Introduction
Cataract is the most common cause of blindness
worldwide and is closely related to age, and oxidative stress and
subsequent formation of reactive oxygen species (ROS) are
considered to be the main cause of age-related cataract (1). Its pathogenesis involves environmental
exposure and genomic mutations that alter epigenetic patterns, and
microRNA is one of them. Studies have found evidence that miRNAs
are involved in a variety of cellular functions, such as cell
proliferation, apoptosis, stress response and aging. A great deal
of research has been carried out on the mechanism of miRNA action
in cancer cells, blood and muscle tissues (2–5). It was
found in a study on cataract that MicroRNA-34a, hereinafter
referred to as miRNA-34a, decreased rapidly in the lucent lens
among the 32 miRNAs that were significantly expressed in the
central epithelial cells of the lucent lens and the cataract lens
(6), indicating that miRNA-34a was
closely related to the pathogenesis of cataract.
miR-34a is associated with many diseases, for
example, it can inhibit the proliferation and migration of smooth
muscle cells in vascular diseases to reduce the formation of
neointima (7), inhibit invasion and
metastasis by targeting tgif2 in gastric cancer (8), and interfere with the cell cycle and
apoptosis through P53 pathway to play a role in regulating aging
(9). Studies have found that miR-34a
can also inhibit the expression of silencing information regulator
1 (SIRT1). SIRT1 has an important protective effect on
H2O2-induced apoptosis of lens epithelial
cells, and its decreased expression is related to the severity of
cataract (10). In addition, some
studies supported that the expression level of miR-34a was
positively correlated with the age, scores of N, C and P of
cataract patients. The N, C and P scores are the degree of lens
opacity, and the nuclear (N), cortical (C) and posterior
subcapsular (P) cataract scores were graded according to the
improved lens opacity grading system. Elderly patients had higher
N, C and P scores. It indicates that the high level of miR-34a is
related to the high degree of lens opacity and severe lens aging
(11). Based on the above, we
hypothesized that the upregulation of expression level of miR-34a
was associated with occurrence of cataract, and the occurrence of
cataract might be caused by the regulation of SIRT1 protein, and
the older the age, the higher the upregulation of expression level
of miR-34a. This experiment aimed to verify the expression of
miR-34a in cataract and its related mechanism through the rat
model.
Materials and methods
Selection of experimental animals
Thirty SD rats with 30 eyes (purchased from Shilaike
Jingda Experimental Animal Co. Ltd.) were selected and divided into
three groups: group A: 2-month-old lucent lens, 10 rats with 10
eyes; group B: 18-month-old lucent lens, 10 rats with 10 eyes;
group C: naturally occurring cataract lens at 18-month-old, 10 rats
with 10 eyes. Group A weighed 160–200 g, group B weighed 390–420 g,
group C weighed 390–420 g, and they were fed for 60 h under the
same feeding conditions.
The study was approved by the Ethics Committee of
The Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China).
Preparation of lens in rats
The rats were sacrificed by dislocation, then the
corneas were cut open with scissors along the corneas of the rats
at 360°. The lens was completely extracted, and then immediately
put into the EP tube and stored at temperature of −80°C. After the
severity of lens opacity, the cells were separated. Part of cells
were made into homogenate to detect the expression of mRNA and
protein, and another part was sent for primary culture to measure
apoptosis rate.
Monitoring indicators
LOC III was used to analyze the severity of lens
opacity. LOC III (12) was used to
classify the degree of cataract opacity in the nucleus (N), cortex
(C) and posterior subcapsular (P) of the lens of rats in the three
groups to determine the severity of cataract. Nuclear (N) opacity
included 4 levels in total: 0 level: nuclear transparency, the
embryo nucleus could be clearly seen. N1, early nuclear opacity;
N2, moderate nuclear opacity; N3, severe nuclear opacity. The
cortex (C) was divided into seven categories: C0, cortical
transparency. C1, a small amount of dot opacity; C1, enlarged dot
opacity range; C2, a small amount of dot opacity in the pupil area;
C3, wheel-like opacity in cortex which was over the second
quadrant; C4, enlarged cortical wheel-like opacity, with ~50% pupil
area in opacity. C5, ~90% of the cortical area in opacity, degree
of opacity in C5 exceeded C4. Posterior subcapsular (P) was
classified into 5 levels: P0, posterior subcapsular transparency;
P1, ~3% posterior subcapsular opacity. P2, ~30% posterior
subcapsular opacity; P3, ~ 50% of posterior capsular opacity; P4,
opacity exceeded P3.
The expression level of miR-34a and mRNA of SIRT1
and P53 in the three groups were detected by qPCR. Total RNA was
extracted firstly: ~50 mg of tissue was put into a 1.5 ml
RNAse-free centrifuge tube. TRIzol (0.5 ml) was added into and
grinded to homogenate with a homogenizer. Then TRIzol (0.5 ml) was
added and left standing for ~0.5 h. Then adding 200 µl of
chloroform per 1 ml of TRIzol, mixed gently for 30 sec and placed
on ice for 5 min. Then centrifuged at 1,500 × g at 4°C for 15 min.
