Introduction
Cerebrovascular occlusion is a common disease of
elderly people, which poses a great threat to human life (1) and has a great impact on patients'
quality of life (2). Cerebral
infarction is caused by cerebral vascular occlusion, which needs
timely opening of the blood vessels to make blood vessel flow
normal, thus to achieve the purpose of treatment (3,4).
Thrombolysis is a common treatment method for cerebrovascular
occlusion in clinical practice and an important way of opening
occlusive cerebral vessels (5). With
the progress of clinical medicine in recent years, interventional
therapy has also been widely used in clinical practice, providing a
new method for cerebral vascular occlusion (6). This was confirmed in the studies of
Tian et al (7) and Lei et
al (8). However, Rao et
al (9) achieved a better effect
than conventional treatment when they applied interventional
therapy to the treatment of aneurysms. Therefore, interventional
therapy has gradually become the first choice for clinical
treatment of cerebrovascular diseases (10). With the wide application of
interventional therapy, however, its disadvantages are gradually
exposed. For example, improper selection of puncture site may not
only result in ineffective treatment, but also cause aggravation of
the disease (11), and it is
particularly important for the more complex structure of the brain
vascularity when selecting puncture site. Therefore, in order to
improve the efficacy of interventional therapy, the relevant
auxiliary examinations are the key to treatment.
Three-dimensional digital subtraction angiography
(3D-DAS) is an extremely effective detection method (12), which digitizes X-ray images taken
before and after the contrast agent to obtain a clear vascular
image (13). Compared with
traditional X-ray examination, 3D-DAS has the characteristics of
high resolution, short examination time and less use of contrast
agent, which is particularly suitable for the organization of
vascular tissues (14). At present,
there are few studies on the application of 3D-DAS combined with
interventional therapy in the treatment of cerebral vascular
occlusion, and its clinical application prospect cannot be
determined. Therefore, this investigation provides a reliable
reference for the future clinical treatment of such diseases by
comparing the use of 3D-DSA combined with interventional therapy
and simple interventional therapy for patients with cerebrovascular
occlusion.
Patients and methods
General data
A total of 129 patients with senile cerebrovascular
occlusion admitted to the Affiliated Hospital of Zunyi Medical
University (Zunyi, China) from August 2015 to September 2017 were
collected as the study subjects. Among them, 69 patients who
underwent neurointerventional catheter thrombolysis under 3D-DSA
were included in the study group, with an average age of 61.6±4.5
years. The 60 patients treated with neurointerventional
thrombolysis were included in the control group, with an average
age of 62.2±4.8 years.
The study was approved by the Ethics Committee of
Affiliated Hospital of Zunyi Medical University. Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Inclusion and exclusion criteria
Inclusion criteria: patients met the diagnostic
criteria for cerebral vascular embolization, patients diagnosed
with cerebral vascular embolization after a series of examinations
in the hospital, patients treated in the hospital after diagnosis
and patients with complete data and who agreed to cooperate with
the investigation of medical staff.
Exclusion criteria: Patients complicated with
multiple tumors, patients complicated with other cardiovascular and
cerebrovascular diseases, patients complicated with autoimmune
diseases, patients suffering from infectious diseases, mental
disorders, other organ dysfunction, and physically disabled
patients who were unable to take care of themselves.
Therapies
Patients in the control group were punctured in the
manner of Seldinger and then placed a guide catheter close to the
diseased vessel. The patients were injected with urokinase
(Guangdong Techpool Biochemical Medicine Co., Ltd.; H44024032,
10,000 units) 200,000 units + 20 ml normal saline mixture. Fifty
milliliters normal saline + 200–500,000 units urokinase was pumped
into the patients with a micro-pump catheter at a rate of 1 ml/min.
Both groups were re-examined with skull CT 24 h after treatment. If
no bleeding changes were found, patients were given aspirin
enteric-coated tablets (Shenyang Original Pharmacolabo Co., Ltd.;
SFDA approval no. H20065051, 50 mg/tablet) at a dose of 100 mg.
Patients in the study group were treated with neurointerventional
thrombolysis under 3D-DSA: Local anesthesia was used with the
assistance of 3D-DSA (Siemens) to puncture and intubate the femoral
artery on the right side of patients, and the artery sheath was
inserted. The whole cerebral vascular angiography was performed
with a digital subtraction angiography to determine the specific
location of the occluded blood vessel, and the subsequent procedure
was consistent with the control group.
