Introduction
Apocynum venetum L. (A. venetum), a
genus of the plant family Apocynaceae, is distributed throughout
the temperate regions of Eurasia and North America (1). The dried leaves of A. venetum
are used as treatments in traditional Chinese medicine (TCM) and
also as a tea. A. venetum leaves have been suggested to
lower cholesterol and blood pressure, and are prescribed in TCM for
sedation, as antidepressants and as anti-anxiety treatments, due to
the reported action of A. venetum on the nervous system
(2). A. venetum has also been
reported to exert an antioxidative effect, the mechanism of which
is thought to be related to free radical scavenging and diuresis
(2–5). The polyphenols found in A.
venetum leaves potentially represent a new class of active
ingredients (2).
Oxidative stress is an endogenous process that
gradually damages the body. Oxidative stress aggravates various
diseases, including hypertension, type 2 diabetes mellitus,
atherosclerosis and dementia (6–8).
Excessive redox-active species and free radicals can also cause
oxidative damage of biological macromolecules, which leads to
oxidative stress in the body. Oxidative stress promotes the
production of hydrogen peroxide in mitochondria, which in turn
increases oxidative damage (9).
Redox regulation is an important focus in the study of oxidative
stress. Maintaining the redox balance and regulating redox-related
genes are important strategies to alleviate oxidative stress
(10).
D-galactose is a commonly used aging-inducing agent
in research that can be used to establish animal models of
oxidative stress. A small amount of D-galactose can be converted to
glucose and metabolized by the body. However, excessive D-galactose
leads to a disordered cellular metabolism, alters the activity of
oxidase in tissues and cells, and produces a large number of
superoxide anions and oxidative products, which results in
oxidative damage to both the structure and function of biological
macromolecules (11). The oxidation
model of D-galactose has been established to verify the antioxidant
effect of antioxidant active substances. This model has been
utilized in the research and development of antioxidant health
products (12).
A previous study indicated that plant polyphenols
have antioxidant and free radical scavenging capacities, due to
their structural characteristics. The phenolic hydroxyl structure,
particularly the ortho-phenolic hydroxyl in catechol or pyrogallol,
is easily oxidized to a quinone structure; this makes it capable of
capturing free radicals, such as reactive oxygen species (2). This structure can reduce or prevent the
oxidation reaction in tissues by binding to lipid free radicals
produced in oxidation (13). Plant
polyphenols have also been shown to exert antioxidative effects in
animals and humans in clinical studies (14,15).
Plant polyphenols act as antioxidants via increases in the
activities of three important antioxidant enzymes: Superoxide
dismutase (SOD), glutathione (GSH) peroxidase (GSH-Px) and catalase
(CAT) (16).
In the present study, A. venetum polyphenol
extract (AVP) was extracted and was administered to mice subjected
to D-galactose-induced oxidative stress. The effects of AVP on the
serum and tissues of oxidized mice were observed, and the mechanism
of AVP-induced prevention of oxidation was studied through the
detection of oxidative stress-related genes. This study provides a
theoretical basis for further research into the use of AVP as a
medical treatment.
Materials and methods
Extraction of AVP
Briefly, 500 g A. venetum (Huake Ecology
Agriculture & Forestry Technology Co., Ltd.) was crushed into a
fine powder. Ethanol solution (50 ml; volume ratio: 45%) was added
to the powder for mixing and extraction at 90°C for 30 min using a
vortex shaker (TRS 140, Shanghai Brave Construction Development
Co., Ltd; Shanghai Dam Industrial Co., Ltd.). After three rounds of
extraction and the mixing, the pH of the extract was adjusted to
6.0 using 2.877 mol/l HCl. A mixed precipitant of AlCl3
(30 g) and ZnCl2 (60 g) was added (in a volume of 800
ml) to the extract for precipitation, followed by centrifugal
separation at 1,700 × g for 10 min at 25°C. Hydrochloric acid
(volume ratio: 12%; 1 l) was added to the precipitate after
centrifugation. The supernatant was separated using a separating
funnel, and then, 100 ml ethyl acetate was added twice for
extraction by vortex shaker. The extract was then subjected to
rotary evaporation to obtain AVP, as previously reported (17).
High-performance liquid chromatography
(HPLC)
HPLC was performed using the Ultimate 3000 system
(Thermo Fisher Scientific, Inc.). The reference substances
neochlorogenic acid, chlorogenic acid, rutin, isoquercitrin,
astragaloside and rosmarinic acid (20 mg of each substance;
Shanghai Yuanye Bio-Technology Co., Ltd.) were weighed and added to
methanol at a chromatographic level of 2 ml. The reference
substances were dissolved by full oscillation and reference
substance reserve solutions were obtained. The two mobile phase
solvents were: Mobile phase A, 0.5% acetic acid water; mobile phase
B, acetonitrile. The flow rate was 0.5 ml/min. Accucore™ C18
chromatography columns (particle size 2.6 µm; dimensions, 4.6×150
mm; Thermo Fisher Scientific, Inc.) were used. The injection volume
was 10 µl and the column temperature was 30°C. The detection
wavelength was 328 nm and the gradient conditions 0–30 min, 12–45%
(phase B); 30–35 min, 45–100% (phase B); 35–40 min, 100% (phase B)
were used to determine the components of the AVP.
Animal oxidation experiment
A total of 50 specific-pathogen-free-grade Institute
of Cancer Research mice (age, 6 weeks; weight, 25±2 g; 25 male and
25 female) were allowed 1 week to adapt to a temperature of 25±2°C
and a relative humidity of 50±5%. All mice received a normal diet
and food and water were freely available. The mice were then
divided into the following five groups: Control group, model group,
AVP low-concentration (AVP-L) group, AVP high-concentration (AVP-H)
group, and vitamin C (Vc) group. There were 10 mice (5 male and 5
female) per group.
During the first 4 weeks, mice in the model group
and the control group received normal diet and drinking water, and
each mouse received 0.2 ml saline per day by gavage. Mice in the
AVP-L and AVP-H groups were treated with AVP at 50 or 100 mg/kg
once per day by gavage. Mice in the Vc group were treated with Vc
(Hefei Bomei Biotechnology Co., Ltd.) at 100 mg/kg daily by gavage.
After 4 weeks, mice in the groups other than the control group were
intraperitoneally injected with D-galactose (Hefei Bomei
Biotechnology Co., Ltd.) at a concentration of 120 mg/kg once per
day for 6 weeks. At the same time, mice in the AVP-L, AVP-H and Vc
groups were also treated with AVP and Vc once per day for 6 weeks
by gavage, whereas mice in the model group and the control group
received the equivalent dose of saline (12). At 10 weeks, all mice were sacrificed
after 24 h of fasting. Mice were euthanized using cervical
dislocation. Blood samples were collected from heart and liver
tissues and stored until further experiments. The organ indices of
the thymus, brain, heart, liver, spleen and kidney were measured,
as follows: Organ index=organ mass (g)/body mass of mouse (kg)
×100. These experiments followed a protocol approved by the Animal
Ethics Committee of Chongqing Collaborative Innovation Center for
Functional Food (approval no. 200802002B).
