Introduction
Sepsis refers to hosts' uncontrolled immune response
to infection, which in turn affects organ functions and causes
death of critically ill patients; it is also one of the main
problems in the global health care system (1–3).
Pathogenesis of sepsis is extremely complex and has not yet been
fully clarified (4). Data show that
the decrease of platelets occur in 35 to 59% of patients with
sepsis. It was found that the symbolic risk factor of the death of
patients with sepsis is thrombocytopenia (TCP), which is induced by
infections, and the recovery of the minimum value of platelet count
is associated with the decrease of mortality (5–7). Studies
have shown that when patients are stimulated by endotoxin, platelet
activation can be induced by some pathways, which makes platelets
play an important role and induce the body to produce an immune
response (8,9).
Interleukin-18 (IL-18), a general pro-inflammatory
cytokine, is widely distributed in the body, and it can facilitate
the production of interferon-γ (IFN-γ) and regulate the initial
immune response of infection and inflammation (10). Studies have found that the severity
and prognosis of sepsis are closely related to IL-18 (11,12).
IL-35 is produced by regulatory T cells and is related to
occurrence and progression of a variety of diseases; it is mainly
involved in immune response, autoimmune diseases, infections and
inflammation, as a new inflammatory factor, IL-35 can be used as a
promising therapeutic target (13,14).
Recent studies have shown that IL-35 can effectively improve
infectious diseases and delay the development of the immune
inflammatory response caused by itself, and infections can promote
Treg and other cells to secrete more IL-35, which enhances the
body's tolerance to inflammatory response caused by infections
(15). Therefore, it has been
speculated that IL-18 and IL-35 are involved in sepsis TCP.
However, there are currently few studies on the expression of IL-18
and IL-35 and the correlation between them and thrombocytopenia in
patients with sepsis TCP.
In this study, the expression of IL-18 and IL-35 in
the serum and in karyocytes in peripheral blood of patients with
sepsis and patients with sepsis TCP were studied, and the
correlation between IL-18, IL-35 and platelet and their clinical
significance were investigated.
Patients and methods
General data
In total, 166 patients who were admitted to Jinan
Central Hospital Affiliated to Shandong University (Jinan, China)
from July 2013 to September 2015 were collected, and they conformed
to the diagnostic criteria of sepsis developed by the American
College of Critical Care Medicine in 2001 (16). Among them, 96 patients who had sepsis
without thrombocytopenia were the sepsis group; there were 55 males
and 41 females, aged 65.12±8.11 years. Seventy patients with sepsis
TCP were the sepsis TCP group; there were 45 males and 25 females,
aged 66.21±10.12 years. Eighty healthy subjects were selected as
the control group, aged 64.20±6.81 years, including 49 males and 31
females.
Inclusion criteria: Patients who did not have
unhealty habits; patients wgo actively cooperated with the
treatment; patients with complete clinicopathological data;
patients with sepsis caused by different pathogen infections
(G-bacteria, G+ bacteria, fungi and no-bud anaerobic bacteria) and
met the latest diagnostic criteria for sepsis; patients with
peripheral blood platelet count ≤50×109/l (the criterion for
TCP).
Exclusion criteria: Patients in gestation period or
puerperium; patients under the age of 18 years; patients had
diseases which affected the formation of platelet, such as primary
thrombocytosis; patients who had a history of malignancy in blood
system; patients with decompensated cirrhosis or failure; patients
who had history of chemotherapy; patients who received therapeutic
anticoagulation or blood transfusion in the prior four weeks;
patients died within 24 h after they were hospitalized.
This study was approved by the Ethics Committee of
Jinan Central Hospital Affiliated to Shandong University, and the
experimental content relating to the patients was described in
detail. The patients and their families agreed and signed an
informed consent form.
The collection of samples
Sequential organ failure assessment (SOFA) and acute
physiology and chronic health evaluation (APACHE II) were carried
out for all the patients within 24 h after they were hospitalized.
Peripheral venous blood (5 ml) was taken immediately, and left at
room temperature for 20 min. Then it was centrifuged at 1,006.2 × g
at 4°C for 10 min, with a centrifugal radius of 10 cm. The
supernatant was collected and placed at −80°C until testing. At the
same time, 5 ml of peripheral venous blood of healthy controls was
collected, centrifuged and stored in a refrigerator at −80°C, and
repeated freeze and thaw were avoided.