Approximately 400–600 µl of the supernatant was transfered to a new
centrifuge tube with a pipette, then 500 µl/1 ml TRIzol of
isopropanol was added, covered, mixing by inversion and left
standing for 10 min. It was centrifuged at 4°C, at 1,500 × g for 10
min. Discarding supernatant, absorbed isopropanol was added with 1
ml of 75% ethanol fully mixing. The RNA was washed at 1,500 × g for
10 min at 4°C. Discarding the supernatant, dried naturally for 5–10
min, and 20 µl of DEPC water was added to fully dissolve the total
RNA. qPCR was performed, and the primers were designed by Shanghai
Sangon Biotech Co., Ltd. The sense and reverse primers are shown in
Table I. Comparing the ratio of the
three groups to the internal reference β-actin, the levels of the
three groups of mRNA were obtained. The details were:
Pre-denaturation at 95°C for 5 min, denaturation at 95°C for 15
sec, annealing at 60°C for 30 sec, with a total of 40 cycles at
60–95°C.
 | Table I.Sense primer and reverse primer of
miR-34a, mRNA and P53. |
Table I.
Sense primer and reverse primer of
miR-34a, mRNA and P53.
| Factors | Sense primer | Reverse primer |
|---|
| miR-34a |
5′-ATGGTTCGTGGGTGGCAGTGTCTTAGCTGG-3′ |
5′-GCAGGGTCCGAGGTATTC-3′ |
| SIRT1 |
5′-CCTTTCAGAACCACCAAAGCGGAA-3′ |
5′-AGTCAGGTATCCCACAGGAAACAG-3′ |
| P53 |
5′-GCCCATCCTTACCATCATCACG-3′ |
5′-TTCTTCCTCTGTCCGACGGTCT3′ |
| β-actin |
5′-ATACGCTGGGATGAGCACTGG-3′ |
5″-TCTTTGCGGATGTCCACGTC-3′ |
Western blotting was used to detect the expression
of SIRT1 and P53 protein in the three groups. The liquid chlorine
was added when grinding the tissues and the RIM cell lysis buffer
was added in a certain proportion. The homogenate was crushed and
centrifuged at a low temperature and high speed. The supernatant
was taken and stored in the refrigerator at −80°C after
subpackaging. The expression levels of SIRT1 and P53 proteins were
detected by western blotting, and the ratios of SIRT1/GAPDH and
P53/GAPDH represented the relative expression levels.
Apoptosis of lens epithelial cells was detected by
flow cytometry. Apoptosis was detected using a cell apoptosis
assay, and operated according to the instructions. BD FACSCalibur
flow cytometry (purchased from Shanghai Pudi Biotechnology Co.,
Ltd.) was used to detect the cells transfected with Annexin V and
PI in 6-well plates for 48 h. The experiment was repeated 3
times.
Statistical methods
SPSS 18.0 was used for data analysis. The
measurement data were expressed as mean ± SD. Multivariate analysis
was used to compare the differences among the groups and one-way
ANOVA was used for testing. Enumeration data were qualified by
chi-square test. P<0.05 was considered statistically
significant.
Results
Comparison of basic data of the three
groups
The basic conditions of rats in group A, B and C
were compared, such as weight, age, length, indoor temperature and
indoor relative humidity. There was no significant difference
between the indoor temperature for feeding and the indoor relative
humidity (P>0.05). However, due to the different age of rats in
the three groups, the length and weight of the group B and group C
were significantly higher than that of A group (P<0.05)
(Table II).
 | Table II.Basic data of four groups of rats mean
± SD (n=10). |
Table II.
Basic data of four groups of rats mean
± SD (n=10).
| Groups | Group A | Group B | Group C | F | P-value |
|---|
| Weight (g) | 168.23±11.24 | 395.94±14.06 | 407.23±12.78 | 1119.00 | <0.0001 |
| Length (cm) | 15.06±1.12 | 18.52±1.42 | 18.46±1.22 | 24.73 | <0.0001 |
| Indoor temperature
(°C) | 23.34±1.02 | 23.78±0.97 | 23.03±1.34 | 1.13 | 0.338 |
| Indoor relative
humidity (%) | 49.23±15.32 | 48.83±16.42 | 50.23±14.45 | 0.022 | 0.978 |
Comparison of LOC III classification
of the three groups
In the classification of opacity degree of N, there
were 9 cases of N0, 1 case of N1, and no cases of N2 and N3 in
group A. N score of group B was basically below N1, with 8 cases of
N0 and 2 cases of N1, without N2 and N3. In group C, there were 2
cases of N0, 1 case of N1, 3 cases of N2 and 5 cases of N3. The
opacity degree of N was higher in group C than in group A and B
(P<0.05). In the classification of opacity degree of C, there
were 8 cases of C0, 1 case of C1 in small amount of dot and 1 case
in C1 with dot, no cases of C2, C3, C4 and C5 in group A. There
were 8 cases of C0, no cases of C1 with small amount of dot, 2
cases of C1 with dot, and no cases of C2, C3, C4 and C5 in group B.