Detection methods
Enzyme linked immunosorbent assay (ELISA) was used
to detect the levels of inflammatory cytokines IL-6, IL-1β and IL-8
in the two groups before treatment (T0), 7 days (7d) after
treatment (T1) and 14 days (14d) after treatment (T2). The
operation process was strictly in accordance with the kit
instructions.
Observational indexes
Main indicators: ELISA was used to detect the levels
of inflammatory cytokines IL-6, IL-1β and IL-8 in the two groups
before treatment (T0), 7d after treatment (T1) and 14d after
treatment (T2). The NIHSS score was used to score the neurological
deficits and compare the clinical efficacy of the two groups before
and after surgery. The National Institutes of Health Stroke Scale
was used for NIHSS score (15). At
14d after treatment, the efficacy assessment standard for stroke
was formulated by referring to the ‘4th national academic
conference on cerebrovascular diseases’ (16). The incidence of adverse reactions
after treatment was compared between the two groups.
Secondary observational indexes: Barthel index (BI)
was used for investigation before and after treatment (17). Patients in both groups were followed
up for 1 year for prognosis, and the recurrence rate of disease in
both groups was recorded within 1 year.
Statistical methods
In this study, SPSS 20.0 (IBM Corp.) medical
statistical analyzer was used for statistical analysis of collected
data. GraphPad Prism 7 (GraphPad Software Co., Ltd.) was used to
image rendering of the collected data. Utilization of enumeration
data (%) was qualified by the Chi-square test and represented by
χ2. The measurement data were expressed by mean ±
standard deviation (mean ± SD). All the measurement data were in
normal distribution. The independent sample t-test was used for
comparison between the two groups, and comparison in group was
qualified by paired t-test and expressed by t. P<0.05 was
considered statistically significant.
Results
Comparison of general data
There were no differences in sex, age, BMI, nitric
oxide sythase (NOS), endothelin-1 (ET-1) pg/ml, vascular
endothelial growth factor (VEGF) pg/ml, 50 sec hemodynamics (SRV)
mPa/sec, living environment, smoking history, drinking history,
