Introduction
Osteoporosis is a major health concern characterized
by a decrease in the density and quality of bones, with high risks
of bone fractures (1). Worldwide,
osteoporosis causes >8.9 million fractures annually, resulting
in an osteoporotic fracture every 3 sec (1). In China, osteoporosis affects ~70
million individuals aged ≥50, and causes ~687,000 hip fractures
annually (2). The overall prevalence
of osteoporosis in mainland China is 7% among adults; 22.5% among
males and 50.1% among females aged ≥50 years (3).
The imbalance between the function of osteoblasts
and osteoclasts contributes to bone disease (4). Osteoblast maturation is a multistep
series of events, including proliferation, differentiation and
mineralization, which are modulated by the interactions of a
variety of cytokines and signaling pathways (4). Since the discovery of insulin receptor
in osteoblasts (5), the role of
insulin signaling in bone has attracted increasing attention.
Previous studies have confirmed that insulin signaling is essential
for osteoblast function (5). Mice
lacking insulin receptors in osteoblasts have been shown to develop
postnatal osteopenia, and IR-deficient osteoblasts in vitro
have been shown to have a lower proliferative and differentiation
capacity (6). Moreover, direct
treatment with insulin promotes osteoblast proliferation,
differentiation and collagen synthesis (7,8). After
insulin binds to its receptor, the insulin receptor substrate (IRS)
family acts as docking proteins between insulin receptors and
intracellular signaling molecules (7). There are four subtype members of the
IRS family, IRS-1, IRS-2, IRS-3 and IRS-4, but only IRS-1 and IRS-2
play important roles in bone development via insulin signal
transduction (9,10). Specifically, genetically modified
mice lacking the IRS-1 or IRS-2 gene exhibit severe osteopenia with
a low bone turnover, and cultured IRS-1−/− and
IRS-2−/− osteoblasts exhibit reduced proliferation,
differentiation and matrix synthesis (10). Furthermore, a previous in
vitro study that suppressed the expression of IRS-1 and IRS-2
in L6 myotubes using small interfering RNA, revealed IRS-1 more
closely regulated glucose uptake and IRS-2 seemed to be more
closely linked to mitogen-activated protein kinase (MAPK)
activation (11). The two main
downstream intracellular components of the insulin signaling
pathway include MAPK, which is mainly responsible for cell
proliferation and differentiation, and PI3K/Akt, primarily
regulating metabolic function (12).
While the MAPK and PI3K/Akt signaling pathways play different roles
in insulin functions, both can control cell growth and
differentiation (12).
Pilose antler peptide (PAP; molecular weight, 7,200;
amino acid residue, 68) is extracted and purified from deer
antlers, and is a well-known Chinese traditional medicine
identified to exert beneficial effects against inflammation and
oxidative injury (13,14). Previous studies have shown that PAP
can protect a number of organs, including the brain, lungs and
liver, from inflammation and oxidative stress (15–18).
However, to the best of our knowledge, only a few studies have
focused on the effects of PAP on bone function, and on the
underlying molecular mechanisms related to the NF-κB pathway, the
classical pathway of inflammation (19,20).
Considering the effects of PAP and the roles of the
insulin signaling pathway in osteoblasts, the present study
hypothesized that PAP may promote osteoblast development in a
dose-dependent manner, and that this may be related to insulin
signaling. To investigate this, MTT assay, alkaline phosphatase
(ALP) activity assay, western blot analysis and reverse
transcription-quantitative PCR (RT-qPCR) for osteogenesis-related
markers and downstream insulin signaling pathway markers were
performed.
Materials and methods
Reagents
PAP was purchased from Shanghai Ai Shuang Commerce
Co., Ltd. and dissolved in DMSO. The final concentration of DMSO
was <0.1% (v/v). The MC3T3-E1 osteoblastic cell subclone 4 cell
line (cat. no. CRL-2593; pre-osteoblast; mouse C57BL/6 calvaria)
was purchased from the American Type Culture Collection. The
BCIP/NBT alkaline phosphatase (ALP) staining kit (SBJ-1049) and
Mineralized nodule staining solution of osteoblasts (Alizarin Red S
staining kit, SBJ-1711) were purchased from SenBeiJia Biological
Technology Co. Ltd. PrimeScript™ RT Master Mix kits (RR036A) were
purchased from Takara Biomedical Technology Co. Ltd.