Determination of nitric oxide (NO) and
malondialdehyde (MDA) concentration, and SOD and GSH-Px activity in
serum and liver tissues
Mouse blood (0.1 ml) was centrifuged at 2,100 × g
for 10 min at 25°C and the serum was collected. The concentrations
of NO (A012-1-2) and MDA (A003-1-2), and the activities of SOD
(A001-3-2) and GSH-Px (A005-1-2) in the serum were determined using
their respective kits, following the instructions of the
manufacturers (Nanjing Jiancheng Bioengineering Institute). In
addition, a total of 2 g liver and brain tissue was placed into a
glass homogenate tube, 18 ml saline was added into the homogenate
tube, and the homogenate was ground up and down at 2,100 r/min
using a tissue masher (TENLIN-C; Jiangsu Tianling Instrument Co.,
Ltd.) for 10 min to produce a 10% homogenate of liver and brain
tissues. The concentrations of NO and MDA, and the activities of
SOD and GSH-Px were determined in these homogenates, using the
aforementioned kits.
Observation of skin, liver and spleen
tissues
Approximately 0.5 cm2 skin, liver and
spleen tissues of mice were removed and fixed in 10% formalin
solution for 48 h at 4°C. Skin, liver and spleen tissues were
dehydrated gradually through 70, 80, 90% and anhydrous ethanol, and
the tissues were paraffin wax embedded sectioned (10 µm) and
permabilized for hematoxylin and eosin (H&E) staining for 25
min at 25°C. Morphological changes in the tissues were observed
(×100) under an optical microscope (BX43; Olympus Corporation). A
section was prepared from the skin, liver and kidney of each mouse
and 3 fields of view were imaged per section.
Reverse transcription-quantitative PCR
(RT-qPCR)
The skin, liver and spleen tissues (0.1g) of mice
were crushed using a tissue masher (TENLIN-C, Jiangsu Tianling
Instrument Co., Ltd.) RNAzol® (Sigma-Aldrich; Merck
KGaA) was used to extract total RNA from tissues, and the
concentration of the extracted total RNA was diluted to 1 µg/µl.
Subsequently, 5 µl diluted total RNA solution was collected, and RT
was conducted according to the instructions of the RT kit (Tiangen
Biotech Co., Ltd.) to obtain the cDNA template at 25°C. cDNA
template (2 µl) was mixed with 10 µl SYBR Green PCR Master Mix
(Thermo Fisher Scientific, Inc.) and 1 µl upstream and downstream
primers (Table I). The reaction
conditions were as follows: 95°C for 60 sec, followed by 40 cycles
of 95°C for 15 sec and 55°C for 30 sec followed by a final step at
72°C for 35 sec. A StepOnePlus Real-Time PCR system (Thermo Fisher
Scientific, Inc.) was used for qPCR. GAPDH was used as an internal
reference. The 2−ΔΔCq method was used to determine the
relative gene expression (18).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Sequence
(5′-3′) |
|---|
| nNOS | Forward:
ACGGCAAACTGCACAAAGC |
|
| Reverse:
CGTTCTCTGAATACGGGTTGTTG |
| eNOS | Forward:
TCAGCCATCACAGTGTTCCC |
|
| Reverse:
ATAGCCCGCATAGCGTATCAG |
| iNOS | Forward:
GTTCTCAGCCCAACAATACAAGA |
|
| Reverse:
GTGGACGGGTCGATGTCAC |
| Cu/Zn-SOD | Forward:
AACCAGTTGTGTTGTCAGGAC |
|
| Reverse:
CCACCATGTTTCTTAGAGTGAGG |
| Mn-SOD | Forward:
CAGACCTGCCTTACGACTATGG |
|
| Reverse:
CTCGGTGGCGTTGAGATTGTT |
| CAT | Forward:
GGAGGCGGGAACCCAATAG |
|
| Reverse:
GTGTGCCATCTCGTCAGTGAA |
| HO-1 | Forward:
ACAGATGGCGTCACTTCG |
|
| Reverse:
TGAGGACCCACTGGAGGA |
| Nrf2 | Forward:
CAGTGCTCCTATGCGTGAA |
|
| Reverse:
GCGGCTTGAATGTTTGTC |
| γ-GCS | Forward:
GCACATCTACCACGCAGTCA |
|
| Reverse:
CAGAGTCTCAAGAACATCGCC |
| NQO1 | Forward:
CTTTAGGGTCGTCTTGGC |
|
| Reverse:
CAATCAGGGCTCTTCTCG |
| GAPDH | Forward:
AGGTCGGTGTGAACGGATTTG |
|
| Reverse:
GGGGTCGTTGATGGCAACA |
Western blotting
Liver tissue (100 mg) was homogenized in 1 ml RIPA
buffer (Thermo Fisher Scientific, Inc.) containing 10 µl
phenylmethylsulfonyl fluoride, and centrifuged at 6,700 × g at 4°C
for 5 min. The middle protein layer was taken to measure the
protein concentration using a bicinchoninic acid kit (Bio-Rad
Laboratories, Inc.). The samples were diluted to 50 µg/µl, mixed
with sample buffer (4:1) and heated at 100°C for 5 min. SDS-PAGE
(12%) was carried out and 10 µg of protein was loaded per lane and
proteins were transferred onto a PVDF membrane. Membranes were
blocked in TBS-0.05% Tween (TBST) containing 5% skim milk for 1 h
at 25°C. After blocking, the membrane was incubated at 25°C for 2 h
with primary antibodies against Cu/Zn-SOD (cat. no. PA5-270240;
1:1,000), Mn-SOD (cat. no. LF-MA0030; 1:1,000) and beta-actin (cat.
no. MA5-15739; 1:1,000). The membrane was then washed with TBST and
incubated at 25°C for 1 h with secondary antibody (cat. no. A32723;
1:500) (17). All antibodies were
supplied by Thermo Fisher Scientific, Inc. SuperSignal™ West Pico
Plus was used to visualize proteins by iBright FL1000 (both Thermo
Fisher Scientific, Inc.). ImageJ version 1.44 (National Institutes
of Health) was used for semi-quantitative analysis of protein
expression.
Statistical analysis
Serum and tissue experiments were conducted three
times in parallel with each mouse, and the mean values were
obtained. SAS® version 9.1 statistical software (SAS
Institute, Inc.) was used to analyze the data. One-way ANOVA
followed by Tukey's test was used for multiple comparisons.
P<0.05 (alpha level) was considered to indicate a statistically
significant difference (19).