Experiment steps
Fluorescent quantitative polymerase
chain reaction (RT-PCR) was used to detect the expression of mRNA
levels of IL-18 and IL-35 in karyocytes in peripheral blood
Expression of mRNA levels of IL-18 and IL-35 in
karyocytes in peripheral blood were detected by RT-PCR, and
mononuclear cells in peripheral blood were isolated by
Ficoll-Hypaque density gradient centrifugation at 400 × g at 4°C
for 40 min. TRIzol extraction reagent was used to extract total
RNA; an ultraviolet spectrophotometer was used to detect the purity
and concentration; reverse transcription kits were used to
transcribe RNA samples into cDNA in strict accordance with the
instructions. ABI PRISM-7500 amplification instrument and SYBR
Premix Ex Taq kit were used for RT-PCR reactions. For PCR
amplification, the reaction system was: at 95°C for 10 min, at 95°C
for 15 sec, at 60°C for 1 min, for 40 cycles. The melting curve was
analyzed by increasing the temperature from 60 to 95°C, with a
temperature transition rate of 0.1°C/sec, three parallel reaction
wells were set for each sample, and the experiment was repeated at
least three times. The primers of the experiment were designed by
Primer Premier 5.0 (Premier Biosoft) primer design software, and
they were synthesized by Tianjin Saier Biotechnology Co., Ltd.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the
internal reference, and the specific sequences are shown in
Table I. In the results, the
fluorescence signal is in the process of amplification of the cycle
Ct value, and the inflection point which started from the
background and entered the exponential growth phase corresponding
to the number of cycles; 2−ΔCt was used to calculate the
relative expression levels of mRNA of IL-18 and IL-35 of each
sample.
 | Table I.Sequences. |
Table I.
Sequences.
| Gene | Upstream | Downstream |
|---|
| IL-18 |
5′-CTTGAATCTAAATTATCAGTC-3′ |
5′-GAAGATTCAAATTGCATCTTAT-3′ |
| IL-35 |
5′-GCTCCCTACGTGCTCAATGTC-3′ |
5′-AGGGTCGGGCTTGATGATGT-3′ |
| GAPDH |
5′-GGAGCGAGATCCCTCCAAAAT-3′ |
5′-GGCTGTTGTCATACTTCTCATGG-3′ |
The levels of IL-18 and IL-35 in the
serum of the samples were detected by enzyme-linked immunosorbent
assay (ELISA)
Standard sample (50 µl) was added into the wells
coated with enzyme label. First, 40 µl of sample diluent was added
into the wells of the sample to be tested, and then 10 µl of the
sample (the dilution ratio of the sample was 5 times) was added,
avoiding touching the wall of the wells during these steps, then
the wells were shaken gently. The reaction wells were sealed with a
sealing film, and then incubated in a water bath kettle or an
incubator for 30 min. After this, the sealing film was carefully
removed, the liquid was discarded; and the wells were dried with
absorbent paper, then each well was filled with washing solution.
After 30 sec, this step was repeated five times and the wells were
dried. Apart from the blank wells (the steps of the blank control
wells were identical to the other steps, but enzyme-labeled reagent
and sample were not added), 50 µl of enzyme-labeled reagent was
added into each well, next they were incubated at 37°C for 30 min
and then they were washed. 50 µl of substrate A and substrate B was
added into each well, and the color was developed at 37°C for 15
min in the dark. Then 50 µl of stop solution was added into each
well, and zero setting was made with a blank well, and the
absorbance (OD value) of each well was detected at the wavelength
of 450 nm in 25 min. The levels of IL-18 and IL-35 in the serum
were calculated.
Experimental instruments and
reagents
SYBR Premix Ex Taq kit, TRIzol extraction kit, cDNA
reverse transcription kit (all from Takara Company), UV
spectrophotometer (Shanghai Mapada Instrument Co., Ltd.), ABI
PRISM-7500 amplification instrument (Shanghai Bajiu Industrial Co.,
Ltd.), IL-18 ELISA kit and IL-35 ELISA kit were from Moshake
Biology Co., Ltd., BS-1101 enzyme-labeled instrument was from
Beijing Linmao Technology Co., Ltd.
Observation indicators
The expression of platelet count (PLT), C-reactive
protein (CRP), creatinine, and total bilirubin was detected. APACHE
II score, SOFA score, infection site and 28-day mortality were
recorded. The expression of mRNA of IL-18 and IL-35 in karyocytes
in peripheral blood and their expressions in the serum were
observed. The correlation between IL-18, IL-35 and platelets in the
serum of the patients with sepsis TCP was analyzed.
Statistical processing
SPSS 19.0 software system (IBM Corp.) was used to
statistically analyze the experiment data. The enumeration data
were expressed in the form of [n(%)], Chi-square test was used in
comparison between groups in this study. The measurement data were
expressed in the form of mean ± SD, t-test was used in the
comparison between two groups; variance analysis followed by LSD-t
test was used in the comparison between groups, Pearson's
correlation coefficient was used in the bivariate normal
distribution data. At P<0.05, the difference was statistically
significant.
Results
Comparison of the clinical basic data
in the three groups
There were no statistically significant differences
in sex and age between the three groups (P>0.05). The
differences in hemoglobin, albumin, creatinine, total bilirubin,
platelet count, whole blood leukocyte count, fibrinogen and
C-reactive protein (CRP) concentration between the three groups
were statistically significant (P<0.05). There were significant
differences in albumin, creatinine, total bilirubin and platelet
count between the sepsis group and the sepsis TCP group (P<0.05)
(Table II).
 | Table II.Comparison of the clinical basic data
in the three groups (mean ± SD)/[n(%)]. |
Table II.