There was 1 case of C0, 2 cases of C2, 2 cases of C3, 2 cases of C4
and 3 cases of C5, no case of C0 in group C. Compared with groups A
and B, opacity degree in group C was higher (P<0.05). In the
classification of opacity degree of P, there were 10 cases of P0,
no case of P1, P2, P3 or P4 in group A. There were 9 cases of P0, 1
case of P1, no case of P2, P3 and P4 in group B. There was no case
of P0 or P1, 1 case of P2, 4 cases of P3 and 5 cases of P4 in group
C. Compared with group A and B, the opacity degree of P was higher
in group C (P<0.05) (Table
III).
 | Table III.LOC III classification of the three
groups (n=10). |
Table III.
LOC III classification of the three
groups (n=10).
| Groups | Group A | Group B | Group C | χ2 | P-value |
|---|
| Grade of N |
|
|
| 20.38 | 0.002 |
| N0 | 9 | 8 | 1 |
|
|
| N1 | 1 | 2 | 1 |
|
|
| N2 | 0 | 0 | 3 |
|
|
| N3 | 0 | 0 | 5 |
|
|
| Grade of C |
|
|
| 27.76 | 0.006 |
| C0 | 8 | 8 | 1 |
|
|
| C1 in
small amount of dot | 1 | 0 | 0 |
|
|
| C1 with dot | 1 | 2 | 0 |
|
|
| C2 | 0 | 0 | 2 |
|
|
| C3 | 0 | 0 | 2 |
|
|
| C4 | 0 | 0 | 2 |
|
|
| C5 | 0 | 0 | 3 |
|
|
| P opacity
degree |
|
|
| 31.58 | 0.0001 |
| P0 | 10 | 9 | 0 |
|
|
| P1 | 0 | 1 | 0 |
|
|
| P2 | 0 | 0 | 1 |
|
|
| P3 | 0 | 0 | 4 |
|
|
| P4 | 0 | 0 | 5 |
|
|
Expression levels of miR-34a and mRNA of SIRT1 and
P53 in the three groups
Expression level of miR-34a in the
three groups
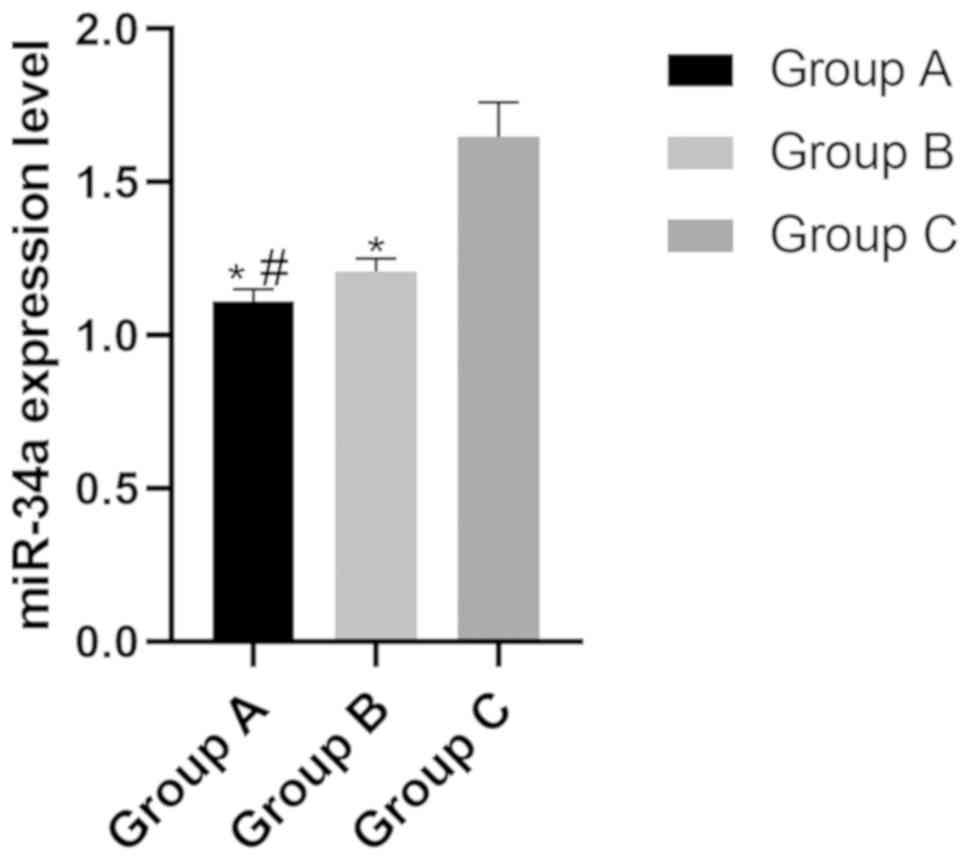
The expression levels of miR-34a in group A, B and C
were (1.11±0.04), (1.21±0.05) and (1.65±0.11), respectively. The
expression level of miR-34a in group C was significantly higher
than that in group A and group B (P<0.05), while the expression
level in group B was significantly higher than that in group A
(P<0.05) (Fig. 1).
