Introduction
Postmenopausal osteoporosis (OP), a common
debilitating disease characterized by low bone mineral density
(BMD) and destruction of bone structure, seriously affects the
health of the elderly, which is caused by osteopenia due to the
decline in the female ovarian function with age. The
pathophysiology of ovary-related bone loss is complicated and
cannot be simply explained by the increase in bone resorption or
decrease in bone formation, and there are various pathogeneses and
regulatory factors (1,2). Currently, it is urgent to search for
satisfactory therapeutic methods for bone diseases such as OP
(3). How to treat bone diseases more
effectively is an urgent problem to be solved. The effects of
multiple genes and other regulatory factors on OP are involved, and
it is a complex and dynamic process regulated by a variety of
cellular components and cytokines. Increasing osteoclast formation
can accelerate cartilage reabsorption and promote bone union, while
inhibiting osteoblast or osteoclast differentiation will suppress
the healing of OP (4,5). The damage to osteoclast absorption will
release organic and inorganic compounds in the bone, the degraded
compound substrates enter the blood circulation in the form of
Ca2+, and the osteoclasts are responsible for cartilage
absorption and remodeling through secreting acid and protease
(6). After OP, cytokines will
initiate osteogenic differentiation, which promotes the benign
development of disease and maintains the benign dynamic balance
(7,8). Once the structural changes happen in
bone tissues and the deposition of the bone matrix is destroyed, OP
and fractures will occur (9). In
recent years, great progress has been made in the treatment and
prevention of female OP, and the treatment mainly focuses on
increasing bone formation or decreasing bone resorption to reduce
its incidence rate (10). Studies
have demonstrated that the osteoblast and osteoclast formation is
regulated by a variety of genes or proteins. The extracellular
matrix proteins, such as Runt-related transcription factor 2
(Runx2), produced by osteoblasts can be positively controlled by
the body and stably secreted, which is the basis to keep bone
homeostasis (11). Therefore, deeply
understanding these molecular regulatory networks is essential for
the treatment of OP, and designing drugs targeting these genes or
proteins will provide new ideas for the OP treatment and fracture
healing.
Postmenopausal ovariectomy will facilitate bone
loss, which is caused by estrogen deficiency (12). In recent years, studies have found
that the growth, maturation, isolation and differentiation of
osteoclasts in bone loss are synchronously regulated by multiple
factors, and the signaling pathways directly involved in bone
resorption play a key role (13). It
has been found that nuclear factor E2-related factor 2 (Nrf2) and
NLR family, pyrin domain containing protein 3 (NLRP3) play
important roles in OP. According to recent studies, Nrf2 is an
important regulator of bone balance in bone cells, and its
activation can enhance the inhibition of endogenous antioxidant
response on reactive oxygen species (ROS), thereby regulating the
occurrence of OP (14,15). Nrf2, through inducing antioxidant and
phase II detoxification enzymes, plays an important role in the
defense of cells against antioxidants and oxidative stress in the
lifespan of the cells. Transcription factors regulate the
expression of marrow stromal antioxidants and phase II enzymes
(16). In addition, Nrf2
downregulates the NLRP3-mediated inflammatory process and innate
immune response (17). The loss of
Nrf2 leads to increased radiation, inducing OP (18). However, the effects of Nrf2 on bone
metabolism and microstructure in vivo and its possible role
in OP are not fully understood yet.
Irisin is a kind of novel actin and adipokine that
is released into the blood circulation through the cleaved
fibronectin type III domain (19).