Results
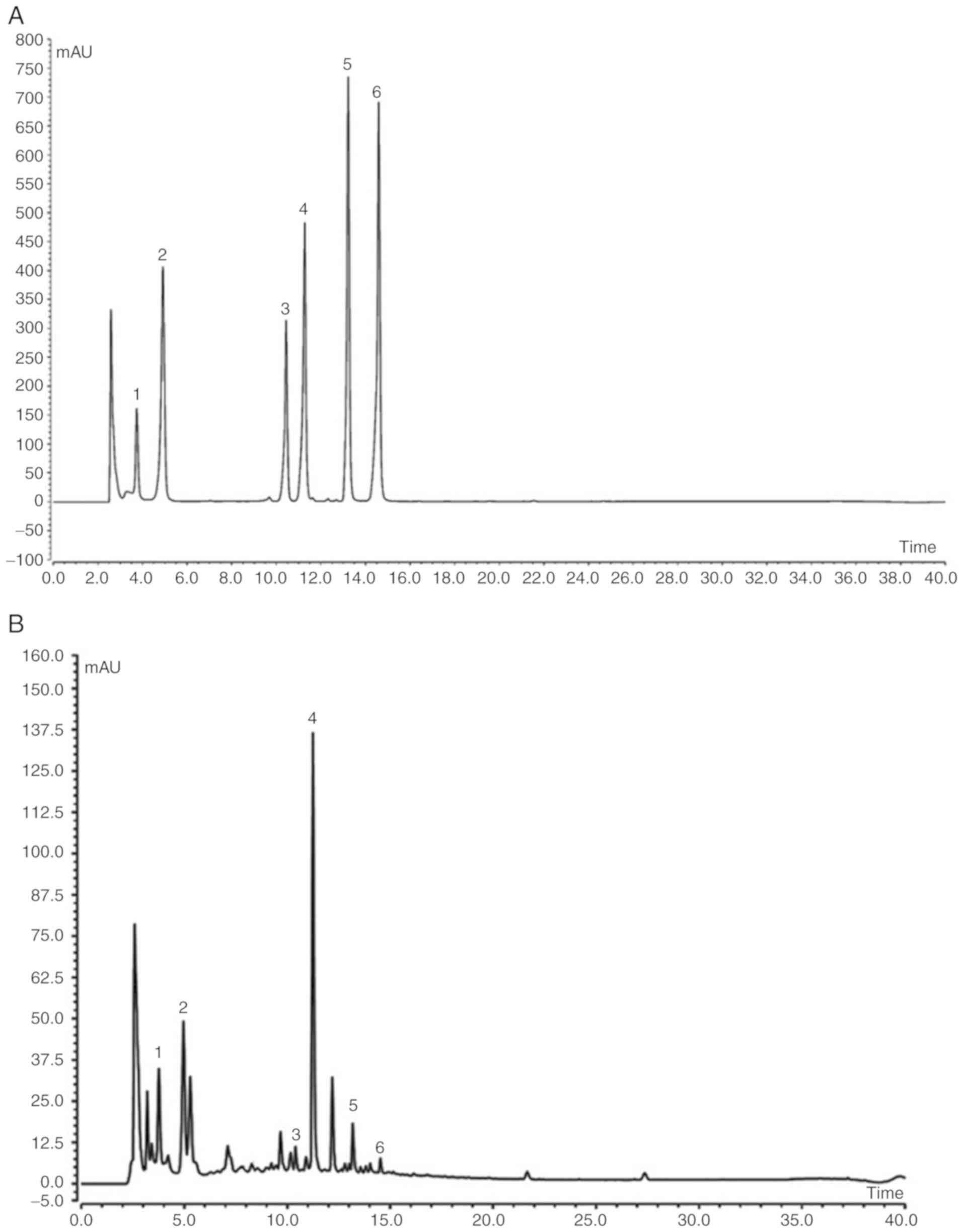
AVP component analysis
Analysis by HPLC identified that AVP contained six
known chemical constituents, namely neochlorogenic acid,
chlorogenic acid, rutin, isoquercitrin, astragalin and rosmarinic
acid (Fig. 1). Among them,
isoquercitrin made up the greatest proportion, followed by
neochlorogenic acid and chlorogenic acid (Table II).
 | Table II.Constituents of Apocynum
venetum polyphenol extract. |
Table II.
Constituents of Apocynum
venetum polyphenol extract.
| Substance | Concentration
(mg/ml) |
|---|
| Neochlorogenic
acid |
9.98 |
| Chlorogenic
acid |
4.94 |
| Rutin |
0.81 |
| Isoquercitrin | 10.87 |
| Astragalin |
0.78 |
| Rosmarinic
acid |
0.21 |
Organ indices of mice
As shown in Table
III, mice in the control group had the highest indices in the
thymus, brain, heart, liver, spleen and kidney, whereas mice in the
model group had the lowest organ indices. Both AVP and Vc
significantly increased organ indices when compared with the model
group (P<0.05). The effect of AVP appeared superior to that of
Vc at the same concentration. These data suggested that AVP could
inhibit the decline of organ indices caused by oxidative
stress-induced tissue atrophy.
 | Table III.Organ indices of mice in each group
(n=10). |
Table III.
Organ indices of mice in each group
(n=10).
| Group | Thymus index | Brain index | Cardiac index | Liver index | Spleen index | Kidney index |
|---|
| Normal |
0.29±0.04b |
5.13±0.22b |
3.57±0.19b |
23.12±1.13b |
1.65±0.11a |
4.50±0.30b |
| Model | 0.16±0.3 | 3.34±0.20 | 1.95±0.19 | 17.17±0.97 | 1.00±0.10 | 2.79±0.17 |
| Vc |
0.23±0.02a |
4.43±0.20b |
2.88±0.09a |
20.90±0.90a |
1.45±0.10a |
3.89±0.15a |
| AVP-L |
0.20±0.04a |
3.79±0.16a |
2.46±0.10a |
19.47±1.05a |
1.32±0.08a |
3.54±0.23a |
| AVP-H |
0.26±0.02a |
4.75±0.22b |
3.14±0.16b |
21.92±0.98a |
1.54±0.08a |
4.13±0.17b |
Levels of NO, SOD, GSH-Px, GSH and
MDA
As shown in Tables
IV–VII, mice in the control
group exhibited the strongest SOD, GSH-Px and GSH activity, and the
lowest NO and MDA levels in the serum, liver, spleen and brain
compared with the other groups. Mice in the model group had the
lowest levels compared with the other groups. Following AVP
treatment, SOD, GSH-Px and GSH activities in mice were
significantly increased compared with the model group (P<0.05),
whereas NO and MDA levels were significantly decreased (P<0.05).
The aforementioned indicators improved until they were close to
those of the control group following AVP-H treatment, and the
effect was markedly better than that of Vc.
 | Table IV.Levels of NO, SOD, GSH-Px, GSH and
MDA in the serum of mice (n=10). |
Table IV.
