Introduction
Breast cancer is the most invasive type of
malignancy in females worldwide, leading to >39,000 deaths in
the USA each year (1). Although a
number of treatments have seen significant improvement over the
years, breast cancer remains a paramount health issue and is at the
forefront of medical research (2).
It can be considered a heterogeneous disease segmented into five
molecular subtypes: Luminal A, luminal B, HER2-enriched, basal-like
and claudin-low (3). Treatment
options for these cases include surgery, chemotherapy and/or
radiotherapy (4). However, breast
cancer remains a leading cause of cancer-associated mortality,
especially among young women (5).
Therefore, the treatments that currently available for patients
with breast cancer require urgent improvement.
Chinese traditional herbs can kill tumor cells by
acting on multiple targets with few adverse effects, making it an
area of great research interest. Matrine (MAT), an alkaloid derived
from Sophora Flavescens, is a traditional Chinese medicine
used for the treatment of aggressive cancers (6). MAT was found to inhibit the progress of
hepatic, cervical and gastric cancer (7), with a plethora of studies focusing on
the pharmacological and clinical applications of MAT (8–10).
To the best of our knowledge, little attention has
previously been paid to the effects of MAT on breast cancer
metastasis. Migration is the driving process of cancer metastasis
and corresponds to poor clinical symptoms, a deterioration in
health and eventual death (11). A
previous study compared different datasets and identified integrin
β1 (ITGB1) as one of the crucial genes involved in breast cancer
cell migration (12). In addition,
ITGB1 is reportedly highly expressed in the claudin-low subtype of
breast cancer (13). However,
whether MAT inhibits the migration of breast cancer cells by
mediating ITGB1 expression remains unclear.
In the present study, it was demonstrated that MAT
dose-dependently inhibits proliferation and induces apoptosis in
MDA-MB-231 cells. In addition, the present data provided novel
evidence of MAT-induced inhibition of cell migration by targeting
ITGB1 and the epithelial-to-mesenchymal transition (EMT) in breast
cancer.
Materials and methods
Reagents
MAT was purchased from Sigma-Aldrich (Merck KGaA)
and stored at 4°C. MAT was later dissolved in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) at a concentration of 20
mg/ml and stored at −20°C. Chloroquine diphosphate salt (CQ) was
purchased from Sigma-Aldrich (Merck KGaA).
Cell culture
The human breast cancer cell lines MDA-MB-231 and
MCF-7 (Shanghai Institute of Cell Biology, Chinese Academy of
Sciences) were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
100 µg/ml penicillin/streptomycin (HyClone; GE Healthcare Life
Sciences) in a humidified atmosphere containing 5% CO2
at 37°C.
Cell proliferation assay
To test the effect of MAT on MDA-MB-231
proliferation, 4×103 cells/well were seeded into 96-well
culture plates (Nunc™; Thermo Fisher Scientific, Inc.) in 100 µl
RPMI-1640 medium and then cultured in a 37°C 5% CO2
incubator overnight. The supernatant was then changed to one that
contained different doses of MAT (0, 1 and 2 mg/ml) and cultured
for 24 and 48 h, followed by another 2 h after 20 µl MTT (5 mg/ml;
Promega Corporation) was added to each well. Optical density values
were obtained using a plate reader at a wavelength of 490 nm.
Cell apoptosis assay
Annexin-V-FITC/PI double staining assays were
performed to detect the effects of apoptosis on MDA-MB-231 cells.
Cells were exposed to MAT (2 mg/ml) or the vehicle control for 48 h
in a 24-well plate (3×105 cells/well), after which each
group was washed with PBS three times followed by staining at room
temperature with an Annexin-V-FITC Apoptosis Detection kit I (RT;
BD Biosciences; Becton, Dickinson and Company). The number of
apoptotic cells was counted using flow cytometry (FACSCanto™; BD
Biosciences; Becton, Dickinson and Company) and the Flowjo Software
(version 8.2.4; FlowJo LLC), according to the manufacturer's
protocol.
Cell migration assay
Migratory abilities of MDA-MB-231 and MCF-7 cells
were determined using a chemotaxis chamber (Corning Life Sciences)
according to the manufacturer's protocol. In this assay, cell
motility was assessed by migration through a membrane (24-well
Transwell® plate, 8-µm pore size) towards a
chemoattractant. Briefly, cells were seeded into the upper chambers
of the Transwell® inserts (3×104 per well in
serum-free medium). Medium with 10% FBS was used as the
chemoattractant in the lower chambers. The medium contained tested
substances or a vehicle. Following incubation at 37°C with 5%
CO2 and 95% air for 16 h, cells were stained with
Calcein-AM (0.2 µg/ml; cat. no. C3100MP; Invitrogen; Thermo Fisher
Scientific, Inc.) at RT for 30 min. The migrated cells were counted
using an eclipse Ti inverted microscope (Nikon Corporation). The
number of cells that had migrated was determined using MetaMorph
image analysis software (version 4.0; Molecular Devices, LLC) and
the results are presented as the mean ± standard deviation
(n=3).
