Introduction
Doxorubicin (DOX) is an anthracycline,
broad-spectrum anti-tumor drug. It has a remarkable therapeutic
effect on cancers, including acute lymphoblastic leukemia, lung
cancer and breast cancer. However, the endotoxicity of DOX
restricts its clinical application. With the accumulation of DOX in
the body, cardiotoxicity eventually leads to apoptosis and necrosis
of cardiomyocytes, mainly manifesting as dilated cardiomyopathy and
heart failure (1–3). Studies have shown that the cumulative
amount of 450–500 mg/m2 DOX leads to 4–5% incidence of
myocardial toxicity, which is up to 18% at 550–660
mg/m2. So far, the mechanism of myocardial damage caused
by DOX has not been fully elucidated. Effective methods for
preventing and treating DOX-induced cardiotoxicity are lacking
(4).
lncRNA is >200-bp long and widely present in
organisms (5–7). It is generally considered to be dark
substance in the human genome without protein-encoding functions
(8,9). Most of lncRNAs are produced in a
similar way to that of mRNAs through cleavage, folding, capping and
polyadenylation. They are involved in regulating chromosome
silencing, epigenetic mediation, genomic imprinting, intranuclear
and transport (10–13). In recent years, lncRNAs were
identified to regulate gene expression at transcriptional and
post-transcriptional levels. In the progression of cardiac
diseases, lncRNAs serve as potential targets for cardiac
regeneration and repair (14,15).
lncRNA HOXB-AS3 encodes an HOXB-AS3 polypeptide that blocks the
tricarboxylic acid cycle of tumor cells. It acts as a switch in the
carbohydrate metabolism pathway. Hence, HOXB-AS3 polypeptide is a
candidate anti-tumor drug, exerting a promising clinical
application (16).
miRNAs are extensively involved in the regulation of
gene expression. Approximately 1/3 of human genes could be
regulated by miRNAs. They exert crucial functions in cellular
behavior and tumorigenesis (17). It
is reported that miRNA-875-3p regulates expression of functional
genes during the synthesis of casein by mediating arginine
(18). It prevents the binding of
arginine to the exon of PKM mRNA, thereby producing PKM1 splicing
instead of PKM2, which in turn mediates the tricarboxylic acid
metabolic pathway (16).
The biological role of lncRNA HOXB-AS3 in
influencing tumor progression has been discovered. However, its
role in DOX-induced cardiotoxicity remains unclear. This study
mainly investigated the role of HOXB-AS3 in protecting DOX-induced
cardiotoxicity and the potential mechanism.
Materials and methods
Cell culture and transfection
PC and H9c2 cells were purchased from American Type
Culture Collection (ATCC). Cells were cultured in Roswell Park
Memorial Institute (RPMI)-1640 containing 10% FBS (Life
Technologies), 100 U/ml penicillin and 100 µg/ml streptomycin.
Until 70–80% confluence, cells were transfected with the vector
using Lipofactamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). Fresh medium was replaced at 6 h. Sequences of transfection
vectors were as follows: sh-HOXB-AS3 1#,
5′-GGUAAACUCGCACCUCUUATT-3′; sh-HOXB-AS3 2#,
5′-GGGUCGUCUGUAUCAAUUUTT-3′; miRNA-875-3p inhibitor,
5′-CACAACCUCGUGUUUCCATT-3′.
RNA extraction
Cells (5×106) were lysed in 1 ml of
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and incubated
with 0.2 ml of chloroform. After 5 min of standing time, the
mixture was centrifuged at 4°C, 10,500 × g for 10 min. The
precipitant was transferred to a new tube, incubated with the
isodose isopropanol and centrifuged again at 4°C, 10,500 × g for 10
min. The precipitant was washed with 75% ethanol and air dried.
Finally, the purified RNA was dissolved in diethyl-pyrocarbonate
(DEPC) water (Beyotime Institute of Biotechnology).