Irisin is mainly synthesized and secreted by skeletal muscles, so
its correlation with muscles has been explored in several studies
(20). Studies have demonstrated
that irisin can serve as a biomarker for sarcopenia in
postmenopausal women. In addition, although there are some studies
on the correlation between irisin and OP and diabetes (21), no definite and consistent results
have been obtained, and the specific molecular mechanism of
treatment has not been fully clarified. Therefore, it is proposed
in this study that irisin can have a positive influence on
osteoblast apoptosis and OP in postmenopausal OP rats through
upregulating Nrf2 and inhibiting NLRP3 inflammasome. The OP model
was established, the biochemical indexes were detected, the content
of inflammatory factors was determined via enzyme-linked
immunosorbent assay (ELISA), and the bone microstructure was
analyzed. Moreover, the changes in Nrf2, NLRP3 inflammasome,
apoptosis and OP molecules were detected through gene and protein
assays, so as to reveal the therapeutic effect of irisin on
postmenopausal OP rats, and explore whether it exerts a regulatory
effect on the recovery of osteoblast apoptosis and OP in
postmenopausal OP rats through upregulating Nrf2 and inhibiting
NLRP3 inflammasome, which provides an experimental basis for the
subsequent research and development of new drugs.
Materials and methods
Animal modeling and grouping
Healthy female Sprague-Dawley rats were selected and
adaptively fed, and then the rat model of postmenopausal OP was
established via ovariectomy. Forty-five rats were divided into OP
model group (OP group, n=15), 1 mmol/l irisin treatment group
(irisin group, n=15) and normal control group (control group,
n=15). After the trial period, the blood was drawn from the caudal
vein and centrifuged, and the serum was collected and stored at
−80°C to detect the serum biochemical indexes. Then the rats were
anesthetized with pentobarbital sodium, and an appropriate number
of bone tissues were taken, one part for ELISA and the other part
was stored at −80°C to detect expression of genes and proteins.
Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) kits were
purchased from Sangon and antibodies from Abcam.
The study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China).
Detection of serum biochemical
indexes
The serum stored at −80°C was taken out, slowly
thawed at −20°C and centrifuged at 120 × g for 10 min at 4°C. The
supernatant was collected and subpackaged into 1.5 ml EP tubes to
detect the content of serum alkaline phosphatase (ALP) using a
full-automatic biochemical analyzer. The raw data were recorded and
then analyzed.
Detection of serum osteocalcin (OC),
TNF-α and IL-6 in serum and bone tissues by ELISA
An appropriate number of bone tissues stored at
−80°C were ground using a mortar, added with lysis buffer (strong)
and centrifuged at 2,000 × g for 10 min at 4°C. Then the
supernatant was collected to detect the changes in TNF-α, OC and
IL-6 levels in tissues according to the instructions. The
absorbance of indexes in each group was detected using a microplate
reader, and the standard curves were plotted to analyze the
changes.
Micro-CT observation of bone
microstructure
The vertebral bone was isolated in vitro and
placed in a Micro-CT sample cup (GE Healthcare) for
three-dimensional CT reconstruction. The trabecular thickness
(Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp)
and Micro-CT BMD were analyzed.
TUNEL apoptosis assay
Apoptosis of paraffin sections was detected using
the in situ cell death detection kit (Roche Diagnostics).
Paraffin sections were fixed, rinsed and infiltrated with 0.1%
Triton X-100, followed by FITC end labeling of apoptotic DNA
fragment using the TUNEL assay kit (Beyotime Institute of
Biotechnology). The FITC-labeled TUNEL-positive cells were observed
under a fluorescence microscope, and they were counted in 10 fields
of view.
Detection of important molecules and
pathway-related genes in bone tissues by RT-PCR
An appropriate number of bone tissues stored at
−80°C were added with liquid nitrogen and ground using a mortar,
followed by homogenization under 4°C at 800 × g for several
seconds. The total RNA was extracted from tissues, and the RNA
purity and concentration were determined qualified. The primer
sequences of target genes and the internal reference GAPDH were
designed according to GenBank (Table
I). RNA was reverse transcribed into cDNA using a kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and the PCR
amplification was performed using 20 µl of system (2 µl of cDNA, 10
µl of qPCR mix, 1 µl of primer F, 1 µl of primer R and 6 µl of
ddH2O): at 95°C for 2 min, 94°C for 20 sec, 60°C for 20
sec and 72°C for 30 sec, a total of 40 cycles. The specific primer
sequences are shown in Table I. The
relative expression levels of related genes in tissues in each
group were calculated using 2−ΔΔCt.
 | Table I.Primer sequences of target genes. |
Table I.