mRNA expression level of SIRT1 in the
three groups
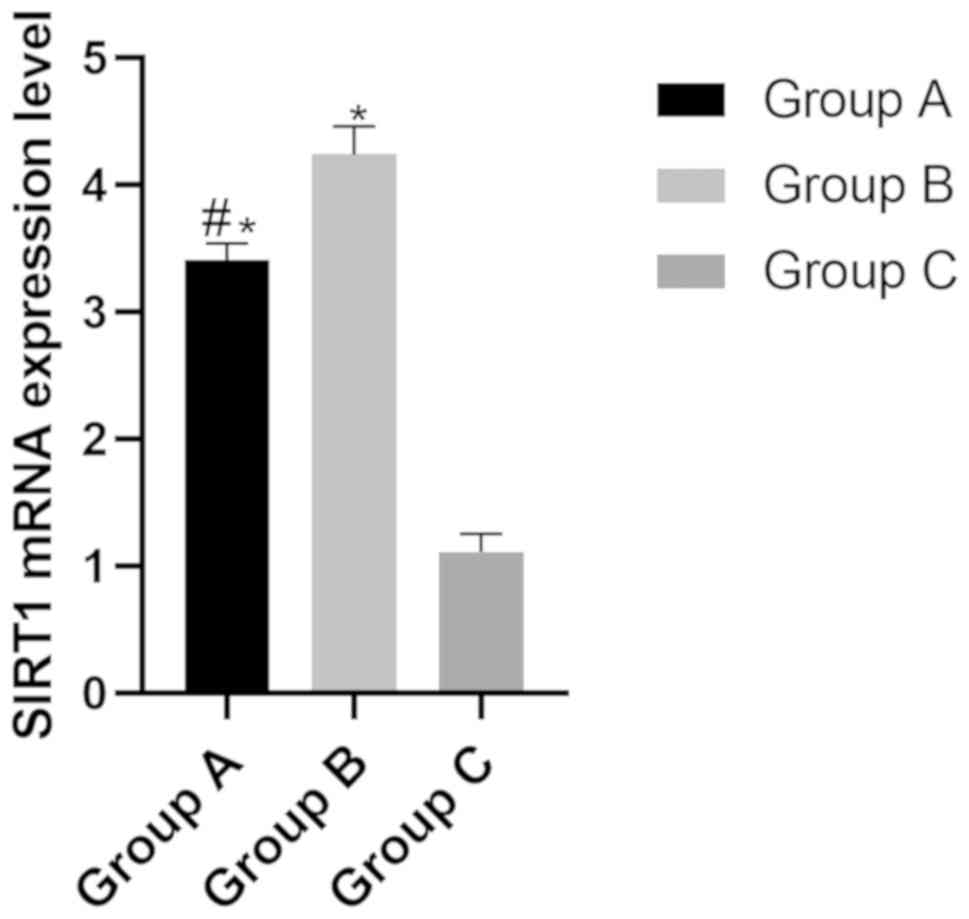
The mRNA expression levels of SIRT1 in group A, B
and C were (3.41±0.13), (4.24±0.22) and (1.11±0.14), respectively.
The mRNA expression level of SIRT1 in group B was significantly
higher than that of group A and group C (P<0.05), while the
expression level of group A was significantly higher than that of
group B and group C (P<0.05) (Fig.
2).