Levels of NO, SOD, GSH-Px, GSH and
MDA in the serum of mice (n=10).
| Group | NO (µmol/l) | SOD (U/ml) | GSH-Px (U/ml) | GSH (mg/l) | MDA (nmol/ml) |
|---|
| Normal |
19.90±0.62b |
220.71±8.13b |
212.09±5.05b |
46.07±2.60b |
3.91±0.36b |
| Model | 60.83±1.04 | 65.20±1.44 | 73.39±3.11 | 11.41±0.56 | 41.60±1.29 |
| Vc |
34.47±0.80b |
159.41±4.17b |
136.67±4.26a |
29.80±0.91a |
19.03±1.17b |
| AVP-L |
47.89±0.40a |
97.60±3.41a |
102.97±3.32a |
20.69±0.71a |
28.61±1.33a |
| AVP-H |
26.42±0.44b |
190.37±4.77b |
175.63±4.43b |
37.02±1.64b |
11.03±0.20b |
 | Table VII.Levels of NO, SOD, GSH-Px, GSH and
MDA in the brains of mice (n=10). |
Table VII.
Levels of NO, SOD, GSH-Px, GSH and
MDA in the brains of mice (n=10).
| Group | NO (µmol/l) | SOD (U/ml) | GSH-Px (U/ml) | GS (mg/l) | MDA (nmol/ml) |
|---|
| Normal |
4.62±0.21b |
127.26±6.32b |
166.39±7.52b |
13.89±0.45b |
0.42±0.11b |
| Model | 12.50±0.61 | 35.10±1.25 | 41.10±2.49 | 2.17±0.18 | 6.32±0.43 |
| Vc |
7.15±0.19a |
79.36±2.89a |
115.25±5.88a |
8.33±0.37a |
1.57±0.10a |
| AVP-L |
9.17±0.44a |
61.25±3.05a |
79.82±4.30a |
4.29±0.31a |
4.08±0.36a |
| AVP-H |
6.03±0.32a |
92.65±4.86b |
137.86±5.38b |
11.27±0.32b |
0.79±0.12b |
Observation of the mice livers and
spleens
As shown in Fig. 2,
the epidermal structure of normal mice was complete and clear, and
the dermis was rich in collagen fibers. The boundary between the
dermis and epidermis was clear and the number of fat vacuoles was
normal. In the model group, the amount of collagen fibers in the
skin was very low and numerous fat vacuoles were visible. The
dermis was inflammatory cells infiltrated, and the boundary between
the dermis and the epidermis was unclear. AVP treatment reduced the
large number of fat vacuoles caused by D-galactose, alleviated the
phenomenon of infiltration, and allowed the boundary between
epidermis and dermis to remain clear.
As shown in Fig. 3,
in the control group, hepatic cell chords were radially arranged
around the central vein, with a regular morphology and uniformly
sized hepatocytes. Aggregation of inflammatory cells could not be
seen. In the model group, the hepatic cells were disordered, with
irregular cellular morphology. The cellular boundaries in this
group were unclear. Cells appeared swollen and several cells
exhibited incomplete nuclei, while a large number of cells
presented inflammatory infiltration. AVP treatment appeared to
promote order of hepatocytes and neatly arranged hepatic cords,
protected the hepatocytes from damage and allowed the cell
structure to remain clear in mice subjected to oxidative stress.
The hepatic morphology of oxidative stress-treated mice became
close to that of the normal group after AVP-H treatment.
As shown in Fig. 4,
the spleen tissues of normal mice were complete, the
corticomedullary junction was clear and the cells were neatly
arranged. In the model group, the spleen structure was unclear as
the shape had become irregular. The red medullary sinus had
expanded and was filled with a large number of red cells. The white
medullary lymphocytes were reduced in number when compared with the
control group, with narrowed red medullary cords and sparsely
arranged cells. AVP effectively alleviated the changes in spleen
tissue morphology caused by D-galactose-induced oxidative stress
and led to normal spleen morphology. The histomorphological
observations of the skin, liver and spleen indicated that AVP could
inhibit the histopathological changes caused by oxidation, and may
serve a preventive and protective role. The effect of AVP exceeded
that of the antioxidant Vc.
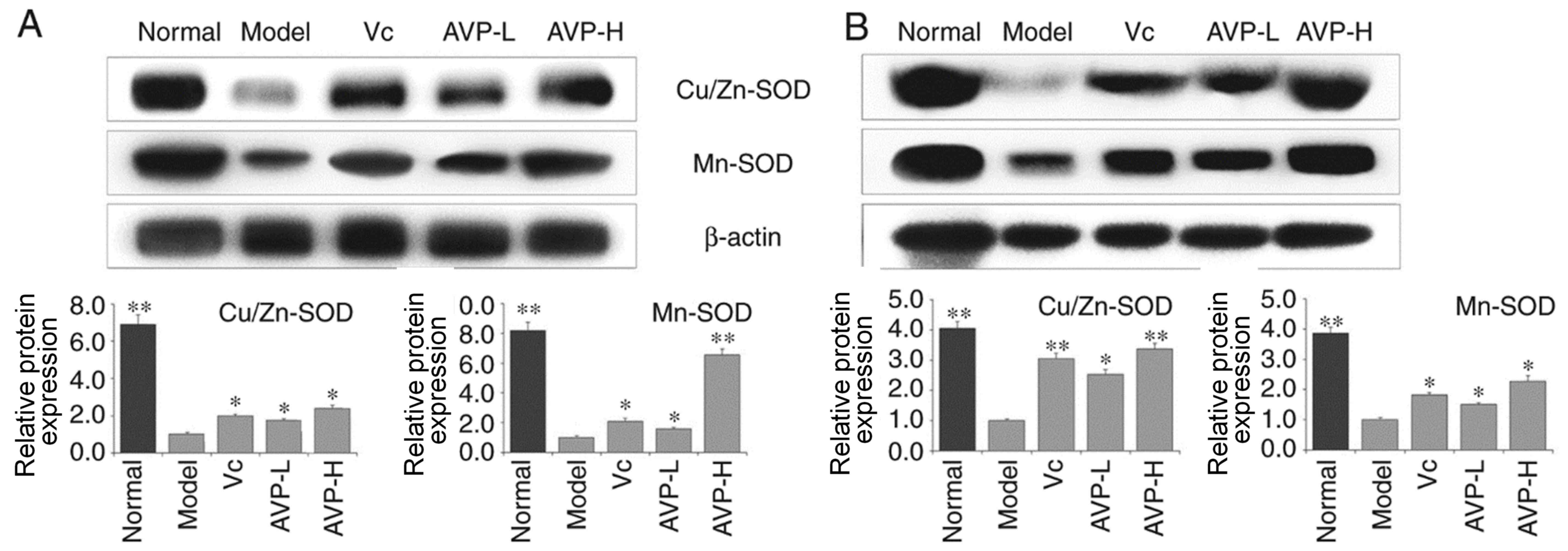
CAT, Cu/Zn-SOD and Mn-SOD expression
in liver and spleen tissues of mice
As shown in Figs. 5
and 6, the expression levels of
Cu/Zn-SOD, Mn-SOD and CAT were strongest in the liver and spleen
tissues of mice in the control group compared with the other
groups. Mice in the model group exhibited the opposite trend to the
normal group; Cu/Zn-SOD, Mn-SOD and CAT expression was the lowest
in the liver and spleen tissues of these mice compared with the
other groups. After AVP and Vc administration, the expression
levels of Cu/Zn-SOD, Mn-SOD and CAT were significantly increased in
the liver and spleen tissues compared with the model group
(P<0.05). Expression levels of these indicators in the liver and
spleen tissues of oxidative stress-induced mice became close to
those of normal mice after AVP-H treatment.