Primer sequences of target genes.
| Target gene | Primer sequence
(5′-3′) |
|---|
| β-actin |
ACATGCCGCCTGGAGAAA |
|
|
GCCCAGGATGCCCTTTAG |
| Bcl-2 |
GTGCTCTTGAGATCTCTGG |
|
|
CATCGATCTTCAGAAGTCTC |
| Caspase-3 |
TACCGCACCCGGTTACTAT |
|
|
TCCGGTTAACACGAGTGAG |
| Runx2 |
TCCAACCCGTAAGGTGACG |
|
|
GCTGCTGAGTCGATGCTAGC |
| OC |
TCGTAGCTAGCTAGTCGAGC |
|
|
CCCTGTGCTAGCTAGCTAGC |
| Nrf2 |
GGCTGGGCCAAGATCCTA |
|
|
ATAGTCCGCAGGTACCTC |
| NLRP3 |
TGTTCACTGTTCCTAATC |
|
|
CTGAAACACTGGCTTAAA |
Western blotting
The tissues stored in the ultra low temperature
refrigerator were added with lysis buffer (strong) prepared
proportionally, incubated at −20°C for 20 min and ground, followed
by centrifugation at 2,000 × g for 10 min at 4°C. The supernatant
was collected and the protein concentration was measured using the
standard curves. After that, western blotting was performed.
Protein (50 µg) was added with buffer to prepare the protein
samples, subjected to water bath for 8 min and centrifuged at 1,000
× g for 5 min at 4°C. Then the protein was loaded for
electrophoresis, transferred onto a membrane, sealed, incubated
with the primary antibody overnight and incubated again with the
secondary antibody for 1 h. Finally, the protein band was scanned
and quantified using the protein scanner (Bio-Rad Laboratories) and
the level of protein to be detected was corrected using GAPDH.
Statistical analysis
All raw data obtained in the experiments were
statistically analyzed using SPSS 20.0 software. The experimental
results were expressed as mean ± standard deviation (mean ± SD).
P<0.05 indicates that the difference was statistically
significant. The bar graph was plotted using GraphPad Prism
7.0.
Results
Results of serum biochemical
indexes
OP can be predicted through early biochemical
indexes to prepare for the treatment and prognosis as early as
possible, so the content of serum ALP was measured and recorded for
analysis. As shown in Fig. 1, the
content of ALP was significantly increased in OP group (P<0.05),
and declined in irisin group.
Levels of OC, TNF-α and IL-6 detected
by ELISA
As shown in Table
II, the content of serum OC was significantly decreased in the
OP group (P<0.05), and increased in the irisin group
(P<0.05). The content of IL-6 and TNF-α was increased in the OP
group (P<0.05), and decreased in the irisin group
(P<0.05).
 | Table II.ELISA results of serum indexes. |
Table II.
ELISA results of serum indexes.
| Group | OC (ng/ml) | IL-6 (mg/l) | TNF-α
(fmol/ml) |
|---|
| Control | 11.26±0.56 | 30.35±5.24 | 18.63±3.28 |
| OP |
4.9±0.45a |
99.64±7.37a |
39.58±5.78a |
| Irisin |
8.99±0.78b |
35.66±3.86b |
22.47±5.85b |
TNF-α and IL-6 levels in bone tissues
detected by ELISA
As shown in Table
III, the content of IL-6 and TNF-α was increased in the OP
group (P<0.05), and declined in the irisin group
(P<0.05).
 | Table III.Results of inflammatory factors. |
Table III.
Results of inflammatory factors.
| Group | IL-6 (mg/l) | TNF-α
(fmol/ml) |
|---|
| Control | 19.34±5.41 | 14.26±4.10 |
| OP |
60.38±6.52a |
29.36±6.52a |
| Irisin |
22.24±3.65b |
19.99±5.58b |
Micro-CT results
To observe the therapeutic effect of irisin on OP
from the microstructure, Tb.Th, Tb.N, Tb.Sp and BMD were detected.