Cell culture
The MC3T3-E1 osteoblastic cell subclone 4 cell line
was cultured in AA-free α-modified Eagle's medium (α-MEM; cat. no.
11900024; Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 µ/ml penicillin and
100 µg/ml streptomycin at 37°C in a humidified atmosphere with 5%
CO2 for 7 days.
MC3T3-E1 cell differentiation and
mineralization
MC3T3-E1 cells at a density of 2×106
cells/ml for 7 days or 1×106 cells/ml for 14 days were
seeded in a 6-well plate. All samples were performed in triplicate.
After the cells reached 80% confluence, the medium was replaced
with α-MEM containing 5 mM β-glycerophosphate and 500 µM ascorbic
acid to facilitate in vitro mineralization. Cells were
treated with various concentrations of PAP (0, 25, 50 and 100 mg/l)
at 37°C in a humidified atmosphere with 5% CO2 for 3, 7
or 14 days. The cells were harvested for cell differentiation,
mineralization and related assays. In some experiments, the ERK
inhibitor PD98059 (PD) and the PI3K inhibitor LY294002 (LY) were
added in advance for 1 h at 37°C before and during exposure to
PAP.
Cell viability and proliferation
assay
Cell viability and proliferation were assessed by
MTT assay. Cells were seeded at 1×105 cells/ml in
96-well plates. Following 24 h of incubation at 37°C, the culture
medium was replaced with α-MEM containing 2.5% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and the cells were treated with PAP (0,
25, 50 and 100 mg/l) for 1, 3, 5 and 7 days. Cell viability assay
was performed after 1 day and cell proliferation assay was
conducted on the indicated days. MTT assay was performed as
previously described (21). Cells
were washed with PBS three times, and 20 µl 5 mg/ml MTT was added
to each well followed by incubation for 4 h at 37°C. Following
solution removal, 200 µl of DMSO was added to each well to dissolve
the purple formazan. The resultant absorbance was measured with
plate reader at a 490 nm test wavelength, using a reference
wavelength of 650 nm.
ALP staining and activity
analysis
ALP staining was performed according to the
manufacturer's instructions provided with the BCIP/NBT kit
(SenBeiJia Biological Technology Co. Ltd). Cells treated with PAP
for 7 days were rinsed with PBS multiple times and fixed with 4%
paraformaldehyde for 30 min at 4°C. The fixed samples (6 µm) were
covered completely with BCIP/NBT working solution for 60 min in the
dark at room temperature. Finally, the cells were washed with PBS
2–3 times and images (×200) were acquired using a light microscope
(IX73, Olympus Corporation).
ALP activity was assayed by the p-Nitrophenyl
Phosphate (PNPP) assay. Protein samples were collected and
quantified using the BCA kit (cat. no. P0012, Beyotime Institute of
Biotechnology). This was followed by the incubation of 20 µl of
protein supernatant with 100 µl PNPP solution in a 96-well plate
for 15 min at 37°C. All samples were performed in triplicate. The
absorbance values were recorded at 405 nm and ALP activity was
determined by optical density (OD) value per mg of total
protein.
Alizarin Red S staining
After 14 days, the cells in 6-well plates were
treated with Alizarin Red S staining solution to visualize bone
nodule formation. The cells were washed twice with PBS and fixed
with 4% paraformaldehyde for 30 min at room temperature, then
rinsed twice with double-distilled H2O. The fixed cells
were then stained with 0.5% Alizarin Red S (pH 4.2) for 30 min at
room temperature with gentle shaking. Finally, the cells were
washed with PBS 2–3 times and images (×200) were acquired using a
light microscope (IX73, Olympus Corporation). The quantification of
Alizarin Red S staining was determined by the addition of a
solution of 20% methanol and 10% acetic acid. The optical density
was measured at an absorbance of 450 nm.