family medical history or ethnicity (P>0.05) (Table I).
 | Table I.Comparison of general data of patients
in the two groups (%). |
Table I.
Comparison of general data of patients
in the two groups (%).
|
| Research group
(n=69) | Control group
(n=60) | t or
χ2 | P-value |
|---|
| Age (years) | 61.6±4.5 | 62.2±4.8 | 0.732 | 0.465 |
| Sex |
|
| 0.305 | 0.581 |
| Male | 48 (69.57) | 39 (65.00) |
|
|
|
Female | 21 (30.43) | 21 (35.00) |
|
|
| BMI
(kg/cm2) | 31.52±5.05 | 30.86±4.72 | 0.763 | 0.447 |
| Nitric oxide synthase
(NOS) U/ml | 0.821 | 0.413 |
|
|
|
| 24.16±3.58 | 23.62±3.89 |
|
|
| Endothelin-1 (ET-1)
pg/ml | 0.751 | 0.454 |
|
|
|
| 92.04±3.54 | 91.57±3.55 |
|
|
| Vascular endothelial
growth factor (VEGF) pg/ml | 0.572 | 0.569 |
|
|
|
| 306.65±32.87 | 309.87±30.75 |
|
|
| 50 sec hemodynamics
(SRV) mPa/sec | 0.919 | 0.360 |
|
|
|
| 6.23±0.32 | 6.17±0.42 |
|
|
| Living
environment |
|
| 0.688 | 0.407 |
| City | 59 (85.51) | 48 (80.00) |
|
|
|
Countryside | 10 (14.49) | 12 (20.00) |
|
|
| Smoking history |
|
| 0.011 | 0.915 |
| With | 34 (49.28) | 29 (48.33) |
|
|
|
Without | 35 (50.72) | 31 (51.67) |
|
|
| Drinking history |
|
| 1.805 | 0.179 |
| With | 38 (55.07) | 40 (66.67) |
|
|
|
Without | 31 (44.93) | 20 (33.33) |
|
|
| Family medical
history |
|
| 0.736 | 0.391 |
| With | 9 (13.04) | 5 (8.33) |
|
|
|
Without | 60 (86.96) | 55 (91.67) |
|
|
| Ethnicity |
|
| 2.956 | 0.086 |
| Han | 59 (85.51) | 66 (94.29) |
|
|
|
Minority | 10 (14.49) | 4 (5.71) |
|
|
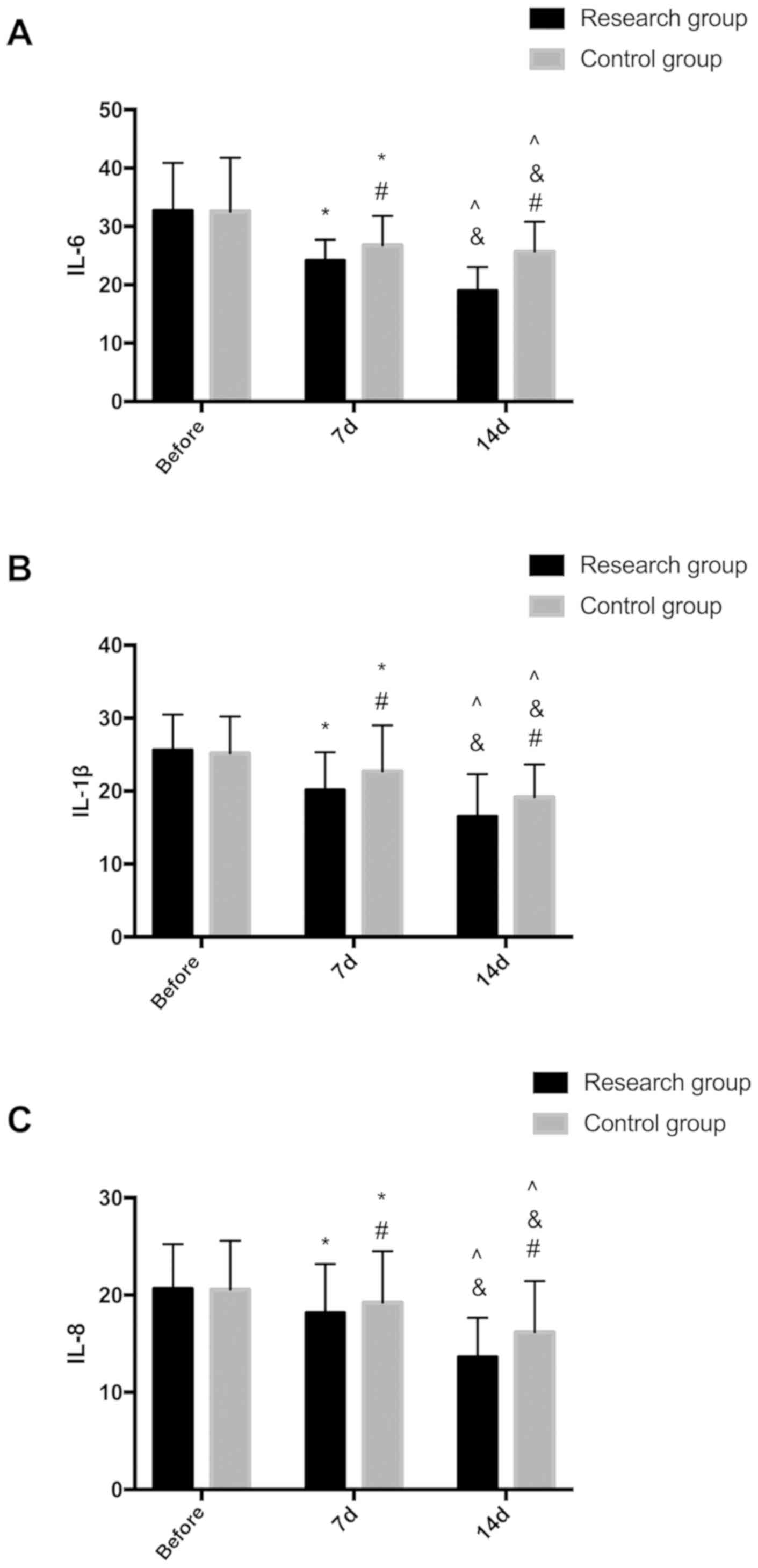
Levels of inflammatory cytokines IL-6, IL-1β and
IL-8 of patients in the two groups before treatment (T0), 7d after
treatment (T1) and 14d after treatment (T2). There were no
significant differences in levels of inflammatory cytokines IL-6,
IL-1β, IL-8 of patients in the two groups (P>0.05). After 7d of
treatment, levels of IL-6, IL-1β, IL-8 in the study group were all
lower than the control group (P<0.05). After 14d of treatment,
levels of IL-6, IL-1β and IL-8 were all lower than these of 7d
after treatment, and levels of IL-6, IL-1β and IL-8 were lower in
the study group than the control group after 14d of treatment
(P<0.05) (Fig. 1).