RNA interference
Oligonucleotides for human ITGB1 siRNA kit was
purchased from Guangzhou RiboBio Co., Ltd. The kit contains three
predesigned duplexes targeting a specific ITGB1 gene. Cells were
transfected with ITGB1 siRNA or NC at the concentration of 50
nmol/l using the opti-MEM plus X-treme GENE siRNA transfection
reagent (Roche Diagnostics) according to the protocol of the
manufacturer. The ITGB1 siRNA sequence was as follows: Forward,
5′-CCAUUCUGAUGAAUCUGAU-3′ and reverse, 5′-AUCAGAUUCAUCAGAAUGG-3′.
After 48 h of post-transfection, western blot analyses were further
performed.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
Briefly, total cellular RNA was extracted using
TRIzol® (Life Technologies; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. Total RNA was
extracted using TRIzol® and a Total RNA kit (Tiangen
Biotech Co., Ltd.). cDNA was generated at 37°C using 1 mg total RNA
and a QuantiTect Reverse Transcription kit (Qiagen GmbH). RT-qPCR
was performed using the SYBR Green (Bio-Rad Laboratories, Inc.)
method on an ABI Prism 7000 Sequence Detection System (Life
Technologies; Thermo Fisher Scientific, Inc.). The primer for ITGB1
was 5′-CCTACTTCTGCACGATGTGATG-3′ (forward) and
5′-CCTTTGCTACGGTTGGTTACATT-3′ (reverse). The primer for β-actin
(control) was 5′-CCACACCCGCCACCAGTTCG-3′ (forward) and
5′-TACAGCCCGGGGAGCATCGT-3′ (reverse). All primers were purchased
from Sangon Biotech Co., Ltd., and diluted in DEPC water. The qPCR
reaction was performed as follows: 95°C For 30 min, 40 cycles of
95°C for 15 sec and 56°C for 20 sec. To confirm the amplification
specificity, the PCR products were subjected to melting curve
analysis. The relative mRNA level of ITGB1 was normalized to the
β-actin mRNA and analyzed by the comparative threshold (Cq) cycle
method (2−ΔΔCq), according to previous research
(14).
Western blot analysis
Protein concentration was measured using a
bicinchoninic acid Protein Assay Reagent (Pierce; Thermo Fisher
Scientific, Inc.). Total protein (20 µg/well) was separated via 10%
SDS-PAGE and then transferred onto PVDF membranes at 250 mA for 1
h. Membranes were blocked at 37°C for 2 h with 5% non-fat milk in
Tris-buffered saline/0.1% Tween-20 and then incubated at 4°C
overnight with the following primary antibodies: Anti-ITGB1
(1:1,000; cat. no. ab183666; Abcam), anti-LC3 II/I (1:1,000; cat.
no. ab128025; Abcam), anti-epithelial (E)-cadherin, anti-neural
(N)-cadherin, anti-vimentin (1:1,000; cat. no. 9782T; EMT Antibody
Sampler kit Cell; Cell Signaling Technology, Inc.), anti-GAPDH
(1:5,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.) and
anti-β-actin (1:5,000; cat. no. 20536-1-AP; ProteinTech Group,
Inc.). Subsequently, membranes were incubated at 37°C for 2 h with
anti-rabbit IgG secondary antibodies (1:3,000; cat. no. 14708S;
Cell Signaling Technology, Inc.) and the immunoblotted proteins
were then detected using an Odyssey Western Blotting Detection
System (Gene Tech Co., Ltd.) and Odyssey software (version
1.2).
Statistical analysis
All data are expressed as the mean ± standard
deviation) based on experiments performed in triplicate, and were
analyzed using SPSS 18.0 statistical analysis software (SPSS,
Inc.). One-way analysis of variance followed by
Student-Newman-Keuls post hoc test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
MAT inhibits MDA-MB-231 cell growth by
inducing apoptosis
To determine the role of MAT in breast cancer,
MDA-MB-231 cells were treated with various concentrations of MAT
for 24 and 48 h, following which an MTT assay was performed to
evaluate proliferation (Fig. 1A).