Western blot analysis
The protein suspension of cells was obtained using a
cell lysis buffer for Western and IP kit (cat. no. P0013; Beyotime
Institute of Biotechnology) and the protein concentrations were
determined by BCA Protein Assay kit (cat. no. P0011; Beyotime
Institute of Biotechnology). For each of the samples, the extracted
total proteins (40 µg) were separated on 10% SDS-PAGE gels and then
transferred onto a nitrocellulose filter membrane using an
electrophoretic transfer system (Bio-Rad Laboratories, Inc.). The
membranes were blocked for 1.5 h at room temperature with 2.5% BSA
in TBST and then incubated with the following Abcam supplied
antibodies: Anti-β-actin (1:3,000; cat. no. ab8224), anti-insulin
receptor (1:2,000; cat. no. ab137747), anti-IRS-1 (1:1,000; cat.
no. ab52167), anti-IRS-2 (1:2,000; cat. no. ab134101),
anti-phosphorylated-InsR (1:1,000; cat. no. ab60946),
anti-phospho-IRS-1 (1:1,000; cat. no. ab5599), anti-phospho-Akt
(1:5,000; cat. no. ab81283), anti-Akt (1:10,000; cat. no.
ab179463), anti-ERK (1:1,000; cat. no. ab17942) and
anti-phospho-ERK (1:1,000; cat. no. ab214362,) overnight at 4°C.
The blots were then incubated with HRP-labeled goat anti-rabbit IgG
(1:500; cat.no. A0208; Beyotime Institute of Biotechnology) or
HRP-labeled Goat Anti-Mouse IgG (1:500; cat.no. A0216; Beyotime
Institute of Biotechnology) for 1 h at room temperature. The bands
were visualized using enhanced chemiluminescence reagent (Beyotime
Institute of Biotechnology). The western blot analysis results are
representative of ≥3 experiments.
RT-qPCR
MC3T3-E1 cell total RNA was extracted using TRIzol
reagent (Beyotime Institute of Biotechnology) for 10 min at 4°C and
quantified spectrophotometrically. Single-stranded cDNA was
synthesized using SuperScript II Reverse Transcriptase (Takara
Biomedical Technology Co., Ltd.) according to the manufacturer's
instructions. SYBR-Green quantitative PCR Biomedical Technology
Co., Ltd.) analysis was performed with an ABI PRISM Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and pre-validated primer sets (Qiagen GmbH). Transcripts were
amplified by 40 cycles of the following: 95°C for 30 sec
(denaturation), 60°C for 30 sec (annealing) and 72°C for 30 sec
(extension). All samples were analyzed in triplicate. Threshold
cycles were placed in the logarithmic portion of the amplification
curve, and the results were normalized to GAPDH. The fold
difference between two samples was determined by 2−ΔΔCq
method (21). The primer sequences
are presented in Table I.
 | Table I.Primer sequence. |
Table I.
Primer sequence.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
AGAAGGTGGTGAAGCAGGCATC |
|
| R:
CGAAGGTGGAAGAGTGGGAGTTG |
| CoI | F:
GAGGCATAAAGGGTCATCGTGG |
|
| R:
CATTAGGCGCAGGAAGGTCAGC |
| OPN | F:
CCAAGCGTGGAAACACACAGCC |
|
| R:
GGCTTTGGAACTCGCCTGACTG |
| OCN | F:
GGTGCAGACCTAGCAGACACCA |
|
| R:
AGGTAGCGCCGGAGTCTATTCA |
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was performed using one-way ANOVA followed by Tukey's test
using GraphPad Prism version 5.0c (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of PAP on cell viability and
proliferation
A previous study revealed that PAP could potentiate
osteoblast differentiation and inhibit osteoclastogenesis at 5–40
mg/l (20). Another previous study
found that PAP protected osteoblasts from inflammatory and
oxidative injury at 25–100 mg/l (19). The present study performed an MTT
assay to investigate whether PAP was cytotoxic to MC3T3-E1 cells.
No cytotoxic effects were observed at 24 h, even at the
concentration 150 mg/l PAP (Fig.
1A). Therefore, 25, 50 and 100 mg/l PAP were classified as low,
middle and high concentrations, respectively.
The present study investigated the effects of PAP on
cell proliferation. MC3T3-E1 osteoblastic cells were cultured in
96-well plate and treated with various concentrations of PAP (0,
25, 50 and 100 mg/l) for 1–7 days. The present results suggested
that when culture time was extended to 5 days, treatment with 50
mg/l PAP significantly increased the proliferation of MC3T3-E1
cells. Furthermore, the maximum cell proliferative ability observed
was with 50 mg/l PAP on day 7 (Fig.
1B).