mRNA expression levels of neuronal
nitric oxide synthase (nNOS), endothelial nitric oxide synthase
(eNOS) and inducible nitric oxide synthase (iNOS) in the liver and
spleen tissues of mice
As shown in Fig. 7,
the mRNA expression levels of nNOS and eNOS in the liver and spleen
tissues in the control group were significantly higher than those
in the other groups (P<0.05), whereas iNOS expression was
significantly lower compared with the other groups (P<0.05).
D-galactose-induced oxidation significantly reduced nNOS and eNOS
expression, and increased iNOS expression in the liver and spleen
tissues of mice in the model group compared with the control group.
AVP significantly inhibited the alterations in mRNA expression
induced by D-galactose (P<0.05) and normalized mRNA expression
in the liver and spleen tissues of oxidative stress-induced mice.
The effect was better than that of Vc at the same
concentration.
 | Figure 7.nNOS, eNOS and iNOS mRNA expression
in the (A) liver and (B) spleen of mice. *P<0.05 and **P<0.01
vs. the model group. Vc, mice treated with 100 mg/kg Vc; AVP-L,
mice treated with a low concentration (50 mg/kg) of AVP; AVP-H,
mice treated with a high concentration (100 mg/kg) of AVP. AVP,
Apocynum venetum polyphenol extract; eNOS, endothelial NOS;
iNOS, inducible NOS; nNOS, neuronal NOS; NOS, nitric oxide
synthase; Vc, vitamin C. |
mRNA expression levels of heme
oxygenase-1 (HO-1), nuclear factor-erythroid 2-related factor 2
(Nrf2), γ-glutamylcysteine synthetase (γ-GCS) and NAD(P)H quinone
dehydrogenase 1 (NQO1) in the liver and spleen tissues of mice
As shown in Fig. 8,
the mRNA expression levels of HO-1, Nrf2, γ-GCS and NQO1 in the
liver and spleen tissues were significantly higher in the control
group than those in the other groups (P<0.05), whereas mice in
the model group exhibited the weakest mRNA expression levels in the
liver and spleen tissues. The expression levels of HO-1, Nrf2,
γ-GCS and NQO1 in the liver and spleen tissues of mice were
significantly increased following treatment with AVP and Vc
compared with the model group (P<0.05); the upregulating effect
of AVP-H on these genes was stronger than that of AVP-L and Vc.
 | Figure 8.HO-1, Nrf2, γ-GCS and NQO1 mRNA
expression in the (A) liver and (B) spleen of mice. *P<0.05 and
**P<0.01 vs. the model group. Vc, mice treated with 100 mg/kg
Vc; AVP-L, mice treated a low concentration (50 mg/kg) of AVP;
AVP-H, mice treated with a high concentration (100 mg/kg) of AVP.
γ-GCS, γ-glutamylcysteine synthetase; AVP, Apocynum venetum
polyphenol extract; HO-1, heme oxygenase-1; Nrf2, nuclear
factor-erythroid 2-related factor 2; NQO1, NAD(P)H quinone
dehydrogenase 1; Vc, vitamin C. |
Discussion
Organ weight and organ indices are indicators of the
status of the animal body. Changes in organ weight and organ
indices can reflect oxidative stress in the body; for example, when
oxidative stress occurs, the thymus and brain will atrophy more
obviously than other organs (20).
The liver and kidneys are the main metabolic organs of mice. A
decrease in organ weight and indices will directly affect the
metabolic capacity of animals. Furthermore, the liver is also an
important immune organ (17). A
change in the hepatic index suggests that the immunity of the body
has been affected (21). The spleen
is related to the cellular immune system in the body and serves an
important role in the immune mechanism. A decline in the quality of
the spleen indicates atrophy of the organ, which can reduce its
immune function (13). Therefore,
determining the spleen index can directly reflect structural and
functional changes in the organ, and therefore has importance for
evaluating the successful establishment of a mouse model of
oxidative stress (22). The results
of this study indicated that D-galactose could reduce the organ
indices of mice under oxidative stress and AVP could alleviate the
decrease of organ indices caused by D-galactose, thus preventing
oxidative stress in mice.
The increased release of excitatory amino acids,
such as glutamic acid, activates NMDA receptors to induce
Ca2+ influx. When intracellular Ca2+
concentration increases, activated NOS generates large amounts of
NO, which accelerates Ca2+ influx and causes an overload
of intracellular Ca2+, directly inhibiting mitochondrial
energy production. Furthermore, the generation of free radicals
O2− and ·OH will be triggered, which
aggravates the damage of brain cells (13). NO and O2− react
with each other to form peroxynitrite anions that decompose into
highly toxic OH− and NO2−, which
further accelerates cell death (23). Repeated infections in the body, even
if not directly related to the central nervous system, can lead to
synthesis of iNOS mRNA and can result in the production of a large
amount of NO, which causes neuronal death in the hippocampus and
memory damage, and accelerates the deterioration of aging neuronal
lesions, such as those found in Alzheimer's disease (17). The synthesis of iNOS mRNA in the
anterior pituitary and pineal glands produces NO, which can
deteriorate the resistance to infections and reduce the production
of melatonin, thus leading to brain aging (24). These studies support the hypothesis
that the regulation of NO levels and the prevention of excessive NO
serve a significant role in controlling aging. Studies have shown
that eNOS in mouse aortic endothelial cells is significantly
reduced under oxidative stress (17,19).
eNOS can inhibit the aging of blood vessels caused by oxidative
stress, as well as dilate and protect blood vessels (25). Under normal physiological conditions,
the nervous system has mechanisms that precisely regulate NO
production, release, diffusion and inactivation, mainly by
regulating the activation and deactivation of nNOS. In addition to
its important function in the nervous system, nNOS is also
expressed in skeletal muscles, the myocardium and smooth muscle
cells, where NO has an important role in regulating blood flow and
muscle contraction (26). A decrease
in the levels of nNOS leads to excessive NO production, which may
lead to the occurrence of neurological diseases, such as cerebral
ischemia injury, Alzheimer's disease and Parkinson's disease
(27). The results of the present
study suggested that AVP may possess the ability to influence the
levels of NO, nNOS, eNOS and iNOS, helping to maintain these at
normal levels and to avoid an imbalance of NO, nNOS, eNOS and iNOS
due to oxidative stress.