The results revealed that in the irisin group, Tb.Th, Tb.N and BMD
were obviously increased compared with those in the OP group
(P<0.05), while Tb.Sp obviously declined compared with that in
the OP group (P<0.05) (Table
IV).
 | Table IV.Micro-CT observation and analysis
results of bone microstructure. |
Table IV.
Micro-CT observation and analysis
results of bone microstructure.
| Group | Tb.Th (mm) | Tb.N
(mm−1) | Tb.Sp (mm) | BMD
(mg/cm3) |
|---|
| Control | 0.18±0.01 | 4.8±0.03 | 0.13±0.01 | 415.2±11.8 |
| OP |
0.07±0.01a |
1.5±0.04a |
0.70±0.02a |
148.6±11.8a |
| Irisin |
0.13±0.02b |
4.2±0.02b |
0.26±0.01b |
385.9±10.2b |
TUNEL apoptosis assay
There were few TUNEL-positive cells and they could
hardly be observed in the control group. The number of
TUNEL-positive cells was remarkably more in the OP group than that
in the other two groups, while it was less in the irisin group
(P<0.05) (Fig. 2), indicating
that irisin can inhibit osteoblast apoptosis in OP.
RT-PCR results of apoptosis and
OP-related genes
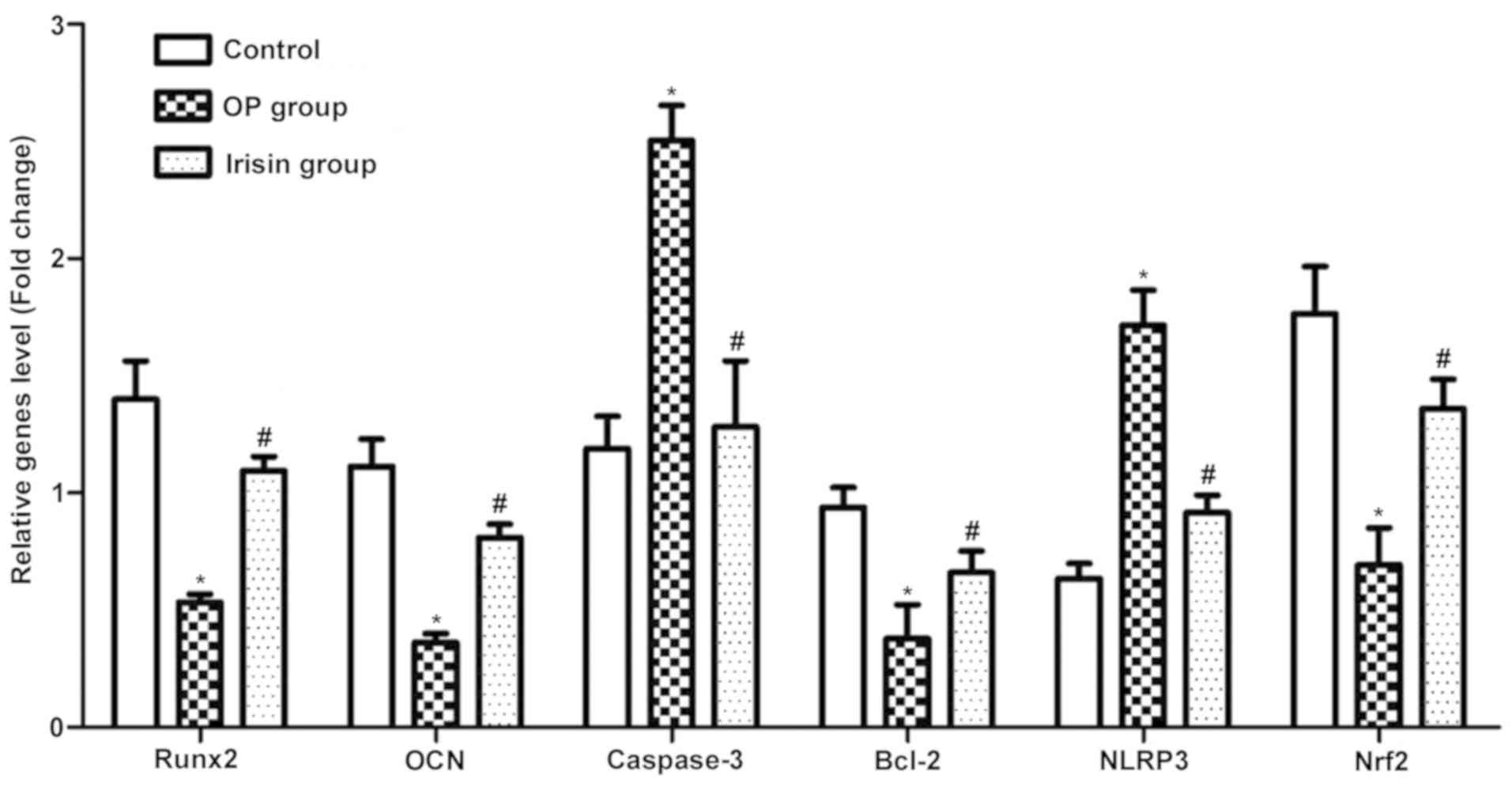
Compared with the OP group, the irisin group had
evidently higher mRNA expression levels of Runx2, OC, Bcl-2 and
Nrf2 (P<0.05), while caspase-3 and NLRP3 displayed the opposite
trends (Fig. 3), suggesting that
irisin can inhibit the incidence of apoptosis in OP and the
occurrence and development of OP.
Western blotting
The protein expression levels of Bcl-2 and Nrf2 in
the irisin group were remarkably higher than those in the OP group,
while that of NLRP3 was the opposite (Fig. 4), demonstrating that irisin not only
inhibits the incidence of apoptosis, but can also treat OP through
upregulating Nrf2 and inhibiting NLRP3 expression.
Discussion
In women, hormonal changes caused by menopause can
lead to muscle atrophy, accelerate bone loss and enhance oxidative
stress. During menopause, therefore, physiological degradation
related to aging and underactivity may be increased, promoting the
occurrence and development of OP (22,23).
Currently, the treatment mainly focuses on increasing bone