Effects of PAP on osteoblast
differentiation and mineralization
The present study investigated the effects of PAP on
osteoblastogenesis in MC3T3-E1 cells. The present ALP staining
results (Fig. 1C) indicated that PAP
increased osteoblast differentiation at day 7. This trend was
identified in the later ALP activity analysis. PAP increased ALP
activity in a dose-dependent manner, and ALP activity in the high
concentration group (100 mg/l) was increased by almost 2–3-fold
(Fig. 1D). At day 14, PAP increased
the cell staining density of Alizarin Red S (Fig. 1E), and subsequent quantitative
analysis further indicated that PAP promoted osteoblast
mineralization (Fig. 1F).
Effects of PAP on the expression
levels of specific genes in MC3T3-E1 cells
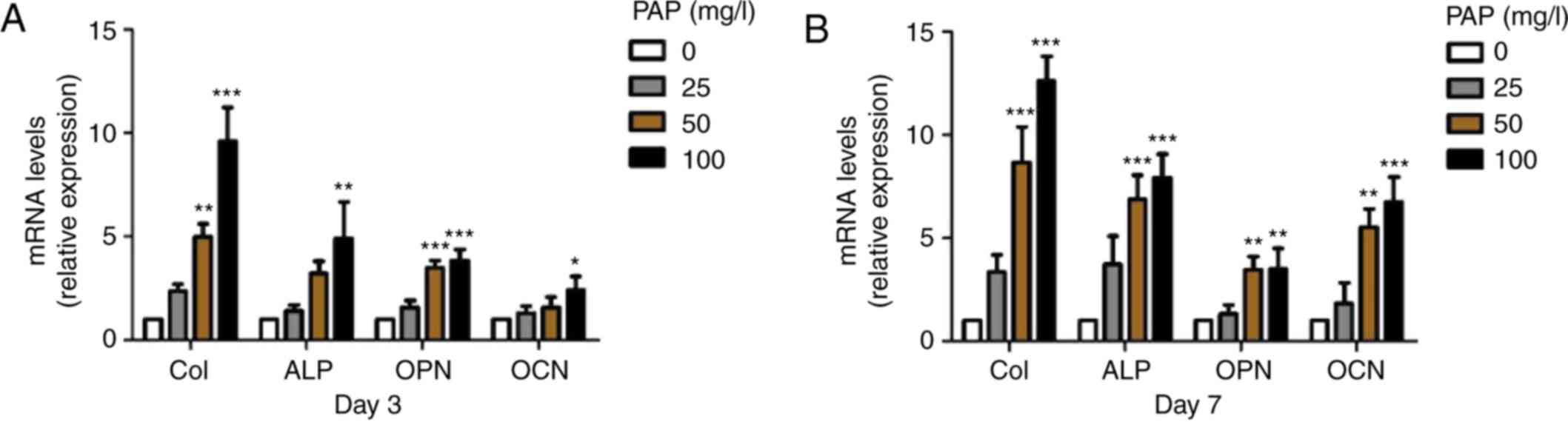
The present study examined the expression levels of
specific marker genes in osteoblasts, including collagen I (CoI),
alkaline phosphatase (ALP), osteopontin (OPN) and osteocalcin (OCN)
by RT-qPCR (21). Collagen I (CoI)
is an early marker of the pre-osteoblast lineage, which
progressively expresses alkaline phosphatase (ALP) during the
maturation stage and then osteopontin (OPN) and osteocalcin (OCN)
during the mineralization phase (22). The present results suggested that PAP
increased the expression levels of the osteogenic-specific genes in
a dose-dependent manner from day 3, with a steady increase until
day 7 (Fig. 2). The present results
suggested that PAP may influence the early stages of osteoblast
differentiation and the late stage of mineralization.
PAP induces the activation of the
insulin signaling pathway
The insulin signaling pathway plays a central role
in osteoblast development (6). After
7 days of treatment, PAP increased the expression level of InsR in
a dose-dependent manner (Fig. 3A).
Treatment with 100 mg/l PAP significantly increased the expression
level of IRS-1, and treatment with 50 mg/l PAP significantly
increased the expression level of IRS-2. The present study
investigated the role of the insulin signaling pathway in the
induction of osteogenic differentiation by PAP by measuring the
phosphorylated and total protein levels of InsR, IRS-1, Akt and ERK
following treatment with 100 mg/l PAP for 0, 15, 30, 60 and 120
min. Treatment with 100 mg/l PAP induced the phosphorylation of
InsR, IRS-1, Akt and ERK (Fig. 3B).