SOD is a type of metal enzyme widely distributed
throughout the biological world. It is a key line of defense
against reactive oxygen species. SOD can be generally divided into
three types depending on the metal co-factors: Cu/Zn-SOD, Mn-SOD
and Fe-SOD. Cu/Zn-SOD is an enzyme that can be predominantly found
in the cytoplasm and chloroplast matrix of eukaryotic cells. In
addition, Cu/Zn-SOD is present in the blood and viscera of animals
(17). Mn-SOD predominantly exists
in prokaryotic cells, eukaryotic cells and the mitochondrial
matrix, whereas Fe-SOD mainly exists in prokaryotic cells and
plants (26). SOD has an effective
role in the prevention and treatment of diseases associated with
superoxide free radicals. When in excess, superoxide anion radicals
cause oxidative stress; in addition, when SOD concentration is low,
oxidative stress may be induced (27). Levels of Cu/Zn-SOD and Mn-SOD
decrease when the body is under oxidative stress (28). CAT is an antioxidant enzyme, which is
abundant in erythrocytes, the cells of numerous tissue types,
mitochondria and the cytoplasm (29). During normal oxidative respiration,
organisms constantly produce reactive oxygen species, which contain
unpaired electrons, and can be removed by CAT, SOD, GSH-Px and
other enzymatic systems. As the first line of defense against
reactive oxygen species, SOD converts O2−
into H2O2, whereas CAT increases the cellular
oxygen content by catalyzing the decomposition of
H2O2 into H2O (30). GSH is an important antioxidant in
mammals that can scavenge free radicals produced in cells, thus
alleviating cell membrane damage caused by the formation of
reactive oxygen species during lipid peroxidation (31). GSH is either directly or indirectly
involved in numerous microbial cell activities. Notably, GSH is
able to build a strong defense against oxidative stress together
with related metabolic enzymes. One of the GSH reductions is
composed of γ-glutamate-cysteine ligase (GSH1), GSH synthetase
(GSH2), GSH reductase, GSH-Px and NADPH. The interaction of various
factors in the system can inhibit oxidative stress and slow down
oxidation (32) MDA is a lipid
peroxide formed by oxidation; the levels of MDA in vivo
directly reflect the degree of oxidation (33). In this study, AVP significantly
regulated the oxidation, increased the levels of SOD, CAT, GSH
(GSH1 and GSH2) and GSH-Px, and reduced the levels of MDA, thus
effectively alleviating oxidative stress caused by D-galactose in
mice.
As a stress protein, HO-1 is involved in
anti-inflammatory and antioxidative processes, in addition to heme
metabolism, and exerts strong protective effects on the
cardiovascular and nervous systems. It has been reported that HO-1
has an antioxidant efficacy in vascular diseases, such as
atherosclerosis, myocardial ischemic injury and hypertension, as
well as neurological diseases, such as Alzheimer's disease,
Parkinson's disease and cerebral ischemic injury (34). Nrf2 is important for the integrity of
the vascular endothelium. A decline in Nrf2 function under
oxidative stress is closely associated with the dysfunction of
vascular endothelial cells. When the level of oxidative stress
increases, Nrf2 is dissociated to promote the transcription and
expression of HO-1, SOD and CAT, thus improving the ability to
scavenge oxygen free radicals in the body (35). Nrf2 also has the function of
regulating γ-GCS. Nrf2 is activated and promotes the expression of
γ-GCS when a large quantity of reactive oxygen species is
generated, and γ-GCS stimulates the synthesis and activation of
GSH, which exerts an antioxidant role (36). When cells are subjected to oxidative
stress, Nrf2 can uncouple from Keapl, which can be activated and
transferred into the nucleus, bind to antioxidant response
elements, regulate the expression of the downstream antioxidant
enzyme gene NQO1, and enhance the tolerance of cells to oxidative
stress. The Nrf2/NQO1 signaling pathway is known to be associated
with oxidative stress, and active substances can exert their
antioxidative effects by regulating the Nrf2/NQO1 signaling pathway
(37). In this study, AVP could
upregulate the expression levels of oxidized Nrf2, and further
enhance the expression of HO-1, γ-GCS and NQO1, thus protecting
oxidized mice and preventing oxidative stress.
Phenolic compounds have phenolic hydroxyl groups, so
they have a strong antioxidant effect, the phenolic hydroxyl group
can provide hydrogen to react with free radicals to form inert
products or more stable free radicals, thus interrupting or slowing
down the chain reaction of free radicals (38). Studies have shown that the
antioxidant activity of polyphenol is much better in a number of
plants than that of Vc (39–41). The difference in the antioxidant
activity of plant polyphenol is not only due to the molecular
structure, but also due to the different three-dimensional
conformation (42,43). A. venetum contains the active
substance of AVP that has a strong antioxidant effect. This study
also confirmed that the antioxidant effect of AVP was stronger than
that of Vc at the same concentration; therefore, A. venetum
could serve a preventive role against oxidative stress with its
active ingredient AVP.
Neochlorogenic acid and chlorogenic acid are found
in numerous types of TCM, and are reported to be beneficial in TCM
treatment of inflammation and diabetes (44,45).
Chlorogenic acid has also been suggested to have an inhibitory
effect on lung cancer, gastric cancer and colon cancer (46,47). In
addition to the suggestion that it has anticancer effects, rutin is
believed to enhance the anticancer effect of other drugs and active
substances (oxaliplatin, paeonol) used in TCM (48,49).
In vitro studies have indicated that isoquercetin has
inhibitory effect on gastric cancer cell proliferation (50,51).
Astragalin and rosmarinic acid have been suggested to possess
antioxidant activity and are bioactive substances (52,53). AVP
contains neochlorogenic acid, chlorogenic acid, rutin,
isoquercitrin, astragalin and rosmarinic acid. It is unclear
whether the effect of the mixture of these six substances is due to
their respective effective effects or combined effects, and further
research is needed to clarify their underlying mechanism of
action.
The results of the present study suggested that AVP
may prevent D-galactose-induced oxidation in mice, and may return
serum, skin, liver and spleen indices of mice under oxidative
stress to close to their normal state. This effect was stronger
than that of the well-known antioxidant Vc. In vivo
experiments suggested that AVP is worthy of further study. The
present preliminary study has begun to elucidate the mechanism of
AVP activity; however, further human studies are required to verify
its precise mechanism. In addition, the specific chemical
composition of AVP needs to be further studied to clarify the
direct relationship between the efficacy of AVP and its
components.