Quantitative real-time polymerase
chain reaction (qRT-PCR)
RNA was reverse transcribed into complementary
deoxyribose nucleic acid (cDNA) using Primescript RT reagent
(Takara Bio). The obtained cDNA underwent qRT-PCR using
SYBR® Premix Ex Taq™ (Takara Bio) at 95°C for 2-min
pre-denaturation, and 40 cycles at 95°C for 1 min, 60°C for 1 min
and 72°C for 1 min. Glyceraldheyde 3-phosphate dehydrogenase
(GAPDH) was used as an internal reference. Each sample was
performed in triplicate, and relative level was calculated by
2−ΔΔCt. Primer sequences were as follows: GAPDH, F,
5′-AGAAGGCTGGGGCTCATTTG-3′; R, 5′-AGGGGCCATCCACAGTCTTC-3′.
CACNA1G-AS1, F, 5′-CGTCCAGCTGCGAGCCAGC-3′; R,
5′-AGCCTTCCTGTGACCTCATC-3′.
5-Ethynyl-2′- deoxyuridine (EdU)
assay
Cells were inoculated into 96-well plates with
1×105 cells per well, and labeled with 100 µl of EdU
reagent (50 µM) per well for 2 h. After washing with
phosphate-buffered saline (PBS), the cells were fixed in 50 µl of
fixation buffer, decolored with 2 mg/ml glycine and permeated with
100 µl of penetrant. After washing with PBS once, cells were
stained with 1X Hoechst 33342 in the dark for 30 min. EdU-positive
ratio was determined under a fluorescent microscope.
Cell Counting Kit (CCK-8)
Cells were seeded in a 96-well plate with
5×103 cells per well. At the appointed time points,
absorbance value at 450 nm of each sample was recorded using the
CCK-8 kit (Dojindo Laboratories) for depicting the viability
curve.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
13.0 (SPSS Inc.) was used for data analyses. Data are expressed as
mean ± standard deviation. Intergroup differences were analyzed by
the t-test. Kaplan-Meier method was introduced for survival
analysis. Two-tailed P<0.05 was considered as statistically
significant.
Results
HOXB-AS3 is upregulated in DOX-treated
cardiomyocytes
PC and H9c2 cells were treated with 0, 2.5, 5, 10
and 20 µM DOX for 24 h. The viability of cardiomyocytes was