formation or decreasing bone resorption to reduce its incidence
rate (24). Physical activity plays
an important role in resisting the physiological degradation
related to aging and menopause, which is a potential mechanism,
possibly because, on the one hand, physical exercise can produce
mechanical signals directly through muscle strength or exert an
anabolic effect on the bones indirectly through endocrine
regulation, but its specific molecular mechanism remains unclear
(25), and, on the other hand,
chronic exercise training can reduce inflammatory and oxidative
damage through enhancing antioxidant capacity and weakening
oxidative stress. In many studies, some plants are used as
traditional medicines with an anti-aging property. Recently, the
potential beneficial effects of these plants on physiological
functions, including the antioxidant capacity, have been studied
increasingly (26). Among them,
irisin is able to resist OP. OP can be predicted through early
biochemical indexes to prepare for the treatment and prognosis as
early as possible, so the classic rat model of postmenopausal OP
was established, and the content of serum ALP was measured in the
present study. The results showed that the content of ALP was
significantly increased in the OP group and declined in the irisin
group. The content of IL-6 and TNF-α was also increased in the OP
group and decreased in the irisin group. Serum OC can indicate the
therapeutic effect of irisin on OP. It was found that the content
of serum OC was significantly decreased in the OP group and
increased in the irisin group, suggesting that irisin has a good
therapeutic effect on OP. In addition, it was observed in bone
microstructure during treatment that both Tb.N and Tb.Th in the
irisin group were obviously increased compared with those in the OP
group. The above results demonstrate that irisin can treat OP
through affecting serum ALP, OC and inflammatory factors,
consistent with the results of Yamaza et al (27) and Yang et al (28).