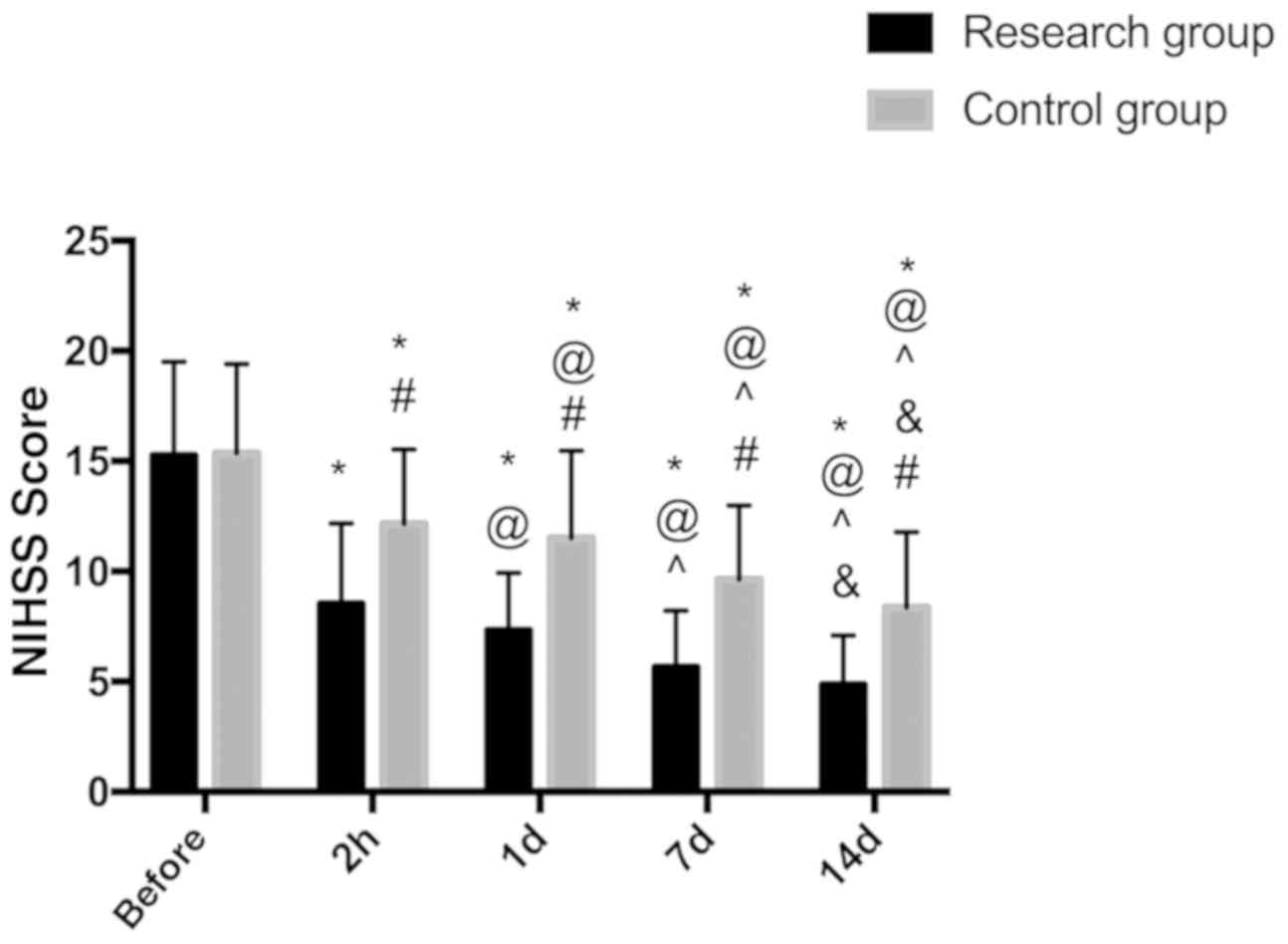
Comparison of NIHSS scores of the two
groups before and after treatment
NIHSS score was used to score neurological defects
before and after surgery. The results showed that there was no
significant difference in NIHSS score between the two groups before
treatment (P>0.05). After treatment, NIHSS scores of the two
groups at the four time periods (2 h, 1d, 7d and 14d after
treatment) were significantly lower than those before treatment,
with statistically significant differences (P<0.05). The
improvement of NIHSS score in the study group was significantly
better than that in the control group, and the difference was
statistically significant (P<0.05) (Fig. 2).