Acknowledgements
Not applicable.
Funding
This research was funded by Chongqing scientific
research institute performance incentive guidance project
(cstc2018jxjlX0003), China.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and XZ performed the experiments. PP and JY
designed and directed the experiments. HG and ZK fed the animals,
performed the experiments and wrote the manuscript. All authors
approved the final manuscript for publication.
Ethics approval and consent to
participate
This study followed a protocol approved by the
Animal Ethics Committee of Chongqing Collaborative Innovation
Center for Functional Food (approval no. 200802002B).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grundmann O, Nakajima J, Seo S and
Butterweck V: Anti-anxiety effects of Apocynum venetum L. in
the elevated plus maze test. J Ethnopharmacol. 110:406–411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan X and Peng Y: Research progress of
Apocynum venetum tea. Mod Chinese Med. 16:666–673. 2014.
|
|
3
|
Kim DW, Yokozawa T, Hattori M, Kadota S
and Namba T: Inhibitory effects of an aqueous extract of
Apocynum venetum leaves and its constituents on
Cu(2+)-induced oxidative modification of low density lipoprotein.
Phytother Res. 14:501–504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong Q, Fan W, Tezuka Y, Adnyana IK,
Stampoulis P, Hattori M, Namba T and Kadota S: Hepatoprotective
effect of Apocynum venetum and its active constituents.
Planta Med. 66:127–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie W, Zhang X, Wang T and Hu J: Botany,
traditional uses, phytochemistry and pharmacology of Apocynum
venetum L. (Luobuma): A review. J Ethnopharmacol. 141:1–8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buford TW: Hypertension and aging. Ageing
Res Rev. 26:96–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chard S, Harris-Wallace B, Roth EG,
Girling LM, Rubinstein R, Reese AM, Quinn CC and Eckert JK:
Successful aging among african american older adults with type 2
diabetes. J Gerontol B Psychol Sci Soc Sci. 72:319–327.
2017.PubMed/NCBI
|
|
8
|
Kitada M, Ogura Y and Koya D: The
protective role of Sirt1 in vascular tissue: Its relationship to
vascular aging and atherosclerosis. Aging (Albany NY). 8:2290–2307.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao KS: Free radical induced oxidative
damage to DNA: Relation to brain aging and neurological disorders.
Indian J Biochem Biophys. 46:9–15. 2009.PubMed/NCBI
|
|
10
|
Hohensinner PJ, Kaun C, Ebenbauer B, Hackl
M, Demyanets S, Richter D, Prager M, Wojta J and Rega-Kaun G:
Reduction of premature aging markers after gastric bypass surgery
in morbidly obese patients. Obes Surg. 28:2804–2810. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Xu M, Shen B, Li M, Gao Q and Wei
SG: Moderate exercise prevents neurodegeneration in
D-galactose-induced aging mice. Neural Regen Res. 11:807–815. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian Y, Zhang J, Zhou X, Yi R, Mu J, Long
X, Pan Y, Zhao X and Liu W: Lactobacillus plantarum CQPC11 isolated
from Sichuan pickled cabbages antagonizes d-galactose-induced
oxidation and aging in mice. Molecules. 23(pii): E30262018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li HY, Xia JQ, Yang LY and Zhong RZ: Plant
polyphenols: Antioxidant capacity and application in animal
production. China J Anim Nutr. 25:2529–2534. 2013.
|
|
14
|
Carluccio MA, Siculella L, Ancora MA,
Massaro M, Scoditti E, Storelli C, Visioli F, Distante A and De
Caterina R: Olive oil and red wine antioxidant polyphenols inhibit
endothelial activation: Antiatherogenic properties of Mediterranean
diet phytochemicals. Arterioscler Thromb Vasc Biol. 23:622–629.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma S, Rana S, Patial V, Gupta M,
Bhushan S and Padwad YS: Antioxidant and hepatoprotective effect of
polyphenols from apple pomace extract via apoptosis inhibition and
Nrf2 activation in mice. Hum Exp Toxicol. 35:1264–1275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Delwing-Dal Magro D, Roecker R, Junges GM,
Rodrigues AF, Delwing-de Lima D, da Cruz JGP, Wyse ATS, Pitz HS and
Zeni ALB: Protective effect of green tea extract against
proline-induced oxidative damage in the rat kidney. Biomed
Pharmacother. 83:1422–1427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan Y, Long X, Yi R and Zhao X:
Polyphenols in Liubao tea can prevent CCl4-induced
hepatic damage in mice through its antioxidant capacities.
Nutrients. 10:12802018. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li GJ, Wang J, Cheng YJ, Tan X, Zhai YL,
Wang Q, Gao FJ, Liu GL, Zhao X and Wang H: Prophylactic effects of
polymethoxyflavone-rich orange peel oil on
Nω-Nitro-L-arginine-induced hypertensive rats. Appl Sci.
8:7522018. View Article : Google Scholar
|
|
20
|
Tang T and He B: Treatment of D-galactose
induced mouse aging with Lycium barbarum polysaccharides and its
mechanism study. Afr J Tradit Complement Altern Med. 10:12–17.
2013.PubMed/NCBI
|
|
21
|
Khan SS, Singer BD and Vaughan DE:
Molecular and physiological manifestations and measurement of aging
in humans. Aging Cell. 16:624–633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manini TM: Energy expenditure and aging.
Ageing Res Rev. 9:12010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tessari P: Nitric oxide in the normal
kidney and in patients with diabetic nephropathy. J Nephrol.
28:257–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wells SM and Holian A: Asymmetric
dimethylarginine induces oxidative and nitrosative stress in murine
lung epithelial cells. Am J Respir Cell Mol Biol. 36:520–528. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukai T, Siegfried MR, Ushio-Fukai M,
Cheng Y, Kojda G and Harrison DG: Regulation of the vascular
extracellular superoxide dismutase by nitric oxide and exercise
training. J Clin Invest. 105:1631–1639. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonthius DJ Jr, Winters Z, Karacay B,
Bousquet SL and Bonthius DJ: Importance of genetics in fetal
alcohol effects: Null mutation of the nNOS gene worsens
alcohol-induced cerebellar neuronal losses and behavioral deficits.
Neurotoxicology. 46:60–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee MH, Hyun DH, Jenner P and Halliwell B:
Effect of proteasome inhibition on cellular oxidative damage,
antioxidant defences and nitric oxide production. J Neurochem.
