AOC3), is a
major VAP-1 in endothelial cell surface adhesion molecule and its
expression in the process of inflammation was obviously raised.
AOC3 can increase the VAP-1 to adjust mediated neutrophil and other
inflammatory cell adhesion, in order to migrate to the area of
inflammation. After this progress endothelial cells can induce
expression of intercellular adhesion molecule-1, IL-8. Kushimoto
et al (22) found that
blocking the expression of VAP-1 can significantly inhibit
angiogenesis of inflammatory mediators, suggesting that VAP-1 can
promote the occurrence and development of inflammation. Research
shows that in many inflammatory diseases, such as psoriasis,
chronic kidney disease, chronic liver disease, multiple sclerosis,
VAP-1 content in peripheral blood increased significantly, which
further prompts VAP-1 involvement in inflammation of the
pathological process (23). In
blood, VAP-1 protein is the carrier of vitamins and endotoxin
playing an important role in vitamin metabolism. VAP-1 proteins may
be combined with compound factor activation, enhance the regulation
of information, regulating immune function, this function in
different cells (initially monocytes and neutrophils) are found;
the protein binds to the surface of the lymphocytes, specifically
it binds to the membrane of the receptor on the lymphocytes
(24). Once bound the protein
complex is activated, and may initiate macrophages. As an
autoimmune disease, SLE eye lesions may not only be the first
manifestation, but may also be a prognostic as well as a predictive
factor for the disease. Unlike connective tissue of the eyes, SLE
can affect almost any part of the eye (eyelid, eye ball and dome
conjunctiva), causing epithelial hyperplasia of fiber, resulting in
conjunctivitis, and may release various inflammatory mediators and
proteolytic enzymes, such as erosion on organization. Therefore, we
hypothesized that the inflammatory response of VAP-1 may be related
with caSLE, and directly or indirectly involved in inflammatory
responses in the blood.
Proteomics offers unique advantages in the field of
life science research, and provides a powerful tool in the study of
many diseases such as SLE, attaining remarkable achievements. With
the further development of proteomics technology, functional
proteomics and biomarkers may be used to study SLE pathogenesis,
targeted therapy and drug development, providing an important
theoretical basis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and HS were responsible for the conception and
design of the study. JZ and PZ contributed to the collection and
assembly of the data. JZ, MK and ND completed data analysis and
interpretation, and HL and HS wrote the manuscript. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Jiamusi University (Heilongjiang,
China), and each patient signed an informed consent to participate
in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VAP-1
|
vascular adhesion protein-1
|
|
caSLE
|
conjunctivitis associated systemic
lupus erythematosus
|
|
2D-DIGE
|
two-dimensional fluorescence
difference gel electrophoresis
|
|
AOC3
|
copper containing 3
|
|
CRP
|
C-reactive protein
|
|
HBB
|
hemoglobin subunit β
|
|
AFAM
|
A-albumin
|
|
ENOA
|
enolase
|
|
IGHM
|
immunoglobulin heavy constant mu
|
|
IRF-1
|
interferon regulatory factor-1
|
|
SAA2
|
serum amyloid A2 protein
|
References
|
1
|
Ferreira TA, de Andrade HM, de Pádua PM,
Carvalho MD, Pires SD, Oliveira IH, Lima BS, Fialho Júnior LC,
Cicarini WB, Chapeourouge DA, et al: Identification of potential
biomarkers for systemic lupus erythematosus diagnosis using
two-dimensional differential gel electrophoresis (2D-DIGE) and mass
spectrometry. Autoimmunity. 50:247–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hung WT, Chen YM, Lan JL, Chen HH, Chen
YH, Chen DY, Hsieh CW and Wen MC: Antinucleosome antibodies as a
potential biomarker for the evaluation of renal pathological
activity in patients with proliferative lupus nephritis. Lupus.
20:1404–1410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ravirajan CT, Rowse L, Mac Gowan JR and
Isenberg DA: An analysis of clinical disease activity and
nephritis-associated serum autoantibody profiles inpatients with
systemic lupus erythematosus: A cross-sectional study. Rheumatology
(Oxford). 40:1405–1412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Förger F, Matthias T, Oppermann M, Becker
H and Helmke K: Clinical significance of anti-ds DNA antibody
isotypes: Ig G/Ig M ratio of anti-ds DNA antibodies as a prognostic
marker for lupus nephritis. Lupus. 13:36–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trendelenburg M, Lopez-Trascasa M,
Potlukova E, Moll S, Regenass S, Frémeaux-Bacchi V, Martinez-Ara J,
Jancova E, Picazo ML, Honsova E, et al: High prevalence of anti-C1q
antibodies in biopsy-proven active lupus nephritis. Nephrol Dial
Transplant. 21:3115–3121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiang YJ and Dai SM: Prevalence of
rheumatic diseases and disability in China. Rheumatol Int.
29:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murata M, Noda K, Fukuhara J, Kanda A,
Kase S, Saito W, Ozawa Y, Mochizuki S, Kimura S, Mashima Y, et al:
Soluble vascular adhesion protein-1 accumulates in proliferative
diabetic retinopathy. Invest Ophthalmol Vis Sci. 53:4055–4062.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solé M and Unzeta M: Vascular cell lines
expressing SSAO/VAP-1: A new experimental tool to study its
involvement in vascular diseases. Biol Cell. 103:543–557. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feldman AS, Banyard J, Wu CL, McDougal WS
and Zetter BR: Cystatin B as a tissue and urinary biomarker of
bladder cancer recurrence and disease progression. Clin Cancer Res.
15:1024–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotte NS and Deyholos MK: A flax fibre
proteome: Identification of proteins enriched in bast fibres. BMC
Plant Biol. 8:522008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Huang P, He Y, Hong WX, Ren X, Yang
X, He Y, Wang W, Zhang R, Yang H, et al: Serum amyloid A and
clusterin as potential predictive biomarkers for severe hand, foot
and mouth disease by 2D-DIGE proteomics analysis. PLoS One.
9:e1088162014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Junker H, Venz S, Zimmermann U, Thiele A,
Scharf C and Walther R: Stage-related alterations in renal cell
carcinoma-comprehensive quantitative analysis by 2D-DIGE and
protein network analysis. PLoS One. 6:e218672011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lappas M: Anti-inflammatory properties of
sirtuin 6 in human umbilical vein endothelial cells. Mediators
Inflamm. 2012:5975142012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan H, Yao YS, Chen GM, Sheng J, Xu L and
Pan HF: Decreased serum levels of T-cell immunoglobulin mucin-1 and
T-cell immunoglobulin mucin-3 in systemic lupus erythematosus
patients. J Biol Regul Homeost Agents. 30:123–129. 2016.PubMed/NCBI
|
|
15
|
Penno MA, Klingler-Hoffmann M, Brazzatti
JA, Boussioutas A, Putoczki T, Ernst M and Hoffmann P: 2D-DIGE
analysis of sera from transgenic mouse models reveals novel
candidate protein biomarkers for human gastric cancer. J
Proteomics. 77:402012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhen Y, Xu N, Richardson B, Becklin R,
Savage JR, Blake K and Peltier JM: Development of an LC-MALDI
method for the analysis of protein complexes. J Am Soc Mass
Spectrom. 15:8032004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng L, Jia HL, Liu CW, Xu YF, Mao LJ, He
CH, Yin GQ, Lin JH, Tao JP and Zhu L: Proteomic analysis of
extremely severe hand, foot and mouth disease infected by
enterovirus 71. BMC Infect Dis. 13:3832013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong MY, Saad S, Pollock CA and Wong MG:
Semicarbazide-sensitive amine oxidase (SSAO) and kidney disease. Am
J Physiol Renal Physiol. 305:1637–1644. 2013. View Article : Google Scholar
|
|
19
|
Yu SL, Kuan WP, Wong CK, Li EK and Tam LS:
Immunopathological roles of cytokines, chemokines, signaling
molecules, and pattern-recognition receptors in systemic lupus
erythematosus. Clin Dev Immunol. 2012:7151902012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Azkargorta M, Arizmendi JM, Elortza F,
Alkorta N, Zubiaga AM and Fullaondo A: Differential proteome
profiles in E2F2-deficient T lymphocytes. Proteomics. 6 (Suppl
1):S42–S50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dunkel J, Aguilar-Pimentel JA, Ollert M,
Fuchs H, Gailus-Durner V, de Angelis MH, Jalkanen S, Salmi M and
Veres TZ: Endothelial amine oxidase AOC3 transiently contributes to
adaptive immune responses in the airways. Eur J Immunol.
44:3232–3239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kushimoto S, Shibata Y, Koido Y, Kawai M,
Yokota H and Yamamoto Y: The clinical usefulness of procalcitonin
measurement for assessing the severity of bacterial infection in
critically ill patients requiring corticosteroid therapy. J Nippon
Med Sch. 74:236–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Apostolidis SA, Lieberman LA, Kis-Toth K,
Crispín JC and Tsokos GC: The dysregulation of cytokine networks in
systemic lupus erythematosus. J Interferon Cytokine Res.
31:769–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trivedi PJ, Tickle J, Vesterhus MN,
Eddowes PJ, Bruns T, Vainio J, Parker R, Smith D, Liaskou E,
Thorbjørnsen LW, et al: Vascular adhesion protein-1 is elevated in
primary sclerosing cholangitis, is predictive of clinical outcome
and facilitates recruitment of gut-tropic lymphocytes to liver in a
substrate-dependent manner. Gut. 67:1135–1145. 2018. View Article : Google Scholar : PubMed/NCBI
|