The phosphorylated InsR expression level was increased over the
first 15 and 30 min, while total InsR expression level exhibited no
significant change. PAP increased Akt phosphorylation at 30 min,
with peak activation being reached at 60 min. IRS-1 and ERK were
activated within 30 min and lasted until 120 min.
 | Figure 3.Effects of PAP on insulin signaling in
MC3T3-E1 cells. (A) Western blot analysis of expression levels of
insulin signaling pathway factors after treatment with the
indicated concentrations of PAP for 7 days. (B) Western blotting
and densitometric analysis of the phosphorylated and total InsR,
IRS-1, Akt and ERK under 100 mg/l PAP treatment at 0, 15, 30, 60
and 120 min. Data are presented as mean ± SD. *P<0.05,
***P<0.001 vs. control group. PAP, pilose antler peptide; IRS,
insulin receptor substrate; InsR, insulin receptor; t-, total; p-,
phosphorylated; Ctrl, control. |
Validation of the regulatory effects
of PAP on the insulin signaling pathway
There are two main downstream insulin signaling
pathways, the PI3K/Akt pathway, which is mainly responsible for
metabolic functions, and the MAPK pathway, which regulates the
expression of certain genes and cooperates with the PI3K/Akt
pathway to control cell growth and differentiation (12). The present western blotting results
suggested that PAP can activate both the PI3K/Akt and the MAPK
pathway. Therefore, PI3K (LY) and ERK (PD) inhibitors were used to
investigate this mechanism. The present results from ALP staining
(Fig. 4A) and activity assays
(Fig. 4B) indicated that the
inhibitors LY and PD reversed the effects of PAP on ALP activity
after 7 days. After 14 days, PD inhibitor significantly inhibited
PAP-induced osteoblast mineralization, while LY inhibitor had no
significant effect (Fig. 4C and D).
In addition, neither LY or PD in the absence of PAP showed any
effect on osteoblast proliferation, differentiation and
mineralization (Figs. 4 and S1). Therefore, the present results
suggested that the MAPK pathway may be involved in the PAP-mediated
osteoblast differentiation and mineralization, and that the
PI3K/Akt pathway may at least in part be involved in this
process.
Discussion
There is increasing evidence to indicate that
diabetes mellitus, characterized by a lack of insulin production or
sensitivity, is associated with reduced bone mineral density and
increased fracture risk and delayed fracture healing (23–25).
Insulin has been proposed as an anabolic agent in bone metabolism
(5). Mice lacking insulin receptors
in osteoblasts have been shown to develop postnatal osteopenia and
an impairment of osteoblast proliferation and differentiation, and
direct treatment with insulin has been shown to promote osteoblast
proliferation, collagen synthesis and alkaline phosphatase
production (5,6). The molecular mechanism of insulin
signaling in osteoblast was found to be related to the MAPK and
PI3K pathway (7). Furthermore,
recent studies found a positive loop between osteocalcin, an
osteoblast-specific hormone and insulin (8). Bone, as an endocrine organ, can secrete
osteocalcin to modulate glucose metabolism, and conversely, high
glucose and insulin resistance downregulate osteocalcin gene
expression and negatively affect osteoblast differentiation
(26). Thus, the role of insulin
signaling in regulating bone growth and metabolism has been
revealed by a number of studies, and agents which can activate
insulin signaling in osteoblasts can potentially be used in the
treatment of diabetes-related osteoporosis and bone disease.
PAP, extracted from the pilose antler, is as
well-known Chinese traditional medicine used to promote
regeneration and fracture healing, and to strengthen sinews and
bone (13). The present results
suggested that PAP improved osteogenic proliferation,
differentiation and mineralization, which was determined by the
number and function of osteoblasts. At day 7, the middle and high
concentration PAP groups showed significantly increased cell
proliferation, and induced ALP activity, particularly at the
concentration of 100 mg/l, and also promoted osteogenic
mineralization at day 14. CoI has an apparent effect on the
biomechanical strength of bone tissue by providing the structural
framework for inorganic molecule deposition (22). OPN, as an intermediate or relatively
earlier marker of osteogenic differentiation, is associated with
the maturation stage of osteoblasts during attachment, and matrix
synthesis before mineralization (22). OCN is related to matrix deposition
and mineralization as a late marker of osteogenic differentiation
(22). The present results indicated
that PAP increased the expression levels of osteogenic-specific
genes in a dose-dependent manner from day 3, with a steady increase
until day 7; the present results were similar to those reported by
Liu et al (20).