The data demonstrated that MAT inhibited the proliferation of
MDA-MB-231 cells in a dose- and time-dependent manner. Tumor growth
was not only associated with abnormal proliferation, but was also
dependent on a reduction in apoptosis. To confirm that the
apoptosis observed in the cancer cells was induced by MAT, an
Annexin-V-FITC/PI apoptosis assay was performed (Fig. 1B). For flow cytometry, MDA-MB-231
cells were treated with or without MAT (2 mg/ml) for 48 h. Cells in
the late and early stages of apoptosis were observed in the upper
and lower right quadrant of the plots (Q2 and Q4 areas),
respectively. These results indicated that treatment with MAT was
able to impair proliferation and induce apoptosis in breast cancer
cells.
 | Figure 1.MAT inhibits MDA-MB-231 and MCF-7 cell
growth by inducing cell apoptosis. Following incubation with
various concentrations of MAT (0–4 mg/ml) for 24 and 48 h, (A)
MDA-MB-231 and (B) MCF-7 cell proliferation was measured by MTT
assay. (C) MDA-MB-231 and (D) MCF-7 cells were treated with or
without MAT (2 mg/ml) for 48 h and stained with Annexin V (5
µg/ml)/PI (10 µg/ml) prior to being analyzed by flow cytometry.
Cells labeled with Annexin V(−) PI(+) are shown in the Q1 area,
cells labeled with Annexin V(+) PI(+) in the Q2 area, cells labeled
with Annexin V(−) PI(−) in the Q3 area and cells labeled with
Annexin V(+) PI(−) in the Q4 area. *P<0.05, **P<0.01 and
***P<0.001 MAT vs. CTL by one-way analysis of variance followed
by Student-Newman-Keuls post hoc test. MAT, matrine; NC, negative
control; PI, propidium iodide; MAT, matrine; FITC, fluorescein
isothiocyanate; CTL, control. |
Role of autophagy in MAT-induced
decrease in cell growth
Previous studies have demonstrated that both
autophagy and apoptosis are involved in the effects observed in
cancer cells treated with MAT, including acute myeloid leukemia
(15) and osteosarcoma (16) cells. It was verified that MAT
inhibits cell proliferation and induces apoptosis. In addition, the
fact that apoptosis often occurs simultaneously with autophagy
prompted the present study to investigate the association between
MAT and autophagy. First, the autophagy inhibitor CQ, a small
alkaline molecule that accumulates in lysosomes and reduces
hydrolysis (17), was used. As shown
in Fig. 2A and B, when MDA-MB-231