Studies have manifested that apoptosis can promptly
remove the garbage produced by cells in the life-sustaining
activity, supply energy for the formation of cellular structure and
metabolism, and maintain cell stability (29). The mechanism and pathway clarified
can be used as important guides for diseases such as OP. In the
present study, the apoptosis level in each group was detected by
TUNEL staining. There were few TUNEL-positive cells and they could
hardly be observed in the control group. The number of
TUNEL-positive cells was remarkably more in the OP group than that
in the other two groups, while it was less in the irisin group,
indicating that irisin can inhibit apoptosis. In OP, bone
morphogenetic protein 2-induced osteogenic differentiation is
significantly damaged (30).
Moreover, bone formation plays a unique and important role in the
body after birth, which ultimately increases the expression of bone
matrix proteins mainly through activating some important
bone-derived transcription factors, such as Runx2. A kind of
connexin, OC1 is found in pre-osteoblasts, which can connect Smad
protein and actin microfilament. The knockout of OC1 can clearly
increase the incidence of OP (31).
The Nrf2 signaling pathway is a key regulatory factor for bone
metabolism (32). Studies have found
that there are more osteoclasts in the bone marrow precursors
cultured in KO, and the deficiency of Nrf2 can also increase the
survival rate of osteoclasts. According to the analysis of bone
parameters of Nrf2 knockout mice and wild-type mice, Nrf2 is
essential for keeping the bone quality (33). Moreover, both BMD and area of
cortical bone decline in Nrf2 knockout mice. Nrf2-induced TESE
changes may lead to OP and fractures. Studies have also manifested
that the ROS production is increased in bone marrow cells in Nrf2
knockout mice receiving sham operation or ovariectomy, confirming
that Nrf2 knockout can increase the number of osteoclasts in
vivo (34). In this study, the
RT-PCR results revealed that the irisin group had evidently higher
mRNA expression levels of Runx2, OC, Bcl-2 and Nrf2 than the other
two groups, while caspase-3 and NLRP3 displayed the opposite
trends, which suggests that irisin can inhibit the incidence of
apoptosis in OP and the occurrence and development of OP. Also, the
protein expression levels of Bcl-2 and Nrf2 in the irisin group
were remarkably higher than those in the OP group, while that of
NLRP3 was the opposite, demonstrating that irisin can not only
inhibit the incidence of apoptosis, but also treat OP through
upregulating Nrf2 and inhibiting NLRP3 expression. Such an effect
was confirmed in the research results. However, the therapeutic
effect of irisin needs further verification in in vivo
experiments.
In conclusion, it was found that after the treatment
of OP with irisin, the related inflammatory factors improved. The
mRNA levels of OP healing-related markers such as Runx2 are
significantly upregulated, osteoblast apoptosis declines, the Nrf2
expression is enhanced and the NLRP3 expression is suppressed.
Therefore, the research results herein confirm that irisin inhibits
the incidence of apoptosis, but also treats postmenopausal OP
through upregulating Nrf2 and inhibiting NLRP3 expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and LS wrote the manuscript. XY and PL were
responsible for establishment of the animal model. LX and LS
performed TUNEL and Western blotting. QW and CL performed ELISA and
other experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang J, Lazarenko OP, Blackburn ML,
Shankar K, Badger TM, Ronis MJ and Chen JR: Feeding blueberry diets
in early life prevent senescence of osteoblasts and bone loss in
ovariectomized adult female rats. PLoS One. 6:e244862011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao X, Wu ZX, Zhang Y, Gao MX, Yan YB,
Cao PC, Zang Y and Lei W: Locally administered perindopril improves
healing in an ovariectomized rat tibial osteotomy model. PLoS One.