The present results suggested that PAP significantly
increased the expression levels of InsR and IRS-1, but had a weak
effect on the expression level of IRS-2 after 7 days of treatment.
The biological effects of insulin signaling are mediated by the
phosphorylation of insulin receptor itself and downstream
substrates, such as IRS-1, Akt and ERK (12). The present results indicated that
treatment with 100 mg/l PAP induced the phosphorylation of InsR at
15 min and that of IRS-1 at 30 min. The PI3K/Akt and MAPK signaling
pathways are involved in the induction of cell proliferation and
differentiation by insulin; therefore, the present study
investigated the activation of Akt and ERK in MC3T3-E1 cells
following treatment with PAP. The present results identified that
treatment with 100 mg/l PAP resulted in the activation of both Akt
and ERK. Thus, the present results suggested that PAP may activate
the insulin signaling pathway by promoting the phosphorylation of
the insulin receptor and downstream substrates, such as IRS-1, Akt
and ERK. Furthermore, the effects of PAP on ALP activity in
osteoblasts after 7 days were inhibited by the ERK inhibitor PD,
and partly by the PI3K inhibitor LY. In addition, bone nodule
formation in the MC3T3-E1 cells stimulated with PAP after 14 days
was attenuated by PD, but not by LY. The present results suggested
that the effects of PAP on osteoblastogenesis via the modulation of
insulin signaling in osteoblasts were mediated by the ERK pathway
and at least partly by the PI3K/Akt pathway.
In conclusion, the present results indicated that
PAP promoted osteoblast proliferation, differentiation and
mineralization in vitro by manipulating the insulin
signaling pathway. The MAPK and PI3K/Akt signaling pathways may be
the mechanisms involved in the effects of PAP. The present results
suggested that PAP may potentially be developed as an alternative
therapy for bone diseases related to diabetes characterized by an
impairment in insulin signaling. PAP has been widely used as
traditional medicine in Asia, and has been demonstrated that have
beneficial effects (13,14). In the present study, all the
experiments were carried out in vitro. Further studies are
ongoing to investigate whether PAP can promote bone remodeling in
an osteoporosis animal model.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Fund of Changzhou
Sci&Tech Program (grant no. CJ20180007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CJY conceived the project, designed and performed
the experiments, analyzed the data and wrote the manuscript. WQ and
JW performed the experiments and analyzed the data. CXY and SJ
performed the experiments and revised the manuscript. JL conceived
the project, designed the experiments and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporos Int. 17:1726–1733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Editorial Board of Osteoporosis prevention
and treatment: (China White Paper). Chin J Health Manage.
3:148–154. 2009.(In Chinese).
|
|
3
|
Wang Y, Tao Y, Hyman ME, Li J and Chen Y:
Osteoporosis in China. Osteoporos Int. 20:1651–1662. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferron M, Wei J, Yoshizawa T, Fattore AD,
DePinho RA, Teti A, Ducy P and Karsenty G: Insulin signaling in
osteoblasts integrates bone remodeling and energy metabolism. Cell.
142:296–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fulzele K, Riddle RC, DiGirolamo DJ, Cao
X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC and
Clemens TL: Insulin receptor signaling in osteoblasts regulates
postnatal bone acquisition and body composition. Cell. 142:309–319.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Zhang X, Wang W and Liu J: Insulin
stimualtes osteoblast proliferation and differentiation through ERK
and PI3K in MG-63 cells. Cell Biochem Funct. 28:334–341. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Shen X, Wan C, Zhao Q, Zhang L,
Zhou Q and Deng L: Effects of insulin and insulin-like growth
factor 1 on osteoblast proliferation and differentiation:
Differential signaling via Akt and ERK. Cell Biochem Funct.