Clinical efficacy of two groups of
patients
The clinical efficacy of the two groups of patients
was compared, and the results showed that the marked efficiency
rate of the study group was significantly higher than that of the
control group on 14d after treatment (P<0.05), and the
difference in the total efficiency of the two groups was not
statistically significant (P>0.05) (Table II).
 | Table II.Comparison of clinical efficacy
between the two groups. |
Table II.
Comparison of clinical efficacy
between the two groups.
| Groups | No. of cases | Cure | Significant
effect | Effective | Invalid | Deterioration | Marked efficiency
rate | Total effective
rate |
|---|
| Research group | 69 | 17 (24.64) | 20 (28.99) | 15 (21.97) | 10 (14.49) | 7 (10.14) | 24.64% | 75.36% |
| Control group | 60 | 6
(10.00) | 21 (35.00) | 15 (25.00) | 10 (16.67) | 8 (13.33) | 10.00% | 70.00% |
| χ2 |
|
|
|
|
|
| 4.694 | 0.467 |
| P-value |
|
|
|
|
|
| 0.030 | 0.495 |
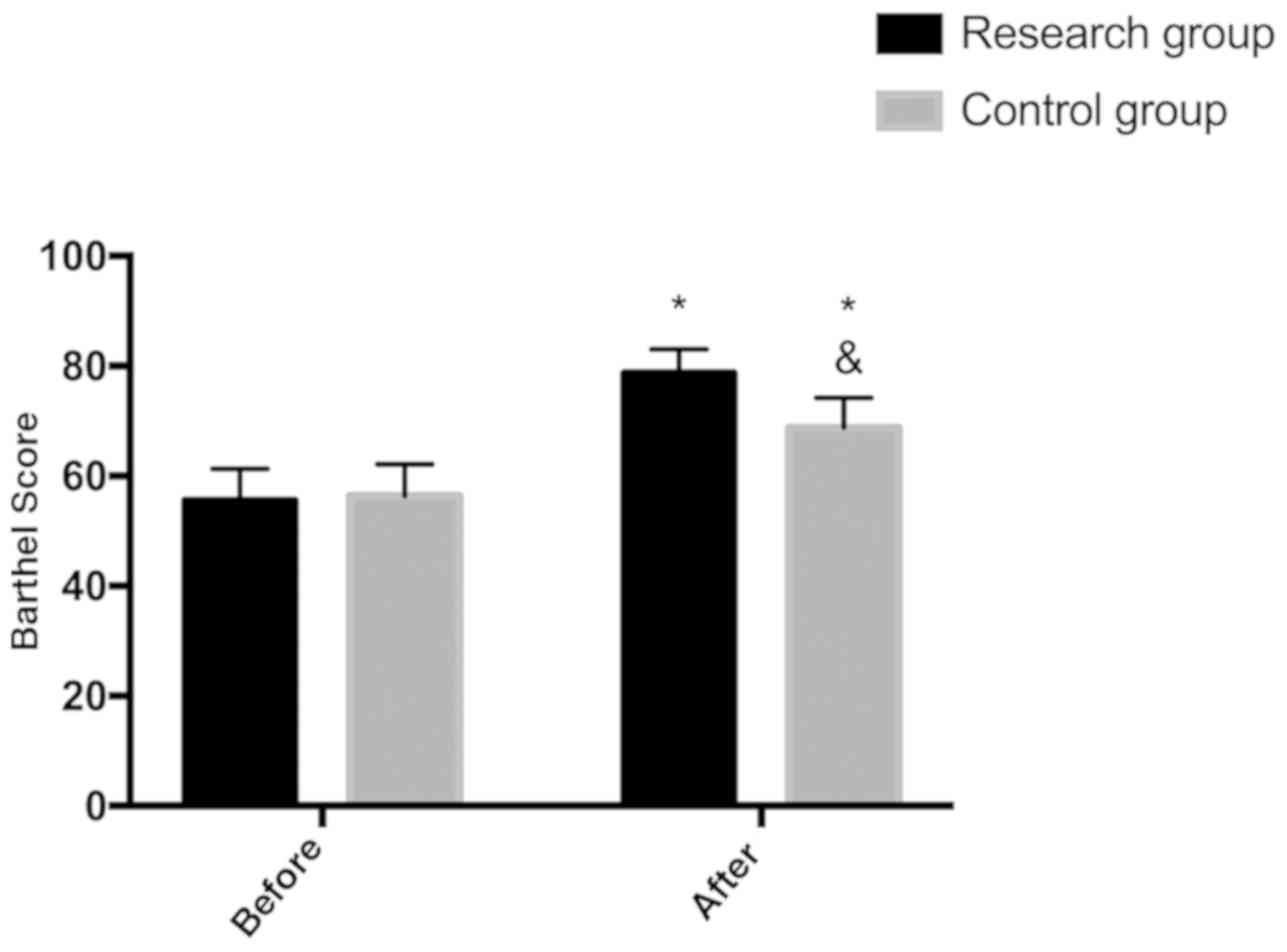
Comparison of Barthel index score
between the two groups
There was no significant difference in Barthel index
score between the two groups before treatment (P>0.05), while
Barthel index score after treatment was higher than that of the
control group (P<0.05) (Fig.