mRNA expression level of P53 in the
three groups
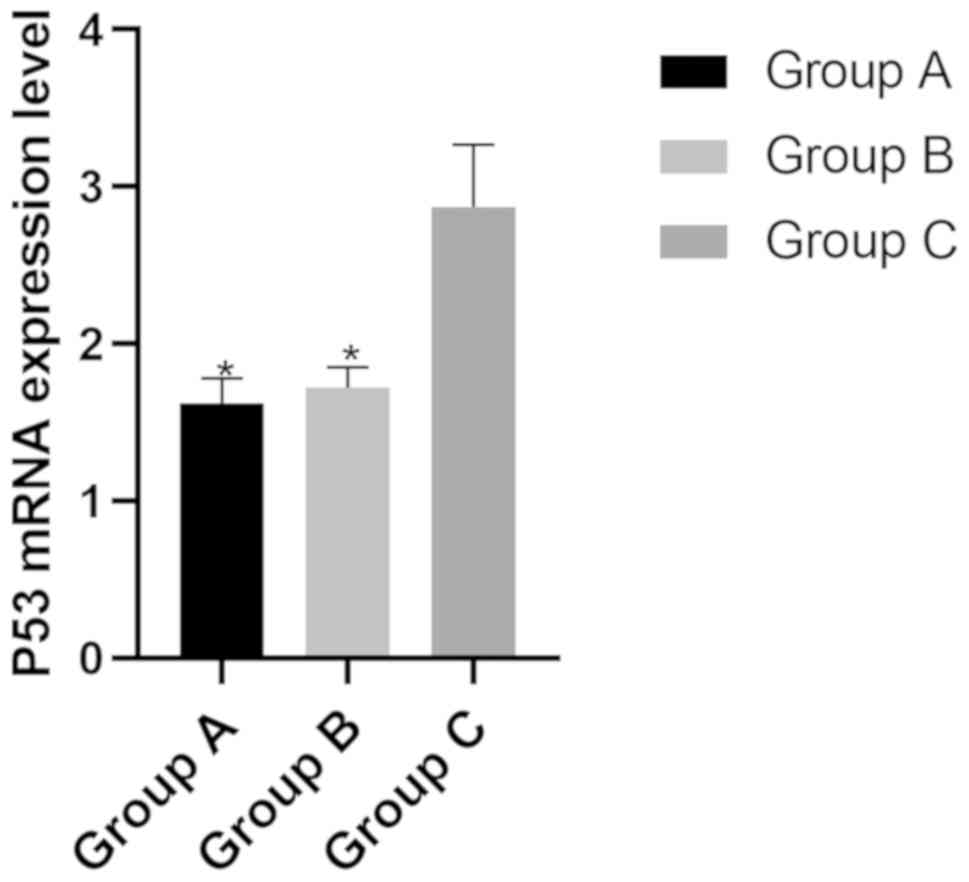
The mRNA expression levels of P53 in group A, B and
C were (1.62±0.16), (1.72±0.13) and (2.87±0.40), respectively. The
mRNA expression level of P53 in group A and B was significantly
lower than that of group C (P>0.05) (Fig. 3).
Expression levels of SIRT1 and P53 in the
three groups
Protein expression level of SIRT in
the three groups
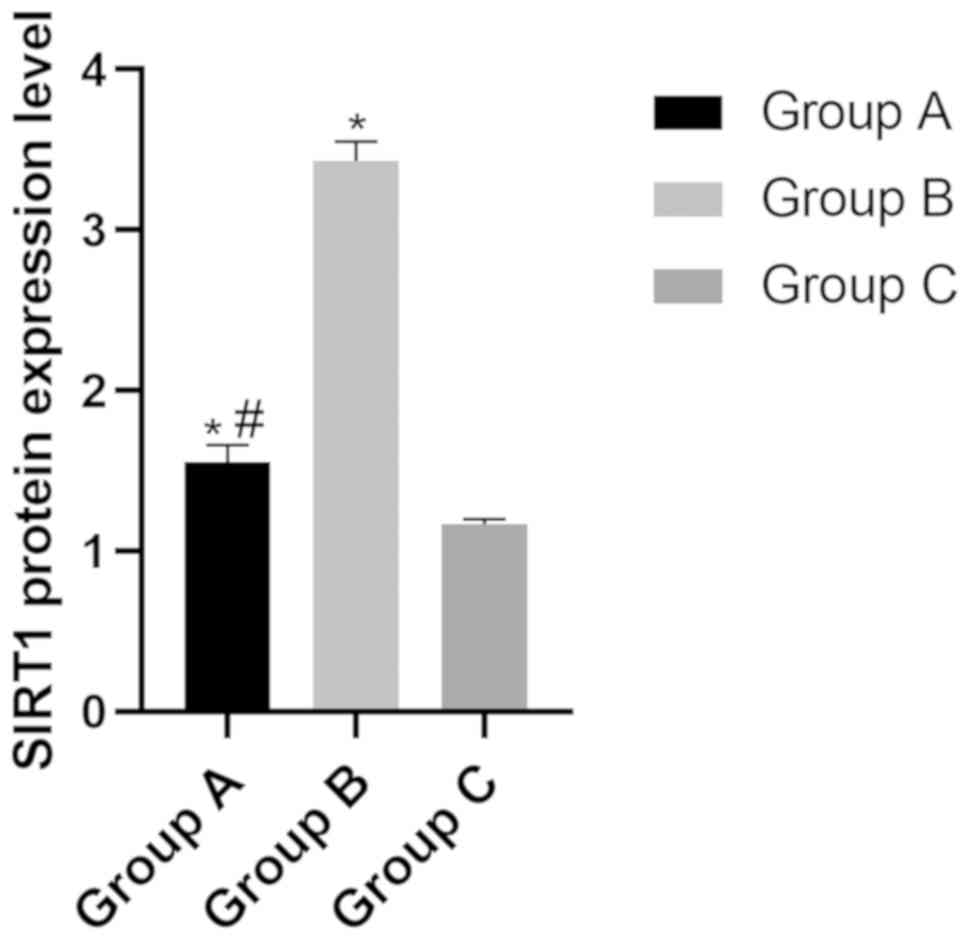
The protein expression level of SIRT in group A, B
and C were (1.55±0.11), (3.43±0.12) and (1.17±0.14), respectively.