and MCF-7 cells were exposed to various doses of MAT with and
without CQ, proliferation was significantly decreased in the MAT+CQ
group, as compared with the MAT alone group (P<0.05).
Next the expression of LC3-II/I, one of the main
autophagy regulatory proteins (18),
was investigated following exposure to MAT in cells pre-treated
with CQ for 1 h. The results showed that the expression of LC3-II/I
was accumulated in MDA-MB-231 and MCF cells treated with MAT or
with MAT+CQ. In addition, LC3-II/I in cells treated with MAT+CQ was
significantly upregulated, as compared with cells treated with MAT
alone (P<0.001; Fig. 2C and D).
These results therefore suggested that autophagy is involved in
MAT-induced breast cancer cell apoptosis.
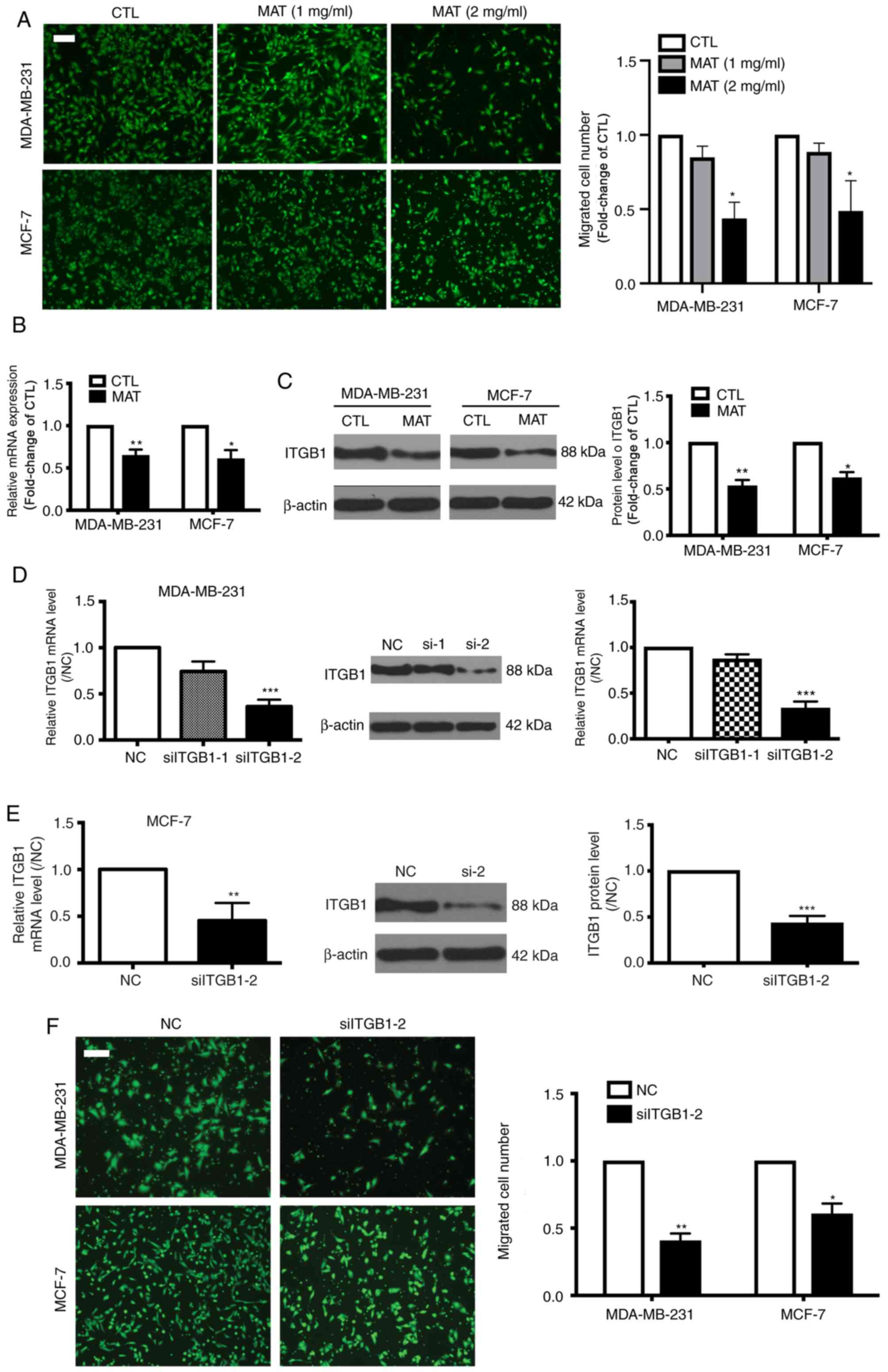
MAT decreases the migratory capacity
of MDA-MB-231 and MCF-7 cells potentially by targeting ITGB1
Metastasis is a primary cause of morbidity and
mortality in patients with cancer (19), and cell migration and invasion are
the most important steps in this complex process. Therefore,
transwell assays were performed to detect the migratory capacity of
breast cancer cells and found that MAT (2 mg/ml) significantly
inhibited the migration of MDA-MB-231 and MCF-7 cells (P<0.05;
Fig. 3A), indicating that MAT may be
a promising anti-metastatic agent for breast cancer.
ITGB1 is reportedly highly expressed in breast
cancer and correlates with cell migration (20). Therefore the levels of ITGB1 in
MDA-MB-231 and MCF-7 cells following treatment with MAT were
investigated. As hypothesized, following incubation with MAT, the
relative mRNA expression of ITGB1 was significantly decreased to
64.3 and 60.3% in MDA-MB-231 and MCF-7 cells, respectively
(P<0.05). The protein activity of ITGB1 also decreased to 53 and
61.6% in MDA-MB-231 and MCF-7 cells, respectively compared with the
control (CTL) group (Fig. 3B). To
further confirm that ITGB1 is involved in MTA-impaired migration,
siRNA was used to silence ITGB1 expression in MDA-MB-231 and MCF-7
cells. ITGB1-silencing was verified by RT-qPCR and western blot
analysis (Fig. 3C and D).
Furthermore, transfection with ITGB1 siRNA decreased the migratory
capacity of MDA-MB-231 and MCF-7 cells (Fig. 3E). Overall, these data indicated that
ITGB1 is involved in MAT-induced inhibition of breast cancer cell
motility.