30:297–302. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oqata N, Chikazu D, Kubota N, Terauchi Y,
Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K and Kawaguchi H:
Insulin receptor substrate-1 in osteoblast is indispensable for
maintaining bone turnover. J Clin Invest. 105:935–943. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akune T, Oqata N, Hoshi K, Kubota N,
Terauchi Y, Tobe K, Takagi H, Azuma Y, Kadowaki T, Nakamura K and
Kawaguchi H: Insulin receptor substrate-2 maintains predominance of
anabolic function over catabolic function of osteoblasts. J Cell
Biol. 159:147–156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang C, Thirone AC, Huang X and Klip A:
Differential contribution of insulin receptor substrates 1 versus 2
to insulin signaling and glucose uptake in L6 myotubes. J Biol
Chem. 280:19426–19435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taniquchi CM, Emanuelli B and Kahn CR:
Critical nodes in signaling pathways: Insights into insulin action.
Nat Rev Mol Cell Biol. 7:85–96. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang ZQ, Zhang Y, Wang BX, Zhou HO, Wang
Y and Zhang H: Purification and partial characterization of
anti-inflammatory peptide from pilose antler of Cervus Nippon
Temminck. Yao Xue Xue Bao. 27:321–324. 1992.PubMed/NCBI
|
|
14
|
Zhang ZQ, Wang Y, Zhang H, Zhang W, Zhang
Y and Wang BX: Anti-inflammatory effects of pilose antler peptide.
Zhongguo Yao Li Xue Bao. 15:282–284. 1994.(In Chinese). PubMed/NCBI
|
|
15
|
Wu T, Yang L, Chen Y, Ni Y, Jiang J, Zhang
W, Zhou Q, Zheng X, Wang Q, Fu Z and Li H: Pilose antler
polypeptides ameliorates hypoxic-ischemic encephalopathy by
activated neurotrophic factors and SDF1/CXCR4 axis in rats. Acta
Biochim Biophys Sin (Shanghai). 50:254–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai L, Shi W, Liu J, Zhao X, Zhang Y, Zhou
Z, Hou W and Chang T: Protective effect of pilose antler peptide on
cerebral ischemia/reperfusion (I/R) injury througe Nrf-2/OH-1/NF-κB
pathway. Int J Biol Macromol. 102:741–748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma C, Long H, Yang C, Cai W, Zhang T and
Zhao W: Anti-inflammatory role of pilose antler peptide in
LPS-induced lung injury. Inflammation. 40:904–912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chunhua M and Hongyan L: Protective effect
of pilose antler peptide on carbon tetrachloride-induced
hepatotoxicity in mice. Int J Biol Macromol. 99:648–654. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chunhui Y, Wenjun C, Hui W, Liquan S,
Changwei Z, Tianzhu Z and Wenhai Z: Pilose antler peptide protects
osteoblasts from inflammatory and oxidative injury through EGF/EGFR
signaling. Int J Biol Macromol. 99:15–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu G, Ma C, Wang P, Zhang P, Qu X, Liu S,
Zhai Z, Yu D, Gao J, Liang J, et al: Pilose antler peptide
potentiates osteoblast differentiation and inhibits
osteoclastogenesis via manipulating the NF-κB pathway. Biochem
Biophys Res Commun. 491:388–395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song L, Zhao J, Zhang X, Li H and Zhou Y:
Icariin induces osteoblast proliferation, differentiation and
mineralization through estrogen receptor-mediated ERK and JNK
signal activation. Eur J Pharmacol. 714:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Starup-Linde J, Frost M, Vesterqaard P and
Abrahamsen B: Epidemiology of fractures in diabetes. Clacif Tissue
Int. 100:109–121. 2017. View Article : Google Scholar
|
|
24
|
Kemink SA, Hermus AR, Swinkels LM,
Lutterman JA and Smals AG: Osteopenia in insulin-dependent diabetes
mellitus; prevalence and aspects of pathophysiology. J Endocrinol
Invest. 23:295–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duarte VM, Ramos AM, Rezende LA, Macedo
UB, Brandão-Neto J, Almeida MG and Rezende AA: Osteopenia: A bone
disorder associated with diabetes mellitus. J Bone Miner Metab.
23:58–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bilotta FL, Arcidiacono B, Messineo S,
Greco M, Chiefari E, Britti D, Nakanishi T, Foti DP and Brunetti A:
Insulin and osteocaclin: Further evidence for a mutual cross-talk.
Endocrine. 59:622–632. 2018. View Article : Google Scholar : PubMed/NCBI
|