7:e332282012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cano A, Chedraui P, Goulis DG, Lopes P,
Mishra G, Mueck A, Senturk LM, Simoncini T, Stevenson JC, Stute P,
et al: Calcium in the prevention of postmenopausal osteoporosis:
EMAS clinical guide. Maturitas. 107:7–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kikuta J and Ishii M: Bone imaging:
Osteoclast and osteoblast dynamics. Methods Mol Biol. 1763:1–9.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu-Lee LY, Yu G, Lee YC, Lin SC, Pan J,
Pan T, Yu KJ, Liu B, Creighton CJ, Rodriguez-Canales J, et al:
Osteoblast-secreted factors mediate dormancy of metastatic prostate
cancer in the bone via activation of the
TGFβRIII-p38MAPK-pS249/T252RB pathway. Cancer Res. 78:2911–2924.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuo K: Cross-talk among bone cells.
Curr Opin Nephrol Hypertens. 18:292–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee WC, Guntur AR, Long F and Rosen CJ:
Energy metabolism of the osteoblast: Implications for osteoporosis.
Endocr Rev. 38:255–266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Black DM and Rosen CJ: Clinical practice.
Postmenopausal osteoporosis. N Engl J Med. 374:254–262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung WH, Miclau T, Chow SK, Yang FF and
Alt V: Fracture healing in osteoporotic bone. Injury. 47 (Suppl
2):S21–S26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanchez C, Mazzucchelli G, Lambert C,
Comblain F, DePauw E and Henrotin Y: Comparison of secretome from
osteoblasts derived from sclerotic versus non-sclerotic subchondral
bone in OA: A pilot study. PLoS One. 13:e01945912018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hara Y, Ghazizadeh M, Shimizu H, Matsumoto
H, Saito N, Yagi T, Mashiko K, Mashiko K, Kawai M and Yokota H:
Delayed expression of circulating TGF-β1 and BMP-2 levels in human
nonunion long bone fracture healing. J Nippon Med Sch. 84:12–18.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Folwarczna J, Zych M and Trzeciak HI:
Effects of curcumin on the skeletal system in rats. Pharmacol Rep.
62:900–909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uemura H, Yasui T, Miyatani Y, Yamada M,
Hiyoshi M, Arisawa K and Irahara M: Circulating profiles of
osteoprotegerin and soluble receptor activator of nuclear factor
kappaB ligand in post-menopausal women. J Endocrinol Invest.
31:163–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ibáñez L, Ferrándiz ML, Brines R, Guede D,
Cuadrado A and Alcaraz MJ: Effects of Nrf2 deficiency on bone
microarchitecture in an experimental model of osteoporosis. Oxid
Med Cell Longev. 2014:7265902014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park CK, Lee Y, Kim KH, Lee ZH, Joo M and
Kim HH: Nrf2 is a novel regulator of bone acquisition. Bone.
63:36–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu H, Zhang L, Itoh K, Yamamoto M, Ross
D, Trush MA, Zweier JL and Li Y: Nrf2 controls bone marrow stromal
cell susceptibility to oxidative and electrophilic stress. Free
Radic Biol Med. 41:132–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maicas N, Ferrándiz ML, Brines R, Ibáñez
L, Cuadrado A, Koenders MI, van den Berg WB and Alcaraz MJ:
Deficiency of Nrf2 accelerates the effector phase of arthritis and
aggravates joint disease. Antioxid Redox Signal. 15:889–901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aw Yeang HX, Hamdam JM, Al-Huseini LM,
Sethu S, Djouhri L, Walsh J, Kitteringham N, Park BK, Goldring CE
and Sathish JG: Loss of transcription factor nuclear
factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to
dysregulation of immune functions, redox homeostasis, and
intracellular signaling in dendritic cells. J Biol Chem.