The level of SIRT in group B was significantly higher than that of
group A and group C (P<0.05), while the level in group C was
significantly lower than that of group A (P<0.05) (Fig. 4).
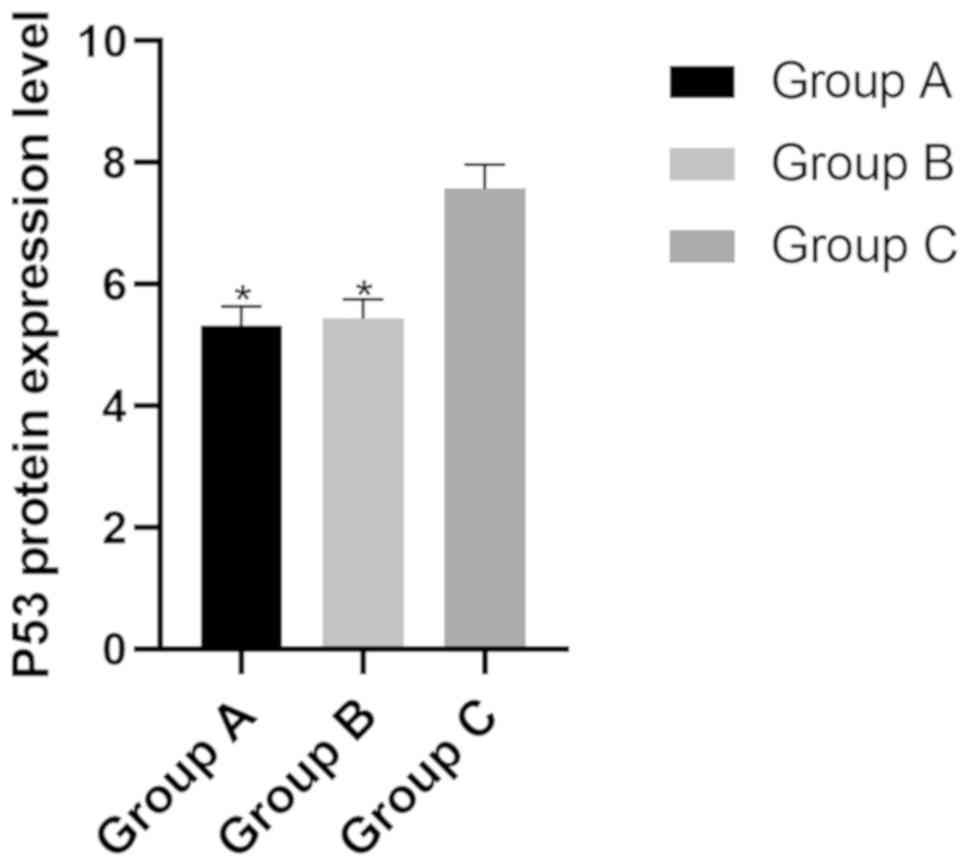
Protein expression level of P53 in the
three groups
The protein expression level of P53 in groups A, B
and C were (5.31±0.32), (5.44±0.31) and (7.57±0.40), respectively.
The protein expression level of P53 in group A and B was
significantly lower than that in group C (P<0.05), while there
was no difference between groups A and B (P>0.05) (Fig. 5).
Comparison of apoptosis rates of lens
epithelial cells in the three groups
The level of apoptosis of P53 in groups A, B and C
were (6.35±0.36)%, (6.45±0.33)%, and (16.07±1.36)%, respectively.
The protein expression level of P53 in group A and B was
significantly lower than that in group C (P<0.05), while the
apoptosis rate of group A and B showed no difference (P>0.05)
(Table IV).
 | Table IV.Rates of lens epithelial cell
apoptosis in the three groups (n=10). |
Table IV.
Rates of lens epithelial cell
apoptosis in the three groups (n=10).
| Groups | Group A | Group B | Group C | F | P-value |
|---|
| Apoptosis rate
(%) |
6.35±0.36a |
6.45±0.33a | 16.07±1.36 | 447.90 | <0.001 |
Discussion
Cataract is the leading cause of blindness. Although
cataract surgery has a high success rate, it still cannot meet the
huge demand for treatment. At present, there is no effective drug
to treat cataract, so we need to find targeted drugs. While miR-34a
has been found to have an effect on human lens epithelial cells,
making its pathogenesis in cataract an important research direction
(13). In this study, the expression
of miR-34a in cataract rats and its related mechanism were studied
through a rat model.