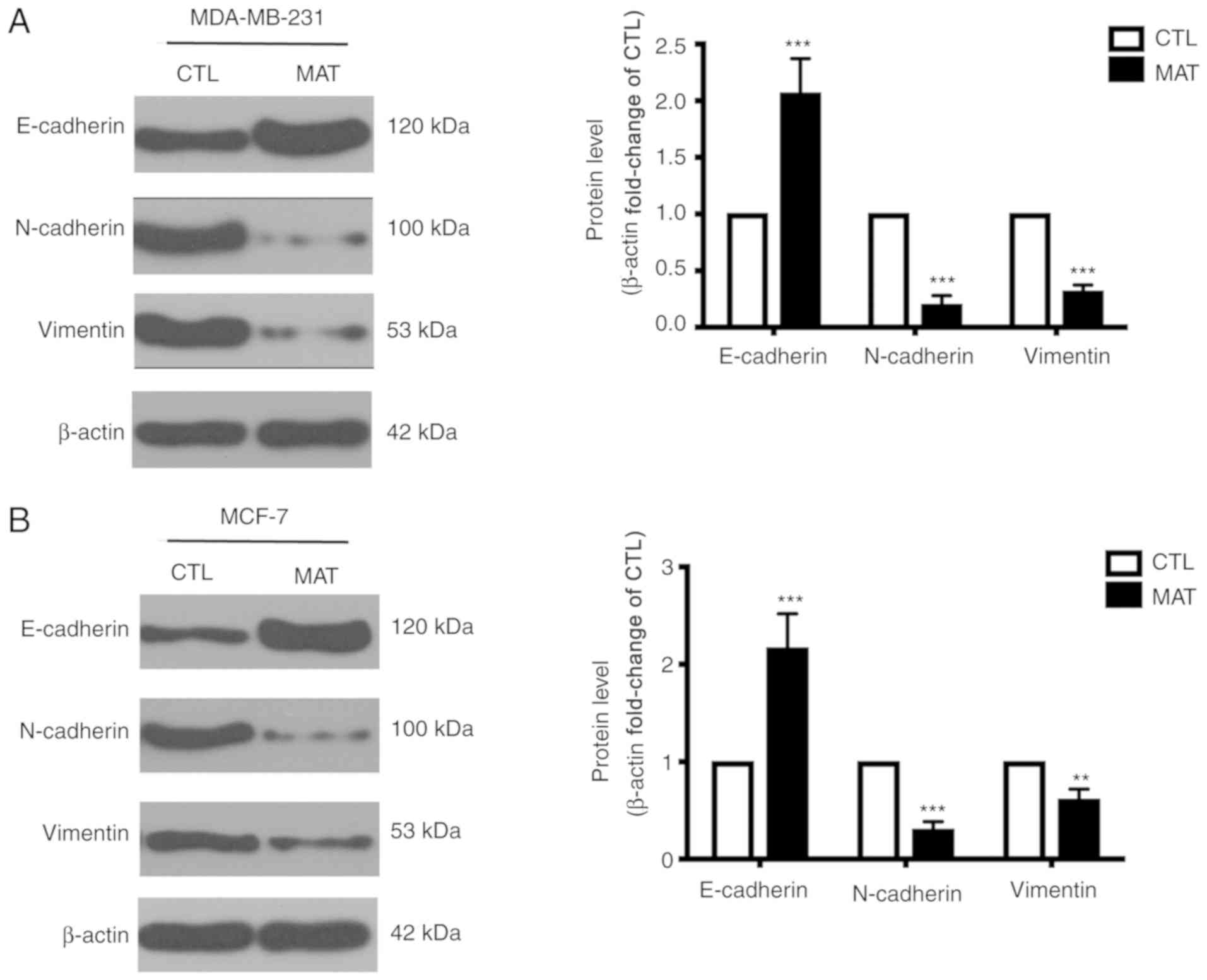
MAT regulates EMT in breast cancer
cells
During EMT, cells lose epithelial characteristics
and obtain mesenchymal properties, including decreased E-cadherin
and increased N-cadherin and vimentin. Considering the significant
effect of EMT on tumor cell migration, as well as that the process
can be mediated by ITGB1 (21), the
levels of certain EMT-associated markers were detected. MDA-MB-231
cells exhibited a mesenchymal phenotype, while MCF-7 cells
exhibited the properties of epithelial cells. Thus, the expression
of E-cadherin, N-cadherin and vimentin between MDA-MB-231 and MCF-7
cells was different in Fig. 4. In
addition, the western blot assays of Fig. 4A and B were not performed on the same
PVDF membranes, so the levels of these proteins in these two cells
cannot be compared due to variation in experimental conditions.
Incubation with MAT can markedly increase the expression of
E-cadherin and reduce the levels of N-cadherin and vimentin
compared with their CTL group. These changes demonstrated that EMT
in MAT-treated MDA-MB-231 and MCF-7 breast cancer cells is blocked,
reducing cell metastasis.