dose-dependently downregulated after DOX treatment (Fig. 1A and B). After 20 µM DOX treatment
for 24 h, HOXB-AS3 level in PC and H9c2 cells was markedly
upregulated (Fig. 1C and D). We
speculate that upregulated HOXB-AS3 may serve as a protective
effect on DOX-induced cardiotoxicity.
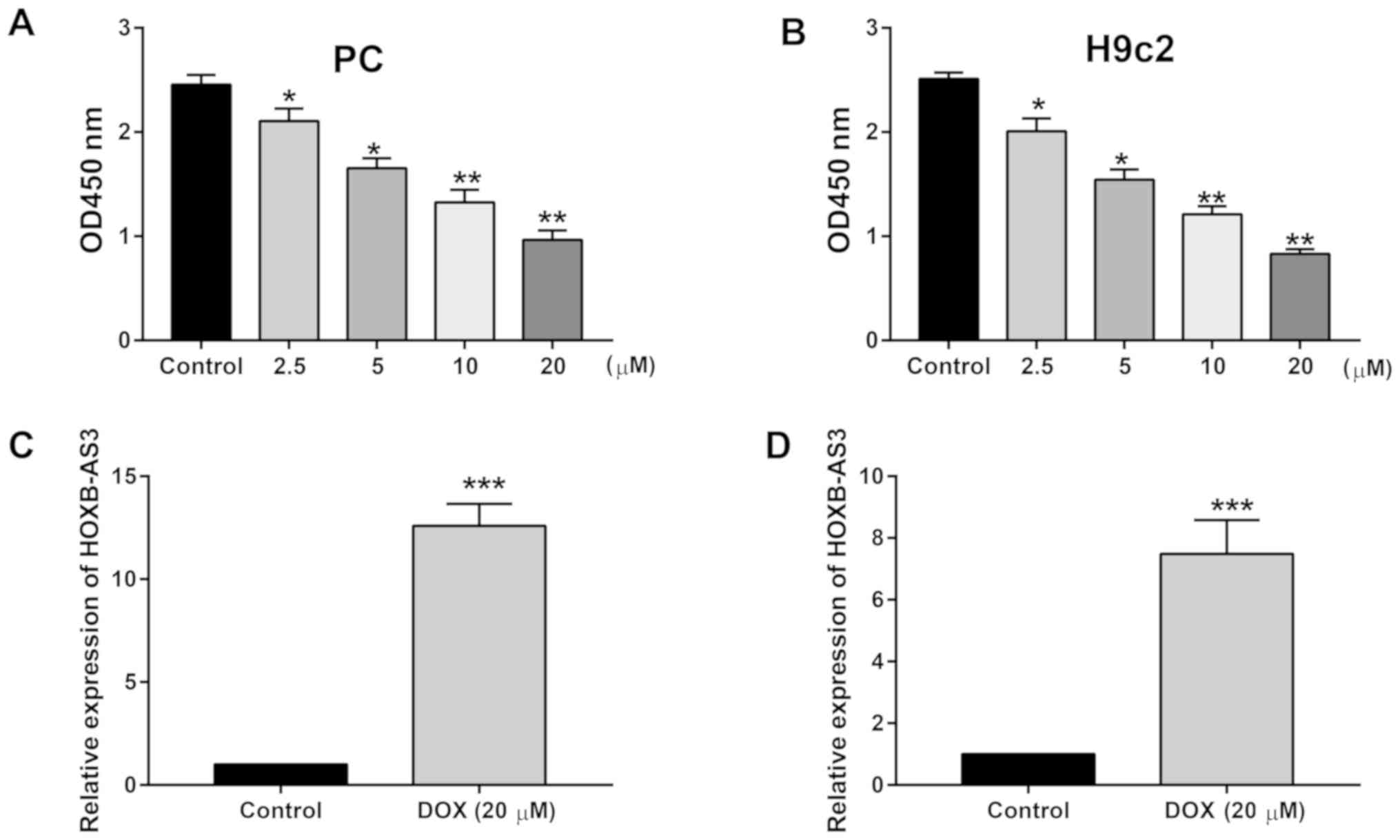 | Figure 1.HOXB-AS3 is upregulated in DOX-treated
cardiomyocytes. (A) Viability in PC cells treated with 0, 2.5, 5,
10 and 20 µM DOX for 24 h. (B) Viability in H9c2 cells treated with
0, 2.5, 5, 10 and 20 µM DOX for 24 h. (C) Relative level of
HOXB-AS3 in PC cells treated with 0 or 20 µM DOX for 24 h. (D)
Relative level of HOXB-AS3 in H9c2 cells treated with 0 or 20 µM
DOX for 24 h. DOX, doxorubicin. Compared with control, *P<0.05,
**P<0.01, ***P<0.001. |
Overexpression of HOXB-AS3 suppresses
cardiomyocyte proliferation
To further investigate the biological function of
HOXB-AS3, sh-HOXB-AS3 1# and sh-HOXB-AS3 2# were constructed.
Transfection of sh-HOXB-AS3 1# or sh-HOXB-AS3 2# markedly
downregulated HOXB-AS3 level in PC and H9c2 cells (Fig. 2A). At 48 and 72 h, the viability in
DOX-treated PC and H9c2 cells transfected with sh-HOXB-AS3 1# or
sh-HOXB-AS3 2# was markedly elevated compared to those in the
controls (Fig. 2B and C). Similarly,
EdU-positive ratio was enhanced after transfection of sh-HOXB-AS3
1# or sh-HOXB-AS3 2# (Fig. 2D and
E). It is concluded that silence of HOXB-AS3 markedly enhanced
the proliferative ability in DOX-treated cardiomyocytes.
miRNA-875-3p is the target of
HOXB-AS3
miRNA-875-3p was found to be downregulated after 20
µM DOX treatment in PC and H9c2 cells (Fig. 3A). Through bioinformatics prediction,
binding sequences between miRNA-875-3p and HOXB-AS3 were discovered
(Fig. 3B). Dual-luciferase reporter
gene assay showed declined luciferase activity after
co-transfection of HOXB-AS3-WT and miRNA-875-3p mimics (Fig. 3C). Moreover, a negative correlation
was identified between expression of miRNA-875-3p and HOXB-AS3.