The severity of cataract opacity was positively
correlated with the level of miR-34a. Wu et al (6) also found in their study that miR-34a
was highly expressed in cataract lens epithelial cells, which was
higher than that in lucent lens. Therefore, we analyzed the opacity
of the lens and the expression level of miR-34a in rats. The
results showed that the opacity degree of N, C and P in group C was
very high, and most cases were above N2, C2 and P2, which was
significantly higher than that in group A and group B. Moreover,
the level of miR-34a in the lens of mice in group C was much higher
than that in group A and group B, while the level in group A was
significantly lower than that in group B, which was similar to the
results of the above experiments. Thus, miR-34a was highly
expressed in cataract and might increase with age.
SIRT1 is a class III nad+ dependent
protein deacetylase that acts on programmed cell death, regulation
of gene expression, DNA repair and aging mechanisms, regulates DNA
stability and ensures cell survival. SIRT1 plays an important role
in the self-renewal and aging of eye stem cells and is associated
with various age-related eye diseases. In human eyes, gene
expression of SIRT1 in lens epithelium and retina of senile
cataract patients has been detected (13,14).
Studies have revealed that miR-34a can inhibit the expression of
SIRT1, and SIRT1 can upregulate the expression of Nrf2 and activate
the Nrf2/antioxidant response element (ES) pathway to protect cells
from oxidative stress. When miR-34 binds to the 3′-untranslated
region (utr) of SIRT1, the downregulation of SIRT1 leads to an
increase in acetylation of P53 that mediates cell cycle and
apoptosis. There was a positive feedback between P53 and miR-34a
(15–17). P53 is a transcription factor whose
main function is to regulate the cell life cycle by controlling the
expression of multiple genes, thus promoting apoptosis (18,19). Ji
et al (20) found that P53
could regulate the cellular differentiation genes in the lens by
regulating αA- and βA3/a1-crystallin genes. Therefore, both SIRT1
and P53 can be used as indicators for cataract detection. In this
study, the expression of SIRT1 and P53 were detected from the
perspective of mRNA and protein levels, and the apoptosis was also
detected. The results showed that the mRNA and protein expression
level of SIRT1 in group C were significantly lower than those in
group A and B, while the P53 level in group C was higher than those
in group A and B, and the level of apoptosis was also significantly
higher than those in group A and B. Kondo et al (21) found in animal models that SIRT1 could
regulate eye aging and protect eye tissues from oxidative stress,
and the increase in its level could prevent age-related cataracts.
In the study of Lu et al (22), it was pointed out that P53 was at a
higher level in age-related cataracts and could aggravate cataracts
through increasing the apoptosis of lens epithelial cells. Zheng
and Lu (23) demonstrated that the
expression of SIRT1 could increase when P53 was suppressed. Yan
et al (24) found that there
were mutual effects between miR-34a and SIRT1/P53 signals, and
miR-34a could decrease SIRT1 protein level and lead to the increase
of P53. These results were similar to the results of this study.
From the above results, it can be concluded that there is a
negative feedback relationship between SIRT1 and P53. In addition,
the level of SIRT1 decreased and P53 increased in the cataract.
miR-34a, on the other hand, increases the expression of P53 by
decreasing the level of SIRT1, leading to increase of apoptosis in
the lens, which further aggravates the cataract.
This study relates to the effects of miR-34a on some
related genes and their proteins, and extrapolated its impact on
age-related cataracts based on these signaling pathways. However,
it does not involve more specific molecular mechanisms, such as the
impact of miR-34a on SIRT1 and P53. Thus, furter study is
necessary.
In conclusion, the upregulation of miR-34a
expression level is related to cataract occurrence in rats, which
may be caused by regulation of SIRT1/P53 pathway. Therefore, the
development of drugs related to cataract can be studied as a new
target for miR-34a.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CX wrote the manuscript. CX and JJ conceived and
designed the study. CX and RS were responsible for the collection
and analysis of the experimental data. JJ and RS interpreted the
data and drafted the manuscript. CX and RS revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chien KH, Chen SJ, Liu JH, Chang HM, Woung
LC, Liang CM, Chen JT, Lin TJ, Chiou SH and Peng CH: Correlation
between microRNA-34a levels and lens opacity severity in
age-related cataracts. Eye (Lond). 27:883–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan F, Zhuang J, Zhou P, Liu X and Luo Y:
MicroRNA-34a promotes mitochondrial dysfunction-induced apoptosis
in human lens epithelial cells by targeting Notch2. Oncotarget.
8:110209–110220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asuthkar S, Velpula KK, Chetty C, Gorantla
B and Rao JS: Epigenetic regulation of miRNA-211 by MMP-9 governs
glioma cell apoptosis, chemosensitivity and radiosensitivity.