Discussion
Natural resources, especially traditional
plant-based medicines, are being increasingly investigated as
anti-tumor agents (22). MAT is a
component of one such traditional plant (Sophora
Flavescens), which has been shown to exert therapeutic effects
on various types of solid tumors (23,24). In
the present study, it was demonstrated that MAT exerts therapeutic
effects on MDA-MB-231 and MCF-7 breast cancer cells through
inhibiting proliferation and migration. Mechanistically, MAT
induces apoptotic cell death, influences ITGB1 expression and
blocks EMT to produce these anti-cancer effects.
Previous reports have demonstrated that MAT inhibits
the growth of various types of tumors by inducing apoptosis and
cell cycle arrest (25,26). The present study reinforced this by
demonstrating the ability of MAT to inhibit MDA-MB-231 and MCF-7
cell growth and induce apoptosis. To investigate the mechanisms of
MAT-induced cell growth inhibition, cell autophagy and LC3-II/I,
two forms of LC3 were focused on. Cytoplasmic LC3-I is conjugated
to phosphatidylethanolamine to form LC3-II, which is closely
associated with autophagosome membranes and serves as a reliable
marker for the monitoring of autophagy (27). First, it was found that cell growth
inhibition was increased following the application of the autophagy
inhibitor CQ, indicating that impaired autophagy aggravates
MAT-induced cell growth inhibition. Secondly, the expression levels
of LC3-II/I were further examined and the present data showed that
the level of LC3-II/I was elevated following MAT treatment. In
addition, its expression markedly increased with CQ co-treatment,
suggesting that cancer cell autophagy and apoptosis could be
targets for enhancing the anti-tumor effects of MAT.
Metastasis is a complex multistep process that
involves cell growth, migration and transportation via the blood
vessels. Therefore, the effects of MAT on cell migration, a crucial
step in breast cancer metastasis, were detected. The
Transwell® assay showed that MAT (1 mg/ml) may have
inhibited cell migration, but no significant differences were
observed. However, MAT (2 mg/ml) exhibited a marked ability to
reduce cell migration in both MDA-MB-231 and MCF-7 cells.
Consistent with present results, other studies reported that MAT is
able to inhibit cell migration in multiple types of cancer
(28–30), suggesting that MAT may be a promising
anti-metastatic drug. Among metastasis-related genes, integrins are
considered to mediate cell-cell crosstalk across the cellular
membrane and play an important role in the maintenance of
extracellular matrix (ECM) macromolecules (31). Furthermore, ITGB1 plays critical
roles in breast cancer cell proliferation and motility, is highly
expressed in aggressive breast tumors and drives metastasis
(32,33). ITGB1 is a major adhesion receptor for
various ECM components; therefore, the present study investigated
the expression of ITGB1 in breast cancer cells following treatment
with MAT. The mRNA and protein expression of ITGB1 was impaired in
MDA-MB-231 and MCF-7 cells following MAT treatment. Based on siRNA
analysis results, the present study hypothesized that ITGB1 and its
downstream signaling network is regulated by MAT. The present data
therefore provided a new target through which MAT can exert
anti-cancer effects.
EMT, a phenotypic cellular process, leads to the
loss of cell-cell adhesion. Consequently, cancer cell motility,
migration and metastasis is triggered (34). Alterations in cadherin expression are
typical in EMT, as is the downregulation of E-cadherin and
upregulation of N-cadherin (35) and
vimentin (as well as other mesenchymal proteins). A western blot
experiment was performed in mesenchymal-like MDA-MB-231 cells and
the effects of MAT on EMT were examined. The results showed that
incubation of MAT in MDA-MB-231 cells increased the expression of
epithelial markers and decreased the expression of mesenchymal
marker. Collectively, these data showed that MAT interfered EMT in
breast cancer cells. Previous studies also showed ZO1 (36) and E-cadherin (37) were upregulated during EMT process in
MDA-MB-231 cells. In the present study, treatment with MAT resulted
in the upregulation of E-cadherin and downregulation of N-cadherin
and vimentin, strongly indicating that EMT is blocked by MAT. In
addition, studies have demonstrated that ITGB1 and ITGB3 exhibit
tumor-promoting effects via facilitating EMT in breast cancer and
nasopharyngeal carcinoma (38,39). The
knockdown of ITGB1 partly increased the expression of E-cadherin
and decreased that of vimentin, fibronectin and N-cadherin in BT549
and Hs578T breast cancer cells (40). In the present study, it was shown
that the induction and regulation of EMT by MAT may involve
multiple molecular mechanisms, including the inhibition of ITGB1
expression.
The limitation of the present study is the lack of
specific mechanism by which MAT regulates ITGB1. The authors will
measure whether ITGB1 is transcriptionally regulated by MAT via
luciferase reporter assays and explore the possibility of reversing
MAT-inhibited cellular proliferation and migration by
overexpressing ITGB1 in MDA-MB-231 and MCF-7 cells in the following
study.