3).
Disease recurrence rate within 1 year
in the two groups
Patients in the two groups were followed up for one
year, and 120 patients were successfully followed up, with a
follow-up success rate of 93.02%. Among them, there were 3 patients
lost to follow-up in the study group and 6 patients in the control
group. The one-year disease recurrence rate of the two groups was
compared, and it was lower in the study group than the control
group (P<0.05) (Table III).
 | Table III.Comparison of disease recurrence rate
within 1 year in two groups. |
Table III.
Comparison of disease recurrence rate
within 1 year in two groups.
|
| Research group
(n=66) | Control group
(n=54) | χ2 | P-value |
|---|
| Recurrence | 7
(10.61) | 14 (25.93) | 4.828 | 0.028 |
| Without
recurrence | 59 (89.39) | 40 (74.07) |
|
|
Discussion
Cerebrovascular occlusion is the main cause of
cerebral infarction (18), its
disability rate and death rate remain high (19). At present, the main clinical
treatment for elder cerebral vascular occlusion is
neurointerventional thrombolysis. Although neurointerventional
thrombolysis has a significant therapeutic effect on vascular
diseases (20), deficiencies of this
method are also very significant. For example, if the puncture
point and the puncture path are artificially selected, there may be
deviations which have certain risks for the complicated tissue of
the blood vessel. It not only causes secondary injury to the
patient, but also causes more serious vascular occlusion (21). Therefore, how to accurately implement
neurointerventional thrombolysis is the key and difficult point of
treatment of cerebrovascular diseases. As shown in this study,
treatment with 3D-DSA assisted neurointerventional thrombolysis is
of great significance for future clinical practice.
The results showed that the levels of inflammatory
cytokines IL-6, IL-1β and IL-8 in the patients after 3D-DSA
combined with neurointerventional thrombolysis were significantly
lower than those in the patients treated with neurointerventional
thrombolysis alone. This suggests that 3D-DSA combined with
neurointerventional thrombolysis can reduce the inflammation caused
by vascular occlusion in patients more effectively. Suzuki et
al (22) aiming at the
application of reconstruction techniques in the diagnosis and
treatment of cerebral aneurysms have shown that 3D-DSA images are
excellent for cerebral aneurysms, and the levels of inflammatory
cytokines are well controlled after treatment. Therefore, it is
speculated that 3D-DSA can make the puncture more accurate and the
intervention channel better when performing cerebral vascular
occlusion surgery, bringing less stress injury and, thus to reduce
inflammatory cytokines. The NIHSS scores of the two groups of
patients before and after treatment were compared, and the results
showed that the improvement of NIHSS scores of the patients treated
with 3D-DSA combined with neurointerventional thrombolysis was
significantly better than that of the patients treated with
neurointerventional thrombolysis alone. This suggests that the use
of 3D-DSA assisted neurointerventional thrombolysis is more
beneficial to the protection of neurological function and
improvement of prognosis of patients. Then the therapeutic effect
of the two groups of patients was observed, and the marked
effective rate of using 3D-DSA as an adjuvant therapy was
significantly higher than that of the patients treated with
neurointerventional thrombolysis alone, indicating that the use of
3D-DSA as an adjuvant therapy has good clinical value and is worthy
of popularization. In assessing the accuracy and practicability of
3D-DSA and 3D-CT angiography for cerebral aneurysms Ishida et
al (23) revealed that 3D-DSA
has a higher diagnostic accuracy, which was conducive to carry out
more effective follow-up treatment for the disease, with better
therapeutic effect. This supports our experimental results. We
speculate that in the treatment of senile cerebrovascular
occlusion, it is precisely because 3D-DSA is detected at any angle
in the brain, which greatly improves the detection efficiency, so
that the neurointerventional thrombolysis can be performed more
effectively and accurately, and the therapeutic effect can be
improved. The study of Muruet et al (24) investigating neurointerventional
thrombolytic therapy for ischemic stroke shows that thrombolytic
therapy can improve long-term survival rate and functional status
after ischemic stroke, its Barthel index is also consistent with
this study. Whereas, in the present study, the curative effect of
3D-DSA- assisted neurointerventional thrombolysis is better than
that of neurointerventional thrombolysis, so the Barthel index of
patients under 3D-DSA-assisted therapy is also higher. The patients
were followed up for one year, and the one-year disease recurrence
rate of patients after 3D-DSA was significantly lower than that of
patients treated with neurointerventional thrombolysis. This
suggests that 3D-DSA adjuvant therapy has a good prognosis for
senile cerebrovascular occlusion. In future clinical practice,
patients with senile cerebrovascular occlusion can be treated by
3D-DSA combined with neurointerventional thrombolysis.
