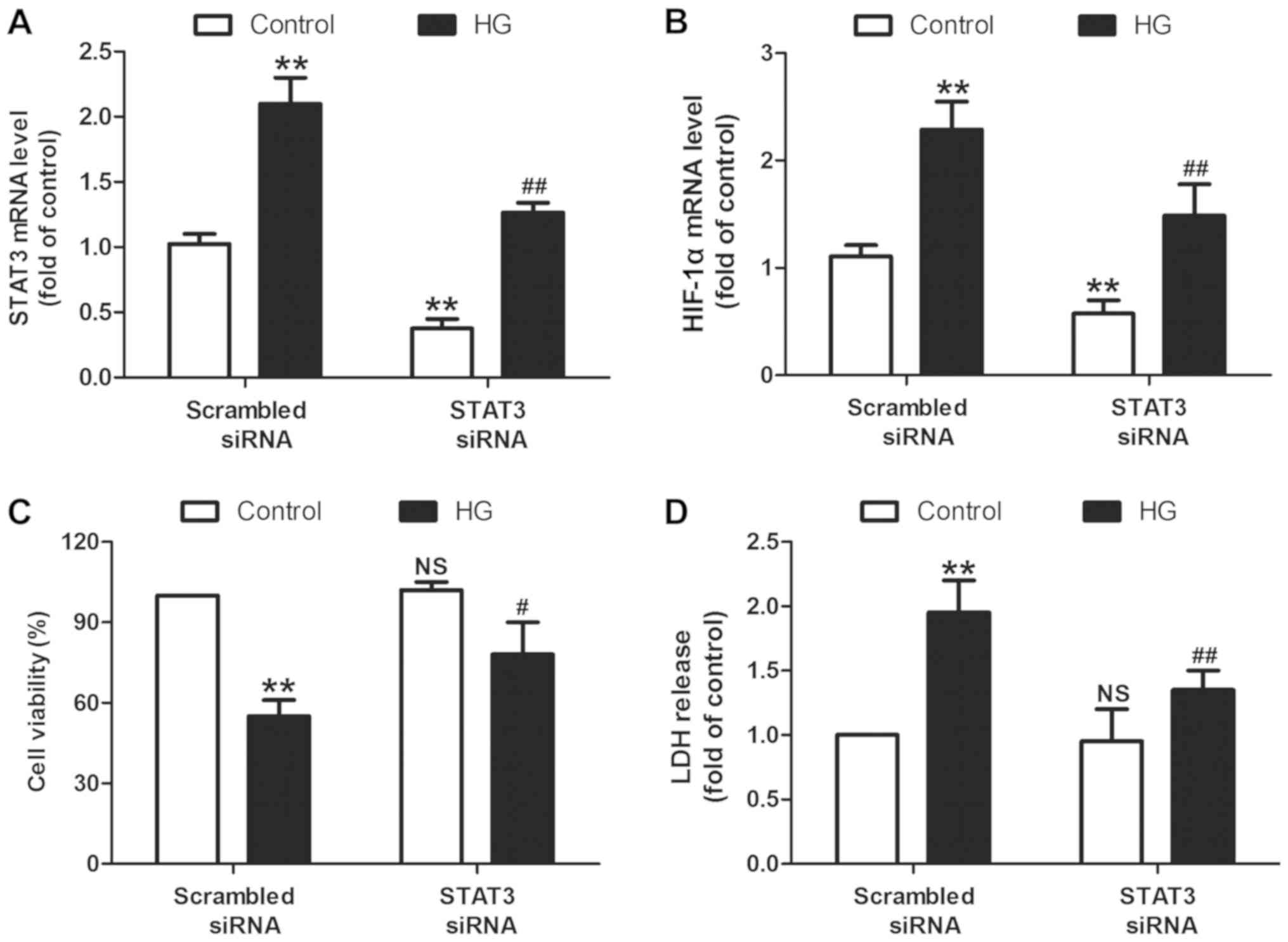
|
1
|
Emerging Risk Factors Collaboration, ;
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio
E, Ingelsson E, Lawlor DA, Selvin E, et al: Diabetes mellitus,
fasting blood glucose concentration, and risk of vascular disease:
A collaborative meta-analysis of 102 prospective studies. Lancet.