In conclusion, the present results revealed that MAT
exerts modulatory effects on apoptotic cell death and that the
inhibition of MAT-induced migration is potentially affected through
the attenuation of ITGB1 and EMT. Therefore, MAT may serve as a
novel suppressor of breast cancer.
Acknowledgements
The authors would like to thank Chief Attending
Physician Dr Qinghua Yao, Zhejiang Cancer Hospital, for her durable
support and constructive guidance.
Funding
The present study was supported by Zhejiang Medical
and Health Science and Technology Plan (grant no. 2013KYB04).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LR wrote the manuscript and performed the
experiment. WM and LW collected and interpreted the data. XW
obtained funding and designed the study. All authors have read and
approved the manuscript for publication.
Ethics approval and consent to
participate
All experimental protocols were performed in
accordance with the regulation of the Helsinki Declaration and were
approved by Ethics Committee of our hospital. Written consent of
the participants was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith RA, Andrews K, Brooks D, DeSantis
CE, Fedewa SA, Lortet-Tieulent J, Manassaram-Baptiste D, Brawley OW
and Wender RC: Cancer screening in the United States, 2016: A
review of current American cancer society guidelines and current
issues in cancer screening. CA Cancer J Clin. 66:96–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller SM, Goulet DR and Johnson GL:
Targeting the breast cancer kinome. J Cell Physiol. 232:53–60.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anastasiadi Z, Lianos GD, Ignatiadou E,
Harissis HV and Mitsis M: Breast cancer in young women: An
overview. Updates Surg. 69:313–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simmons A, Burrage PM, Nicolau DV Jr,
Lakhani SR and Burrage K: Environmental factors in breast cancer
invasion: A mathematical modelling review. Pathology. 49:172–180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou H, Xu M, Gao Y, Deng Z, Cao H, Zhang
W, Wang Q, Zhang B, Song G, Zhan Y and Hu T: Matrine induces
caspase-independent program cell death in hepatocellular carcinoma
through bid-mediated nuclear translocation of apoptosis inducing
factor. Mol Cancer. 13:592014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
Inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng F, Wang J, Ding F, Xie Y, Zhang Y and
Zhu J: Neuroprotective effect of matrine on MPTP-induced
Parkinson's disease and on Nrf2 expression. Oncol Lett. 13:296–300.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu
XS, Xu XR, Liu BZ and He YJ: Effects of matrine on proliferation
and differentiation in K-562 cells. Leuk Res. 25:793–800. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
11
|
Sciacovelli M and Frezza C: Metabolic
reprogramming and epithelial-to-mesenchymal transition in cancer.
FEBS J. 284:3132–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klahan S, Huang WC, Chang CM, Wong HS,
Huang CC, Wu MS, Lin YC, Lu HF, Hou MF and Chang WC: Gene
expression profiling combined with functional analysis identify
integrin beta1 (ITGB1) as a potential prognosis biomarker in triple
negative breast cancer. Pharmacol Res. 104:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zawistowski JS, Nakamura K, Parker JS,
Granger DA, Golitz BT and Johnson GL: MicroRNA 9-3p targets β1
integrin to sensitize claudin-low breast cancer cells to MEK
inhibition. Mol Cell Biol. 33:2260–2274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Hu G, Dong Y, Ma R, Yu Z, Jiang S,
Han Y, Yu K and Zhang S: Matrine induces Akt/mTOR signalling
inhibition-mediated autophagy and apoptosis in acute myeloid
leukaemia cells. J Cell Mol Med. 21:1171–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma K, Huang MY, Guo YX and Hu GQ:
Matrine-induced autophagy counteracts cell apoptosis via the ERK
signaling pathway in osteosarcoma cells. Oncol Lett. 12:1854–1860.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu X, Sheng J, Shen L, Su J, Xu Y, Xie Q,
Wu Y, Zhang X and Sun L: Autophagy inhibitor chloroquine increases
sensitivity to cisplatin in QBC939 cholangiocarcinoma cells by
mitochondrial ROS. PLoS One. 12:e01737122017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo S-H, Tang G, Ma K, Babij R, Cortes E,
Vonsattel JP, Faust PL, Sulzer D and Louis ED: Macroautophagy
abnormality in essential tremor. PLoS One. 7:e530402012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li WX, Sha RL, Bao JQ, Luan W, Su RL and
Sun SR: Expression of long non-coding RNA linc-ITGB1 in breast
cancer and its influence on prognosis and survival. Eur Rev Med
Pharmacol Sci. 21:3397–3401. 2017.PubMed/NCBI
|
|
21
|
Xie G, Ji A, Yuan Q, Jin Z, Yuan Y, Ren C,
Guo Z, Yao Q, Yang K, Lin X and Chen L: Tumour-initiating capacity
is independent of epithelial-mesenchymal transition status in
breast cancer cell lines. Br J Cancer. 110:2514–2523. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Li X, Bai M, Suo Y, Zhang G and Cao
X: Matrine inhibited proliferation and increased apoptosis in human
breast cancer MCF-7 cells via upregulation of Bax and
downregulation of Bcl-2. Int J Clin Exp Pathol. 8:14793–14799.