However, we did not make a comparative study on
3D-DSA combined with other therapeutic methods, and it is still
unclear whether 3D-DSA combined with other therapeutic methods have
the same efficacy in treating senile cerebrovascular occlusion. As
the number of subjects in this study is small and the research
period is short further study is required.
In conclusion, 3D-DSA combined with
neurointerventional thrombolysis in patients with cerebrovascular
occlusion can significantly reduce the expression of inflammatory
cytokines, improve quality of life, and has a high clinical
value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJ observed and compared the levels of inflammatory
cytokines and wrote the manuscript. LG and ZW conceived and
designed the study. LZ and JH were responsible for the collection
and analysis of the experimental data. BT and SY interpreted the
data and drafted the manuscript. SJ and ZW revised the manuscript
critically for important intellectual content. All the authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Zunyi Medical University (Zunyi, China).
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients or
the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Horie N, Tateishi Y, Morikawa M, Morofuji
Y, Hayashi K, Izumo T, Tsujino A, Nagata I and Matsuo T: Acute
stroke with major intracranial vessel occlusion: characteristics of
cardioembolism and atherosclerosis-related in situ
stenosis/occlusion. J Clin Neurosci. 32:24–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park MG, Yoon CH, Baik SK and Park KP:
Susceptibility vessel sign for intra-arterial thrombus in acute
posterior cerebral artery infarction. J Stroke Cerebrovasc Dis.
24:1229–1234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jayaraman MV, Hussain MS, Abruzzo T,
Albani B, Albuquerque FC, Alexander MJ, Ansari SA, Arthur AS,
Baxter B, Bulsara KR, et al: Embolectomy for stroke with emergent
large vessel occlusion (ELVO): Report of the Standards and
Guidelines Committee of the Society of NeuroInterventional Surgery.
J Neurointerv Surg. 7:316–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrer I and Vidal N: Neuropathology of
cerebrovascular diseases. Handb Clin Neurol. 145:79–114. 2018.
View Article : Google Scholar
|
|
5
|
Vedantham S, Piazza G, Sista AK and
Goldenberg NA: Guidance for the use of thrombolytic therapy for the
treatment of venous thromboembolism. J Thromb Thrombolysis.
41:68–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindekleiv H, Berge E, Bruins Slot KM and
Wardlaw JM: Percutaneous vascular interventions versus intravenous
thrombolytic treatment for acute ischaemic stroke. Cochrane
Database Syst Rev. Oct 26–2018.(Epub ahead of print). doi:
10.1002/14651858.CD009292.pub2. View Article : Google Scholar
|
|
7
|
Tian Q, Zhu G and Dong S: Clinical
efficacy of neurointerventional catheter thrombolysis for cerebral
infarction. J Clin Nurs Res. Nov 30–2019.(Epub ahead of print).
doi: 10.26689/jcnr.v3i6.867.
|
|
8
|
Lei X, Zheng H, Wang Y and Liu C:
Observation of the clinical effect of interventional therapy of
acute cerebral infarction with digital subtraction angiography.