375:2215–2222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jia G, Whaley-Connell A and Sowers JR:
Diabetic cardiomyopathy: A hyperglycaemia- and
insulin-resistance-induced heart disease. Diabetologia. 61:21–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mishra PK, Ying W, Nandi SS, Bandyopadhyay
GK, Patel KK and Mahata SK: Diabetic cardiomyopathy: An
immunometabolic perspective. Front Endocrinol (Lausanne). 8:722017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian J, Zhao Y, Liu Y, Liu Y, Chen K and
Lyu S: Roles and Mechanisms of herbal medicine for diabetic
cardiomyopathy: Current status and perspective. Oxid Med Cell
Longev. 2017:82145412017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faria A and Persaud SJ: Cardiac oxidative
stress in diabetes: Mechanisms and therapeutic potential. Pharmacol
Ther. 172:50–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kayama Y, Raaz U, Jagger A, Adam M,
Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM and Tsao
PS: Diabetic cardiovascular disease induced by oxidative stress.
Int J Mol Sci. 16:25234–25263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huynh K, Kiriazis H, Du XJ, Love JE, Gray
SP, Jandeleit-Dahm KA, McMullen JR and Ritchie RH: Targeting the
upregulation of reactive oxygen species subsequent to hyperglycemia
prevents type 1 diabetic cardiomyopathy in mice. Free Radic Biol
Med. 60:307–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
Mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang R: The gasotransmitter role of
hydrogen sulfide. Antioxid Redox Signal. 5:493–501. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Powell CR, Dillon KM and Matson JB: A
review of hydrogen sulfide (H2S) donors: Chemistry and
potential therapeutic applications. Biochem Pharmacol. 149:110–123.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calvert JW, Coetzee WA and Lefer DJ: Novel
insights into hydrogen sulfide-mediated cytoprotection. Antioxid
Redox Signal. 12:1203–1217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Li M, Li Y, Sun W, Wang Y, Bai S, Li
H, Wu B, Yang G, Wang R, et al: Exogenous H2S contributes to
recovery of ischemic post-conditioning-induced cardioprotection by
decrease of ROS level via down-regulation of NF-κB and JAK2-STAT3
pathways in the aging cardiomyocytes. Cell Biosci. 6:262016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu MH, Lin XL, Zhang Y, He J, Tan TP, Wu
SJ, Liu J, Tian W, Chen L, Yu S, et al: Hydrogen sulfide attenuates
doxorubicin-induced cardiotoxicity by inhibiting reactive oxygen
species-activated extracellular signal-regulated kinase 1/2 in H9c2
cardiac myocytes. Mol Med Rep. 12:6841–6848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian LL, Liu XY, Chai Q and Wang RX:
Hydrogen sulfide in diabetic complications: Focus on molecular
mechanisms. Endocr Metab Immune Disord Drug Targets. 18:470–476.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong F, Chai R, Jiang H and Dong B: In
vitro/vivo drug release and anti-diabetic cardiomyopathy properties
of curcumin/PBLG-PEG-PBLG nanoparticles. Int J Nanomedicine.
13:1945–1962. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong
S, Wu J, Zhao Y, Xu C, Zhang W and Lu F: Exogenous H2S protects
against diabetic cardiomyopathy by activating autophagy via the
AMPK/mTOR pathway. Cell Physiol Biochem. 43:1168–1187. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Yu X, Li T, Wu J, Zhao Y, Liu J,
Sun A, Dong S, Wu J, Zhong X, et al: Exogenous H2S
regulates endoplasmic reticulum-mitochondria cross-talk to inhibit
apoptotic pathways in STZ-induced type I diabetes. Am J Physiol
Endocrinol Metab. 312:E190–E203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, An G and Lu X: Hydrogen sulfide
attenuates the development of diabetic cardiomyopathy. Clin Sci
(Lond). 128:325–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haghikia A, Ricke-Hoch M, Stapel B, Gorst
I and Hilfiker-Kleiner D: STAT3, a key regulator of cell-to-cell
communication in the heart. Cardiovasc Res. 102:281–289. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szczepanek K, Chen Q, Larner AC and
Lesnefsky EJ: Cytoprotection by the modulation of mitochondrial
electron transport chain: The emerging role of mitochondrial STAT3.
Mitochon. 12:180–189. 2012. View Article : Google Scholar
|
|
21
|
He Y, Khan M, Yang J, Yao M, Yu S and Gao
H: Proscillaridin A induces apoptosis, inhibits STAT3 activation
and augments doxorubicin toxicity in prostate cancer cells. Int J
Med Sci. 15:832–839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Sullivan KE, Breen EP, Gallagher HC,
Buggy DJ and Hurley JP: Understanding STAT3 signaling in cardiac
ischemia. Basic Res Cardiol. 111:272016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Li H, Wang S, Mao X, Yan D, Wong
SS, Xia Z and Irwin MG: Repeated non-invasive limb ischemic
preconditioning confers cardioprotection through PKC-ε/STAT3
signaling in diabetic rats. Cell Physiol Biochem. 45:2107–2121.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan
L, Su W, Liu H, Xu J, Liu Z, et al: Selective inhibition of PTEN
preserves ischaemic post-conditioning cardioprotection in
STZ-induced type 1 diabetic rats: Role of the PI3K/AkT and
JAK2/STAT3 pathways. Clin Sci (Lond). 130:377–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Owais K, Huang T, Mahmood F, Hubbard J,
Saraf R, Bardia A, Khabbaz KR, Li Y, Bhasin M, Sabe AA, et al:
Cardiopulmonary bypass decreases activation of the signal
transducer and activator of transcription 3 (STAT3) pathway in
diabetic human myocardium. Ann Thorac Surg. 100:1636–1645. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT
and Chen ZC: Ginsenoside Rh2 improves cardiac fibrosis via
PPARδ-STAT3 signaling in type 1-like diabetic rats. Int J Mol Sci.