Oncotarget. 3:1439–1454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung AK and Sharp PA: MicroRNA functions
in stress responses. Mol Cell. 40:205–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noguchi S, Mori T, Otsuka Y, Yamada N,
Yasui Y, Iwasaki J, Kumazaki M, Maruo K and Akao Y: Anti-oncogenic
microRNA-203 induces senescence by targeting E2F3 protein in human
melanoma cells. J Biol Chem. 287:11769–11777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu C, Lin H, Wang Q, Chen W, Luo H, Chen W
and Zhang H: Discrepant expression of microRNAs in transparent and
cataractous human lenses. Invest Ophthalmol Vis Sci. 53:3906–3912.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Q, Yang F, Guo M, Wen G, Zhang C,
Luong A, Zhu J, Xiao Q and Zhang L: miRNA-34a reduces neointima
formation through inhibiting smooth muscle cell proliferation and
migration. J Mol Cell Cardiol. 89A:75–86. 2015. View Article : Google Scholar
|
|
8
|
Hu Y, Pu Q, Cui B and Lin J: MicroRNA-34a
inhibits tumor invasion and metastasis in gastric cancer by
targeting Tgif2. Int J Clin Exp Pathol. 8:8921–8928.
2015.PubMed/NCBI
|
|
9
|
Wang B, Li D and Kovalchuk O: p53 Ser15
phosphorylation and histone modifications contribute to IR-induced
miR-34a transcription in mammary epithelial cells. Cell Cycle.
12:2073–2083. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin TJ, Peng CH, Chiou SH, Liu JH, Lin
C-W, Tsai CY, Chuang JH and Chen SJ: Severity of lens opacity, age,
and correlation of the level of silent information regulator T1
expression in age-related cataract. J Cataract Refract Surg.
37:1270–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu X, Zheng H, Chan MT and Wu WKK:
MicroRNAs: New players in cataract. Am J Transl Res. 9:3896–3903.
2017.PubMed/NCBI
|
|
12
|
Chylack LT Jr, Wolfe JK, Singer DM, Leske
MC, Bullimore MA, Bailey IL, Friend J, McCarthy D and Wu SY; The
Longitudinal Study of Cataract Study Group, : The lens opacities
classification system III. Arch Ophthalmol. 111:831–836. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang W, Lin H, Wang Q and Chen W, Liu Z,
Chen H, Zhang H and Chen W: miR-34a suppresses proliferation and
induces apoptosis of human lens epithelial cells by targeting E2F3.
Mol Med Rep. 14:5049–5056. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mimura T, Kaji Y, Noma H, Funatsu H and
Okamoto S: The role of SIRT1 in ocular aging. Exp Eye Res.
116:17–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li QL, Zhang HY, Qin YJ, Meng QL, Yao XL
and Guo HK: MicroRNA-34a promoting apoptosis of human lens
epithelial cells through down-regulation of B-cell lymphoma-2 and
silent information regulator. Int J Ophthalmol. 9:1555–1560.
2016.PubMed/NCBI
|
|
16
|
Huang K, Huang J, Xie X, Wang S, Chen C,
Shen X, Liu P and Huang H: Sirt1 resists advanced glycation end
products-induced expressions of fibronectin and TGF-β1 by
activating the Nrf2/ARE pathway in glomerular mesangial cells. Free
Radic Biol Med. 65:528–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López Valverde G, Garcia Martin E, Larrosa
Povés JM, Polo Llorens V, Fernández Mateos J, Pablo Júlvez LE and
González Sarmiento R: Study of association between pre-senile
cataracts and the polymorphisms rs2228000 in XPC and rs1042522 in
p53 in Spanish population. PLoS One. 11:e01563172016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volker M, Moné MJ, Karmakar P, van Hoffen
A, Schul W, Vermeulen W, Hoeijmakers JH, van Driel R, van Zeeland
AA and Mullenders LH: Sequential assembly of the nucleotide
excision repair factors in vivo. Mol Cell. 8:213–224. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji WK, Tang XC, Yi M, Chen PQ, Liu FY, Hu
XH, Hu WF, Fu SJ, Liu JF, Wu KL, et al: p53 directly regulates αA-
and βA3/A1-crystallin genes to modulate lens differentiation. Curr
Mol Med. 13:968–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo A, Goto M, Mimura T and Matsubara M:
Silent information regulator T1 in aqueous humor of patients with
cataract. Clin Ophthalmol. 10:307–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu B, Christensen IT, Ma LW, Wang XL,
Jiang LF, Wang CX, Feng L, Zhang JS and Yan QC: miR-24-p53 pathway
evoked by oxidative stress promotes lens epithelial cell apoptosis
in age-related cataracts. Mol Med Rep. 17:5021–5028.
2018.PubMed/NCBI
|
|
23
|
Zheng T and Lu Y: Upregulation of Sirt1
protects lens epithelial cells in oxidative conditions and cataract
formation in humans. Invest Ophthalmol Vis Sci. 52:5291. 2011.
|
|
24
|
Yan S, Wang M, Zhao J, Zhang H, Zhou C,
Jin L, Zhang Y, Qiu X, Ma B and Fan Q: MicroRNA-34a affects
chondrocyte apoptosis and proliferation by targeting the SIRT1/p53
signaling pathway during the pathogenesis of osteoarthritis. Int J
Mol Med. 38:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|