Chin J Primary Med Pharm. 24:3152–3155. 2017.(In Chinese).
|
|
9
|
Rao ACA, Shah SA, Sim BW, Yun ST, Jain NS,
Kalani Y and Francis IC: Neuroradiological endovascular
intervention for diplopia in a case of aneurysmal aberrant
regeneration of the third nerve. Cureus. 9:e13402017.PubMed/NCBI
|
|
10
|
Xianxian Z, Chengsong Y, Qiang M, Fei W,
Lin S, Huiyan D and Zili G: The efficiency analysis of thrombolytic
rt-PA combined with intravascular interventional therapy in
patients with acute basilar artery occlusion. Int J Biol Sci.
13:57–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taslakian B, Sebaaly MG and Al-Kutoubi A:
Patient evaluation and preparation in vascular and interventional
radiology: What every interventional radiologist should know (part
2: patient preparation and medications). Cardiovasc Intervent
Radiol. 39:489–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mine B, Tancredi I, Aljishi A, Alghamdi F,
Beltran M, Herchuelz M and Lubicz B: Follow-up of intracranial
aneurysms treated by a WEB flow disrupter: A comparative study of
DSA and contrast-enhanced MR angiography. J Neurointerv Surg.
8:615–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papafaklis MI, Bourantas CV, Yonetsu T,
Vergallo R, Kotsia A, Nakatani S, Lakkas LS, Athanasiou LS, Naka
KK, Fotiadis DI, et al: Anatomically correct three-dimensional
coronary artery reconstruction using frequency domain optical
coherence tomographic and angiographic data: Head-to-head
comparison with intravascular ultrasound for endothelial shear
stress assessment in humans. EuroIntervention. 11:407–415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Athanasiou L, Rigas G, Sakellarios AI,
Exarchos TP, Siogkas PK, Bourantas CV, Garcia-Garcia HM, Lemos PA,
Falcao BA, Michalis LK, et al: Three-dimensional reconstruction of
coronary arteries and plaque morphology using CT angiography -
comparison and registration with IVUS. BMC Med Imaging. 16:92016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeyaseelan RD, Vargo MM and Chae J:
National Institutes of Health Stroke Scale (NIHSS) as an early
predictor of poststroke dysphagia. PM R. 7:593–598. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albers GW, Lansberg MG, Kemp S, Tsai JP,
Lavori P, Christensen S, Mlynash M, Kim S, Hamilton S, Yeatts SD,
et al: A multicenter randomized controlled trial of endovascular
therapy following imaging evaluation for ischemic stroke (DEFUSE
3). Int J Stroke. 12:896–905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prodinger B, O'Connor RJ, Stucki G and
Tennant A: Establishing score equivalence of the Functional
Independence Measure motor scale and the Barthel Index, utilising
the International Classification of Functioning, Disability and
Health and Rasch measurement theory. J Rehabil Med. 49:416–422.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buschmann EE, Hillmeister P, Bondke
Persson A, Liebeskind DS, Schlich L, Kamenzky R, Busjahn A,
Buschmann IR, Bramlage P, Hetzel A, et al: Short-term external
counterpulsation augments cerebral blood flow and tissue
oxygenation in chronic cerebrovascular occlusive disease. Eur J
Neurol. 25:1326–1332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nerva JD and Levitt MR: Medical and
surgical treatment of cerebrovascular occlusive disease. Principles
Neurol Surg. Content Repository Only. 241–253. e3:2018.
|
|
20
|
Pulicherla KK and Verma MK: Targeting
therapeutics across the blood brain barrier (BBB), prerequisite
towards thrombolytic therapy for cerebrovascular disorders - an
overview and advancements. AAPS PharmSciTech. 16:223–233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tocci G and Presta V: Increased arterial
stiffness and haemorrhagic transformation in ischaemic stroke after
thrombolysis: A new marker of risk for cerebrovascular events and
complications. Int J Cardiol. 243:471–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki K, Ikeda S, Ueda K, Nakamura T,
Okabe M, Kadomura T, Baba R and Colbeth RE: Development of
angiography system with cone-beam reconstruction using large-area
flat-panel detector. Proc SPIE. 5368:488–498. 2004. View Article : Google Scholar
|
|
23
|
Ishida F, Kawaguchi K, Mizuno M, Hoshino
T, Murao K and Taki W: The accuracy and usefulness of 3D-DSA and
3D-CT angiography for cerebral aneurysms. Interv Neuroradiol. 7
(Suppl 1):181–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muruet W, Rudd A, Wolfe CDA and Douiri A:
Long-term survival after intravenous thrombolysis for ischemic
stroke: A propensity score-matched cohort with up to 10-year
follow-up. Stroke. 49:607–613. 2018. View Article : Google Scholar : PubMed/NCBI
|