18(pii): E13642017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong A and Liu Y: Targeting hypoxia
inducible factors-1α as a novel therapy in fibrosis. Front
Pharmacol. 8:3262017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Norouzirad R, González-Muniesa P and
Ghasemi A: Hypoxia in obesity and diabetes: Potential therapeutic
effects of hyperoxia and nitrate. Oxid Med Cell Longev.
2017:53502672017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu G, Briggs J, Deng J, Ma Y, Lee H,
Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R and Yu H: Signal
transducer and activator of transcription 3 is required for
hypoxia-inducible factor-1alpha RNA expression in both tumor cells
and tumor-associated myeloid cells. Mol Cancer Res. 6:1099–1105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Whiteman M, Guan YY, Neo KL, Cheng
Y, Lee SW, Zhao Y, Baskar R, Tan CH and Moore PK: Characterization
of a novel, water-soluble hydrogen sulfide-releasing molecule
(GYY4137): New insights into the biology of hydrogen sulfide.
Circulation. 117:2351–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu KM, Hsu YM, Ying MC, Tsai FJ, Tsai CH,
Chung JG, Yang JS, Tang CH, Cheng LY, Su PH, et al: High-density
lipoprotein ameliorates palmitic acid-induced lipotoxicity and
oxidative dysfunction in H9c2 cardiomyoblast cells via ROS
suppression. Nutr Metab (Lond). 16:362019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Li J and Li D: Losartan reduces
myocardial interstitial fibrosis in diabetic cardiomyopathy rats by
inhibiting JAK/STAT signaling pathway. Int J Clin Exp Pathol.
8:466–473. 2015.PubMed/NCBI
|
|
34
|
Niu G, Briggs J, Deng J, Ma Y, Lee H,
Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R and Yu H: Signal
transducer and activator of transcription 3 is required for
hypoxia-inducible factor-1alpha RNA expression in both tumor cells
and tumor-associated myeloid cells. Mol Cancer Res. 6:1099–1105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lassègue B, San Martín A and Griendling
KK: Biochemistry, physiology, and pathophysiology of NADPH oxidases
in the cardiovascular system. Circ Res. 110:1364–1390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du S, Huang Y, Jin H and Wang T:
Protective mechanism of hydrogen sulfide against
chemotherapy-induced cardiotoxicity. Front Pharmacol. 9:322018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagpure BV and Bian JS: Interaction of
hydrogen sulfide with nitric oxide in the cardiovascular system.
Oxid Med Cell Longev. 2016:69043272016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun Y, Tian Z, Liu N, Zhang L, Gao Z, Sun
X, Yu M, Wu J, Yang F, Zhao Y, et al: Exogenous H2S
switches cardiac energy substrate metabolism by regulating SIRT3
expression in db/db mice. J Mol Med (Berl). 96:281–299. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang Z, Dong X, Zhuang X, Hu X, Wang L
and Liao X: Exogenous hydrogen sulfide protects against high
glucose-induced inflammation and cytotoxicity in H9c2 cardiac
cells. Mol Med Rep. 14:4911–4917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei WB, Hu X, Zhuang XD, Liao LZ and Li
WD: GYY4137, a novel hydrogen sulfide-releasing molecule, likely
protects against high glucose-induced cytotoxicity by activation of
the AMPK/mTOR signal pathway in H9c2 cells. Mol Cell Biochem.
389:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alikhah A, Pahlevan Kakhki M, Ahmadi A,
Dehghanzad R, Boroumand MA and Behmanesh M: The role of lnc-DC long
non-coding RNA and SOCS1 in the regulation of STAT3 in coronary
artery disease and type 2 diabetes mellitus. J Diabetes
Complications. 32:258–265. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Andreadou I, Efentakis P, Balafas E,
Togliatto G, Davos CH, Varela A, Dimitriou CA, Nikolaou PE, Maratou
E, Lambadiari V, et al: Empagliflozin limits myocardial infarction
in vivo and cell death in vitro: Role of STAT3, mitochondria, and
redox aspects. Front Physiol. 8:10772017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Das A, Salloum FN, Filippone SM, Durrant
DE, Rokosh G, Bolli R and Kukreja RC: Inhibition of mammalian
target of rapamycin protects against reperfusion injury in diabetic
heart through STAT3 signaling. Basic Res Cardiol. 110:312015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Papadakis AI, Paraskeva E, Peidis P,
Muaddi H, Li S, Raptis L, Pantopoulos K, Simos G and Koromilas AE:
eIF2{alpha} kinase PKR modulates the hypoxic response by
Stat3-dependent transcriptional suppression of HIF-1{alpha}. Cancer
Res. 70:7820–7829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng SC, Quintin J, Cramer RA, Shepardson
KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao
NA, Aghajanirefah A, et al: mTOR- and HIF-1α-mediated aerobic
glycolysis as metabolic basis for trained immunity. Science.
345:12506842014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT
and Chen ZC: Ginsenoside Rh2 improves cardiac fibrosis via
PPARδ-STAT3 signaling in type 1-like diabetic rats. Int J Mol Sci.
18(pii): E13642017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pipicz M, Demján V, Sárközy M and Csont T:
Effects of cardiovascular risk factors on cardiac STAT3. Int J Mol
Sci. 19(pii): E35722018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma A, Tate M, Mathew G, Vince JE,
Ritchie RH and de Haan JB: Oxidative stress and NLRP3-inflammasome
activity as significant drivers of diabetic cardiovascular
complications: Therapeutic implications. Front Physiol. 9:1142018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan B, Ren J, Zhang Q, Gao R, Zhao F, Wu J
and Yang J: Antioxidative effects of natural products on diabetic
cardiomyopathy. J Diabetes Res. 2017:20701782017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gilca GE, Stefanescu G, Badulescu O,
Tanase DM, Bararu I and Ciocoiu M: Diabetic cardiomyopathy: Current
approach and potential diagnostic and therapeutic targets. J
Diabetes Res. 2017:13102652017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Movafagh S, Crook S and Vo K: Regulation
of hypoxia-inducible factor-1a by reactive oxygen species: New
developments in an old debate. J Cell Biochem. 116:696–703. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye P, Gu Y, Zhu YR, Chao YL, Kong XQ, Luo
J, Ren XM, Zuo GF, Zhang DM and Chen SL: Exogenous hydrogen sulfide
attenuates the development of diabetic cardiomyopathy via the FoxO1
pathway. J Cell Physiol. 233:9786–9798. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang R, Jia Q, Liu XF, Gao Q, Wang L and
Ma SF: Effect of hydrogen sulfide on oxidative stress and
endoplasmic reticulum stress in diabetic cardiomyopathy. Zhongguo
Ying Yong Sheng Li Xue Za Zhi. 32:8–12. 2016.(In Chinese).
PubMed/NCBI
|
|
54
|
Stuhlmiller TJ, Zawistowski JS, Chen X,
Sciaky N, Angus SP, Hicks ST, Parry TL, Huang W, Beak JY, Willis
MS, et al: Kinome and transcriptome profiling reveal broad and
distinct activities of erlotinib, sunitinib, and sorafenib in the
mouse heart and suggest cardiotoxicity from combined signal
transducer and activator of transcription and epidermal growth
factor receptor inhibition. J Am Heart Assoc. 6(pii):
e0066352017.PubMed/NCBI
|
|
55
|
Li J, Xiang X, Gong X, Shi Y, Yang J and
Xu Z: Cilostazol protects mice against myocardium
ischemic/reperfusion injury by activating a PPARγ/JAK2/STAT3
pathway. Biomed Pharmacother. 94:995–1001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lamont K, Nduhirabandi F, Adam T, Thomas
DP, Opie LH and Lecour S: Role of melatonin, melatonin receptors
and STAT3 in the cardioprotective effect of chronic and moderate
consumption of red wine. Biochem Biophys Res Commun. 465:719–724.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cao L, Cao X, Zhou Y, Nagpure BV, Wu ZY,
Hu LF, Yang Y, Sethi G, Moore PK and Bian JS: Hydrogen sulfide
inhibits ATP-induced neuroinflammation and Ab1-42
synthesis by suppressing the activation of STAT3 and cathepsin S.
Brain Behav Immun. 73:603–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang M, Tang W, Xin H and Zhu YZ:
S-Propargyl-cysteine, a novel hydrogen sulfide donor, inhibits
inflammatory hepcidin and relieves anemia of inflammation by
inhibiting IL-6/STAT3 pathway. PLoS One. 11:e01632892016.
View Article : Google Scholar : PubMed/NCBI
|