2015.PubMed/NCBI
|
|
23
|
Chen F and Huang K: Effects of the Chinese
medicine matrine on experimental C. parvum infection in BALB/c mice
and MDBK cells. Parasitol Res. 111:1827–1832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao H, Yang B, Hu R and Wang Y: Matrine
effectively inhibits the proliferation of breast cancer cells
through a mechanism related to the NF-κB signaling pathway. Oncol
Lett. 6:517–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL and Jiang HC: Matrine inhibits
breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells.
Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Hou C, Zhang S, Xie H, Zhou W,
Jin Q, Cheng X, Qian R and Zhang X: Matrine upregulates the cell
cycle protein E2F-1 and triggers apoptosis via the mitochondrial
pathway in K562 cells. Eur J Pharmacol. 559:98–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu KY, Wang YP, Wang LH, Jian Y, Zhao XD,
Chen JW, Murao K, Zhu W, Dong L, Wang GQ and Zhang GX:
Mitochondrial KATP channel involvement in angiotensin II-induced
autophagy in vascular smooth muscle cells. Basic Res Cardiol.
109:4162014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Zhou J, Cai D and Li M: Matrine
inhibits the metastatic properties of human cervical cancer cells
via downregulating the p38 signaling pathway. Oncol Rep.
38:1312–1320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan
H, Luan G, Yagasaki K and Zhang G: Effects of matrine against the
growth of human lung cancer and hepatoma cells as well as lung
cancer cell migration. Cytotechnology. 59:191–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Wang T, Wen X, Wei Y, Peng X, Li
H and Wei L: Effect of matrine on HeLa cell adhesion and migration.
Eur J Pharmacol. 563:69–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Z, Zou L, Ma G, Wu X, Huang F, Feng T,
Li S, Lin Q, He X, Liu Z and Cao X: Integrin β1 is a critical
effector in promoting metastasis and chemo-resistance of esophageal
squamous cell carcinoma. Am J Cancer Res. 7:531–542.
2017.PubMed/NCBI
|
|
33
|
Jahangiri A, Nguyen A, Chandra A, Sidorov
MK, Yagnik G, Rick J, Han SW, Chen W, Flanigan PM,
Schneidman-Duhovny D, et al: Cross-activating c-Met/β1 integrin
complex drives metastasis and invasive resistance in cancer. Proc
Natl Acad Sci USA. 114:E8685–E8694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang R, Zhang C, Liu G, Gu R and Wu H:
MicroRNA-126 inhibits proliferation, migration, invasion and EMT in
osteosarcoma by targeting ZEB1. J Cell Biochem. 118:3765–3774.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangiocarcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Hao Y, Mao W, Xue X, Xu P, Liu L,
Yuan J, Zhang D, Li N, Chen H, et al: LincK contributes to breast
tumorigenesis by promoting proliferation and
epithelial-to-mesenchymal transition. J Hematol Oncol. 12:192019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Zhao J, Zhang PY, Zhang Y, Sun SY,
Yu SY and Xi QS: MicroRNA-10b targets E-cadherin and modulates
breast cancer metastasis. Med Sci Monit. 18:BR299–BR308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding Y, Pan Y, Liu S, Jiang F and Jiao J:
Elevation of MiR-9-3p suppresses the epithelial-mesenchymal
transition of nasopharyngeal carcinoma cells via down-regulating
FN1, ITGB1 and ITGAV. Cancer Biol Ther. 18:414–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang YY, Kong LQ, Zhu XD, Cai H, Wang CH,
Shi WK, Cao MQ, Li XL, Li KS, Zhang SZ, et al: CD31 regulates
metastasis by inducing epithelial-mesenchymal transition in
hepatocellular carcinoma via the ITGB1-FAK-Akt signaling pathway.
Cancer Lett. 429:29–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu
L, Tang X, Du YE, Hu P and Liu M: Twist induces
epithelial-mesenchymal transition and cell motility in breast
cancer via ITGB1-FAK/ILK signaling axis and its associated
downstream network. Int J Biochem Cell Biol. 71:62–71. 2016.
View Article : Google Scholar : PubMed/NCBI
|