Transfection of miRNA-875-3p mimics downregulated HOXB-AS3 level in
PC and H9c2 cells and conversely, transfection of miRNA-875-3p
inhibitor upregulated the level of HOXB-AS3 (Fig. 3D and E).
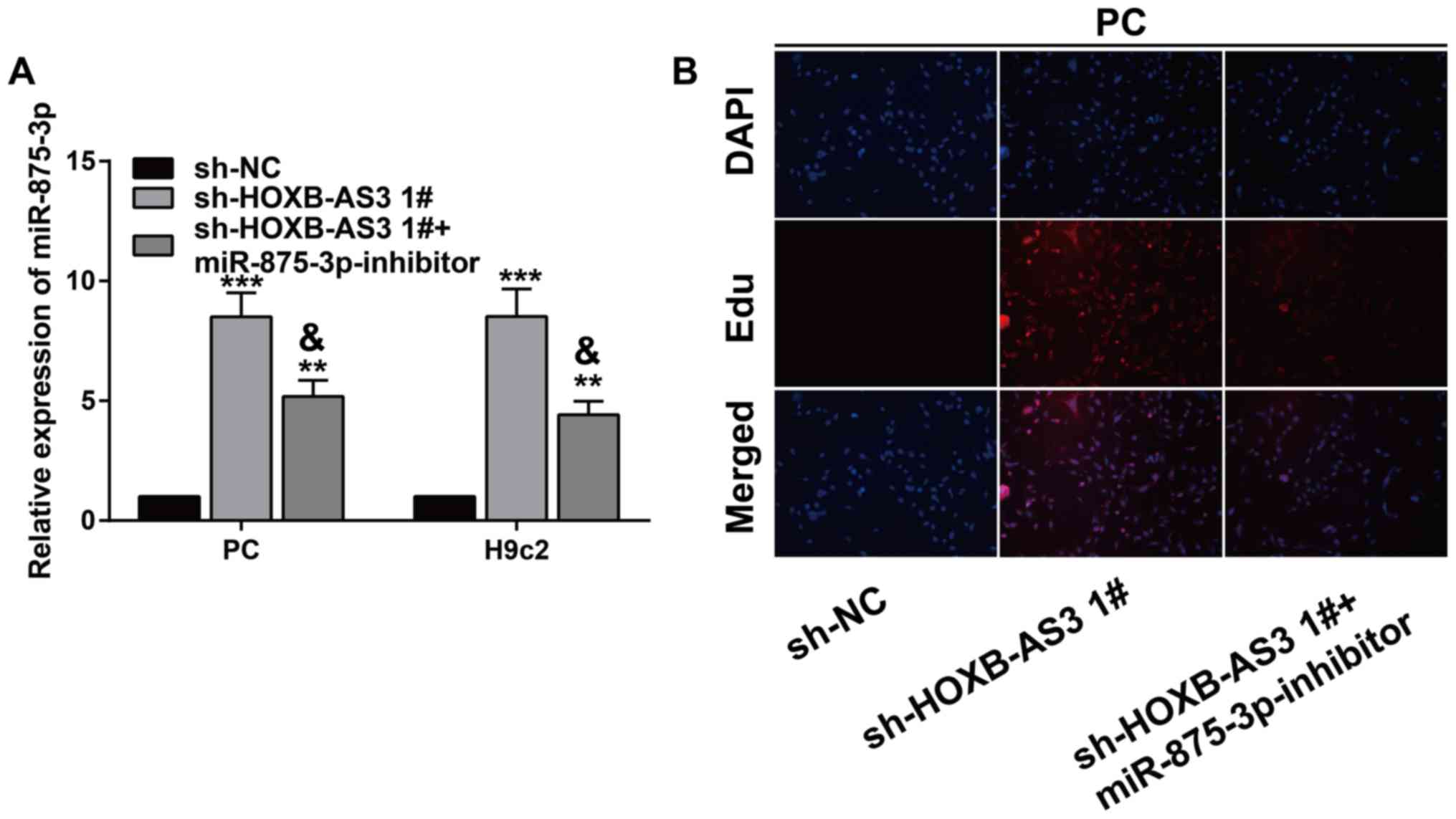
miRNA-875-3p partially reverses the
regulatory effect of HOXB-AS3 on cardiomyocytes
It is speculated that miRNA-875-3p may be involved
in HOXB-AS3-mediated cardiomyocyte proliferation. In PC and H9c2
cells transfected with sh-HOXB-AS3 1#, miRNA-875-3p level was
markedly upregulated, which was partially downregulated by
co-transfection of miRNA-875-3p inhibitor (Fig. 4A). Interestingly, the elevated
EdU-positive ratio in PC cells transfected with sh-HOXB-AS3 1# was
partially reversed by knockdown of miRNA-875-3p (Fig. 4B). Hence, HOXB-AS3/miRNA-875-3p
complex was determined to protect cardiomyocytes from DOX-induced
cardiotoxicity.
Discussion
DOX is one of the important chemotherapeutic drugs
applied in the treatment of various solid tumors. However,
dose-dependence and cardiotoxicity of DOX severely restrict its
clinical application (19).
Currently, lncRNAs are found to be abnormally expressed in patients
with myocardial diseases, and able to alleviate cardiomyocyte
apoptosis and improve cardiac function (20). Li et al (21) reported that lncRNA NRF regulates the
programmed necrosis of cardiomyocytes, which may be utilized as a
target for the treatment of cardiac remodeling following myocardial
infarction. In non-ischemic cardiac diseases (such as DOX-induced