78:32–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosenko EA, Tikhonova LA, Alilova GA,
Montoliu C, Barreto GE, Aliev G and Kaminsky YG: Portacaval
shunting causes differential mitochondrial superoxide production in
brain regions. Free Radic Biol Med. 113:109–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Selvaratnam JS and Robaire B: Effects of
aging and oxidative stress on spermatozoa of superoxide-dismutase
1- and catalase-null mice. Biol Reprod. 95:602016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pawlak W, Kedziora J, Zolynski K,
Kedziora-Kornatowska K, Blaszczyk J, Witkowski P and Zieleniewski
J: Effect of long term bed rest in men on enzymatic antioxidative
defence and lipid peroxidation in erythrocytes. J Gravit Physiol.
5:P163–P164. 1998.PubMed/NCBI
|
|
31
|
Berndt C and Lillig CH: Glutathione,
glutaredoxins, and iron. Antioxid Redox Signal. 27:1235–1251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vázquez-Medina JP, Zenteno-Savín T, Forman
HJ, Crocker DE and Ortiz1 RM: Prolonged fasting increases
glutathione biosynthesis in postweaned northern elephant seals. J
Exp Biol. 214:1294–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hosen MB, Islam MR, Begum F, Kabir Y and
Howlader MZ: Oxidative stress induced sperm DNA damage, a possible
reason for male infertility. Iran J Reprod Med. 13:525–532.
2015.PubMed/NCBI
|
|
34
|
Sue YM, Cheng CF, Chang CC, Chou Y, Chen
CH and Juan SH: Antioxidation and anti-inflammation by haem
oxygenase-1 contribute to protection by tetramethylpyrazine against
gentamicin-induced apoptosis in murine renal tubular cells. Nephrol
Dial Transplant. 24:769–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong C, Cao J, Wu CF, Kadioglu O,
Schüffler A, Kauhl U, Klauck SM, Opatz T, Thines E, Paul NW and
Efferth T: The Chinese herbal formula Free and Easy Wanderer
ameliorates oxidative stress through KEAP1-NRF2/HO-1 pathway. Sci
Rep. 7:115512017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwayama K, Kusakabe A, Ohtsu K, Nawano T,
Tatsunami R, Ohtaki KI, Tampo Y and Hayase N: Long-term treatment
of clarithromycin at a low concentration improves hydrogen
peroxide-induced oxidant/antioxidant imbalance in human small
airway epithelial cells by increasing Nrf2 mRNA expression. BMC
Pharmacol Toxicol. 18:152017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang XP, Tang JY, Xu Z, Han P, Qin ZQ,
Yang CD, Wang SQ, Tang M, Wang W, Qin C, et al: Sulforaphane
attenuates di-N-butylphthalate-induced reproductive damage in
pubertal mice: Involvement of the Nrf2-antioxidant system. Environ
Toxicol. 32:1908–1917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang SX, Li AM, Zhang JJ, Ou SY, Huang XS
and Zhang GW: HPLC-ESI-MS analysis of phenolic compounds in
different solvent fractions of ethanol extract of longan seeds and
their antioxidant activities. Food Sci. 32:196–203. 2011.
|
|
39
|
Ramful D, Tarnus E, Aruoma OI, Bourdon E
and Bahorun T: Polyphenol composition, vitamin C content and
antioxidant capacity of Mauritian citrus fruit pulps. Food Res Int.
44:2088–2099. 2011. View Article : Google Scholar
|
|
40
|
Wittayarat M, Kimura T, Kodama R, Namula
Z, Chatdarong K, Techakumphu M, Sato Y, Taniguchi M and Otoi T:
Long-term preservation of chilled canine semen using vitamin C in
combination with green tea polyphenol. Cryo Lett. 33:318–326.
2012.
|
|
41
|
Bai JW, Gao ZJ, Xiao HW and Wang XT:
Polyphenol oxidase inactivation and vitamin C degradation kinetics
of Fuji apple quarters by high humidity air impingement blanching.
Int J Food Sci Technol. 48:1135–1141. 2013. View Article : Google Scholar
|
|
42
|
Yilixiati XKY and Li XJ: Inhibitory effect
of epigallocatechin gallate on the proliferation of lewis lung
cancer mice and A549 and Calu-3 lung cancer cells. Chin Pharm J.
47:585–589. 2012.
|
|
43
|
Shen SR, Jin CF, Yang XQ and Zhao BL:
Study on the scavenging effects of EGCG and GCG on singlet oxygen
with ESR method. J Tea Sci. 20:19–21. 2000.
|
|
44
|
Zhang LW, Ji T, Su SL, Shang RX, Guo S,
Guo JM, Qian DW and Duan JA: Pharmacokinetics of Mori Folium
flavones and alkaloids in normal and diabetic rats. Zhongguo Zhong
Yao Za Zhi. 42:4218–4225. 2017.(In Chinese). PubMed/NCBI
|
|
45
|
Hou N, Liu N, Han J, Yan Y and Li J:
Chlorogenic acid induces reactive oxygen species generation and
inhibits the viability of human colon cancer cells. Anticancer
Drugs. 28:59–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamagata K, Izawa Y, Onodera D and Tagami
M: Chlorogenic acid regulates apoptosis and stem cell
marker-related gene expression in A549 human lung cancer cells. Mol
Cell Biochem. 441:9–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lukitasari M, Nugroho DA and Widodo N:
Chlorogenic acid: The conceivable chemosensitizer leading to cancer
growth suppression. J Evid Based Integr Med.
23:2515690X187896282018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Q, Ren LQ, Wang YD and Chen XX: Effects
of rutin combined with oxaliplatin on the proliferation and
apoptosis of human gastric cancer SGC-7901 cells. Chin J Clin Pharm
Ther. 22:1099–1105. 2017.
|
|
49
|
Zhan P, Peng XS, Xu XM, Zhou YF, Zhang XP,
He R and Zhou G: The anti-cancer study on combinating paeonol and
rutin. Chin Arch Tradit Chin Med. 28:1710–1712. 2010.
|
|
50
|
Liu KY, Liu HJ, Wu JZ and Zhang TJ:
Studies on inhibitory effect of active constituents from Tussilago
farfara L. on lung cancer cells LA795 proliferation. J Fudan Univ
(Nat Sci). 48:125–129. 2009.
|
|
51
|
Li YY, Zhao SJ, Bai CZ, Zhang LW and Wang
ZH: Effect of isoquercetin from Fagopyrum tataricum on the
proliferation and apoptosis of human gastric carcinoma cell line
SGC-7901. Food Sci. 35:193–197. 2014.
|
|
52
|
Kim MS and Kim SH: Inhibitory effect of
astragalin on expression of lipopolysaccharide-induced inflammatory
mediators through NF-κB in macrophages. Arch Pharm Res.
34:2101–2107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tepe B, Eminagaoglu O, Akpulat HA and
Aydin E: Antioxidant potentials and rosmarinic acid levels of the
methanolic extracts of Salvia verticillata (L.) subsp.
Verticillataand S. verticillata (L.) subsp. Amasiaca (Freyn &
Bornm.) Bornm. Food Chem. 100:985–989. 2007. View Article : Google Scholar
|