287:10556–10564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee P, Linderman JD, Smith S, Brychta RJ,
Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et
al: Irisin and FGF21 are cold-induced endocrine activators of brown
fat function in humans. Cell Metab. 19:302–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee MJ, Lee SA, Nam BY, Park S, Lee SH,
Ryu HJ, Kwon YE, Kim YL, Park KS, Oh HJ, et al: Irisin, a novel
myokine is an independent predictor for sarcopenia and carotid
atherosclerosis in dialysis patients. Atherosclerosis. 242:476–482.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palermo A, Strollo R, Maddaloni E,
Tuccinardi D, D'Onofrio L, Briganti SI, Defeudis G, De Pascalis M,
Lazzaro MC, Colleluori G, et al: Irisin is associated with
osteoporotic fractures independently of bone mineral density, body
composition or daily physical activity. Clin Endocrinol (Oxf).
82:615–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Velders M and Diel P: How sex hormones
promote skeletal muscle regeneration. Sports Med. 43:1089–1100.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bednarek-Tupikowska G, Tupikowski K,
Bidzińska B, Bohdanowicz-Pawlak A, Antonowicz-Juchniewicz J,
Kosowska B and Milewicz A: Serum lipid peroxides and total
antioxidant status in postmenopausal women on hormone replacement
therapy. Gynecol Endocrinol. 19:57–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tu KN, Lie JD, Wan CKV, Cameron M, Austel
AG, Nguyen JK, Van K and Hyun D: Osteoporosis: A review of
treatment options. PT. 43:92–104. 2018.
|
|
25
|
Zhang L, Yin X, Wang J, Xu D, Wang Y, Yang
J, Tao Y, Zhang S, Feng X and Yan C: Associations between VDR gene
polymorphisms and osteoporosis risk and bone mineral density in
postmenopausal women: A systematic review and meta-analysis. Sci
Rep. 8:981–989. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saei-Dehkordi SS, Tajik H, Moradi M and
Khalighi-Sigaroodi F: Chemical composition of essential oils in
Zataria multiflora Boiss. from different parts of Iran and
their radical scavenging and antimicrobial activity. Food Chem
Toxicol. 48:1562–1567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K,
Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, et al:
Pharmacologic stem cell based intervention as a new approach to
osteoporosis treatment in rodents. PLoS One. 3:e26152008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang C, Zeng YP and Yang T: Irisin in
restraining IL-1, IL-6, TNF-α and M-CSF expression and its effect
in preventing post-menopause osteoporosis. China Pharmaceuticals.
24:15–17. 2015.
|
|
29
|
Klionsky DJ: Autophagy: From phenomenology
to molecular understanding in less than a decade. Nat Rev Mol Cell
Biol. 8:931–937. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thirunavukkarasu K, Halladay DL, Miles RR,
Yang X, Galvin RJ, Chandrasekhar S, Martin TJ and Onyia JE: The
osteoblast-specific transcription factor Cbfa1 contributes to the
expression of osteoprotegerin, a potent inhibitor of osteoclast
differentiation and function. J Biol Chem. 275:25163–25172. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu H, Wu F, Zhang H, Yang C, Li K, Wang H,
Yang H, Liu Y, Ding B, Tan Y, et al: Actin cytoskeleton mediates
BMP2-Smad signaling via calponin 1 in preosteoblast under simulated
microgravity. Biochimie. 138:184–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanzaki H, Shinohara F, Kajiya M and
Kodama T: The Keap1/Nrf2 protein axis plays a role in osteoclast
differentiation by regulating intracellular reactive oxygen species
signaling. J Biol Chem. 288:23009–23020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pellegrini GG, Morales CC, Wallace TC,
Plotkin LI and Bellido T: Avenanthramides prevent osteoblast and
osteocyte apoptosis and induce osteoclast apoptosis in vitro in an
Nrf2-independent manner. Nutrients. 8:423–436. 2016. View Article : Google Scholar
|
|
34
|
Ibáñez L, Ferrándiz ML, Brines R, Guede D,
Cuadrado A and Alcaraz MJ: Effects of Nrf2 deficiency on bone
microarchitecture in an experimental model of osteoporosis. Oxid
Med Cell Longev. 2014:7265902014. View Article : Google Scholar : PubMed/NCBI
|