myocardial necrosis), lncRNAs also has crucial functions.
It negatively regulates the expression of target
genes by binding to the mRNAs. Studies have demonstrated that
miRNAs are differentially expressed in tissues under pathological
conditions, which are widely applied as disease markers (22). Serum level of miRNA-875-3p is found
to be downregulated in children with primary dilated heart disease
(23). In this study, DOX-induced
cardiotoxicity upregulated the level of HOXB-AS3 and downregulated
miRNA-875-3p.
Genomic sequencing and bioinformatics analysis
technology contribute to identification of the differentially
expressed lncRNAs in tumor cells, which help to develop therapeutic
targets for tumor diseases (22).
Previous studies illustrated the switch function of HOXB-AS3 in
glycolysis pathway (19–21). Our study showed that DOX treatment
remarkably suppressed the viability in cardiomyocytes, confirming
the cardiotoxicity of DOX. Silence of HOXB-AS3 enhanced the
viability and EdU-positive ratio in DOX-treated cardiomyocytes,
indicating an elevated proliferation. Hence, we considered that
HOXB-AS3 protected cardiotoxicity induced by DOX treatment.
Subsequently, miRNA-875-3p was verified to be the direct target of
HOXB-AS3. Notably, knockdown of miRNA-875-3p could reverse the
regulatory effect of HOXB-AS3 on the cardiomyocyte proliferation.
As a result, HOXB-AS3/miRNA-875-3p complex was proved to alleviate
DOX-induced cardiotoxicity.
In conclusion, HOXB-AS3 protects DOX-induced
suppression in the proliferation of cardiomyocytes through
targeting and downregulating miRNA-875-3p. HOXB-AS3/miRNA-875-3p
complex shows potential as drug targets for alleviating
cardiotoxicity.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology Foundation of Shannxi Province (nos. 2016HM-04,
2016JM-8038 and S2017-ZDYF-ZDCXL-SF-0054).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QL and JH designed the study and performed the
experiments, QL and PL collected the data, LB and AM analyzed the
data, QL prepared the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Lipshultz SE, Franco VI, Miller TL, Colan
SD and Sallan SE: Cardiovascular disease in adult survivors of
childhood cancer. Annu Rev Med. 66:161–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Volkova M and Russell R III: Anthracycline
cardiotoxicity: Prevalence, pathogenesis and treatment. Curr
Cardiol Rev. 7:214–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SY, Kim SJ, Kim BJ, Rah SY, Chung SM,
Im MJ and Kim UH: Doxorubicin-induced reactive oxygen species
generation and intracellular Ca2+ increase are
reciprocally modulated in rat cardiomyocytes. Exp Mol Med.
38:535–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neilan TG, Blake SL, Ichinose F, Raher MJ,
Buys ES, Jassal DS, Furutani E, Perez-Sanz TM, Graveline A,
Janssens SP, et al: Disruption of nitric oxide synthase 3 protects
against the cardiac injury, dysfunction, and mortality induced by
doxorubicin. Circulation. 116:506–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu X, Sood AK, Dang CV and Zhang L: The
role of long noncoding RNAs in cancer: The dark matter matters.
Curr Opin Genet Dev. 48:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun T: Long noncoding RNAs act as
regulators of autophagy in cancer. Pharmacol Res. 129:151–155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim TK, Hemberg M and Gray JM: Enhancer
RNAs: A class of long noncoding RNAs synthesized at enhancers. Cold
Spring Harb Perspect Biol. 7:a0186222015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun W, Yang Y, Xu C and Guo J: Regulatory
mechanisms of long noncoding RNAs on gene expression in cancers.
Cancer Genet. 216-217:105–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang Y, Cheung BB, Atmadibrata B, Marshall
GM, Dinger ME, Liu PY and Liu T: The regulatory role of long
noncoding RNAs in cancer. Cancer Lett. 391:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu B and Shan G: Functions of long
noncoding RNAs in the nucleus. Nucleus. 7:155–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y and Cao X: Long noncoding RNAs in
innate immunity. Cell Mol Immunol. 13:138–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Viereck J and Thum T: Long noncoding RNAs
in pathological cardiac remodeling. Circ Res. 120:262–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang JZ, Chen M, Chen D, Gao XC, Zhu S,
Huang H, Hu M, Zhu H and Yan GR: A peptide encoded by a putative
lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell.
68:171–184.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CJ, Yang JH, Huang FZ, Yang JH, Liu
CP, Mao XH, Yi WM, Shen XB, Peng C, Chen MF, et al: The role of
miR-99b in mediating hepatocellular carcinoma invasion and
migration. Eur Rev Med Pharmacol Sci. 22:2273–2281. 2018.PubMed/NCBI
|
|
18
|
Yang J, Li C, Li H and E C: lncRNA
CACNA1G-AS1 facilitates hepatocellular carcinoma progression
through the miR-2392/C1 or f61 pathway. J Cell Physiol.
234:18415–18422. 2019.PubMed/NCBI
|
|
19
|
Yu SY, Liu L, Li P and Li J: Rapamycin
inhibits the mTOR/p70S6K pathway and attenuates cardiac fibrosis in
adriamycin-induced dilated cardiomyopathy. Thorac Cardiovasc Surg.
61:223–228. 2013.PubMed/NCBI
|
|
20
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Chen C, Fan J, Yin Z, Ni L,
Cianflone K, Wang Y and Wang DW: Identification of cardiac long
non-coding RNA profile in human dilated cardiomyopathy. Cardiovasc
Res. 114:747–758. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enes Coşkun M, Kervancıoğlu M, Öztuzcu S,
Yılmaz Coşkun F, Ergün S, Başpınar O, Kılınç M, Temel L and Coşkun
MY: Plasma microRNA profiling of children with idiopathic dilated
cardiomyopathy. Biomarkers. 21:56–61. 2016. View Article : Google Scholar : PubMed/NCBI
|