Comparison of the clinical basic data
in the three groups (mean ± SD)/[n(%)].
| Clinical
features | Control group
(n=80) | Sepsis group
(n=96) | Sepsis TCP group
(n=70) | F/χ2 value | P-value |
|---|
| Sex |
|
|
| 0.85 | 0.65 |
| Male | 49 (61.3) | 55 (57.3) | 45 (64.3) |
|
|
|
Female | 31 (38.7) | 41 (42.7) | 25 (35.7) |
|
|
| Age (years) | 64.20±6.81 | 65.12±8.11 | 66.21±10.12 | 1.08 | 0.34 |
| Hemoglobin
(g/dl) | 13.93±3.13 |
10.12±2.42a |
10.45±2.33a | 52.15 | <0.01 |
| Albumin (g/dl) | 3.65±1.21 |
3.03±0.78a |
2.32±0.62a,b | 40.12 | <0.01 |
| Creatinine
(mg/dl) | 1.22±0.65 |
1.51±0.87a |
1.94±1.03a,b | 13.26 | <0.01 |
| Total bilirubin
(mg/dl) | 0.21±0.12 |
0.50±0.20a |
0.65±0.31a,b | 80.32 | <0.01 |
| Platelet count
(×109/l) | 186.11±21.31 |
178.34±13.21a |
72.36±20.12a,b | 654.3 | <0.01 |
| Whole blood
leukocyte count (×109/l) | 9.34±1.02 |
10.45±1.32a |
10.32±1.41a | 19.19 | <0.01 |
| Fibrinogen
(g/l) | 3.47±1.00 |
4.78±1.32a |
4.98±1.48a | 32.73 | <0.01 |
| CRP (mg/dl) | 4.23±3.01 |
9.32±6.68a |
10.11±7.21a | 22.94 | <0.01 |
Comparison of infection site and organ
damage and other baseline data of the patients in the sepsis group
and the sepsis TCP group
There was no difference in APACHE II score and SOFA
score between the patients in the sepsis group and the sepsis TCP
group (P>0.05); there was no significant difference in the
source of infection, the site of infection, the number of organ
damage and the type of organ damage between the two groups
(P>0.05); there were significant differences in presence and
absence of shock, ICU mortality, and 28-day mortality between the
two groups (P<0.05); the rate of patients with shock, ICU
mortality and 28-day mortality in the sepsis group were lower than
those in the sepsis TCP group (Table
III).
 | Table III.Comparison of infection site and
organ damage and other baseline data of the patients in the sepsis
group and the sepsis TCP group (mean ± SD)/[n(%)]. |
Table III.
Comparison of infection site and
organ damage and other baseline data of the patients in the sepsis
group and the sepsis TCP group (mean ± SD)/[n(%)].
| Baseline data | Sepsis group
(n=96) | Sepsis TCP group
(n=70) | χ2/t value | P-value |
|---|
| APACHE II
score | 24.12±7.21 | 22.79±8.99 | 1.06 | 0.29 |
| SOFA score | 12.76±4.27 | 11.56±4.12 | 1.82 | 0.07 |
| Source of
infection |
|
| 0.45 | 0.80 |
| Gram
negative bacteria | 39 (40.6) | 27 (38.6) |
|
|
| Gram
positive bacteria | 34 (35.4) | 23 (32.8) |
|
|
|
Others | 23 (24.0) | 20 (28.6) |
|
|
| Site of
infection |
|
| 0.40 | 1.00 |
|
Pulmonary infection | 31 (32.3) | 21 (30.0) |
|
|
|
Abdominal infection | 11 (11.4) | 9 (12.9) |
|
|
| Blood
infection | 10 (10.4) | 8 (11.4) |
|
|
| Urinary
tract infection | 12 (12.5) | 10 (14.3) |
|
|
| Soft
tissue infection | 9 (9.4) | 7 (10.0) |
|
|
|
Others | 23 (24.0) | 15 (21.4) |
|
|
| Presence or absence
of shock |
|
| 13.84 | <0.01 |
|
Yes | 14 (14.6) | 28 (40.0) |
|
|
| No | 82 (85.4) | 42 (60.0) |
|
|
| The number of organ
damage |
|
| 0.06 | 0.80 |
| ≤2 | 54 (56.2) | 38 (54.3) |
|
|
|
>2 | 42 (43.8) | 32 (45.7) |
|
|
| Type of organ
damage |
|
| 0.16 | 1.00 |
|
Arterial hypotension | 13 (13.5) | 9 (12.9) |
|
|
|
Arterial hypoxemia | 17 (17.7) | 12 (17.1) |
|
|
| Acute
lung injury | 12 (12.5) | 8 (11.4) |
|
|
| Acute
renal failure | 24 (25.0) | 18 (25.7) |
|
|
| Acute
respiratory failure | 16 (16.7) | 13 (18.6) |
|
|
| Nerve
injury | 14 (14.6) | 10 (14.3) |
|
|
| ICU mortality | 5 (5.2) | 14 (20.0) | 8.74 | 0.003 |
| 28-day
mortality | 31 (32.3) | 45 (64.3) | 16.69 | <0.01 |
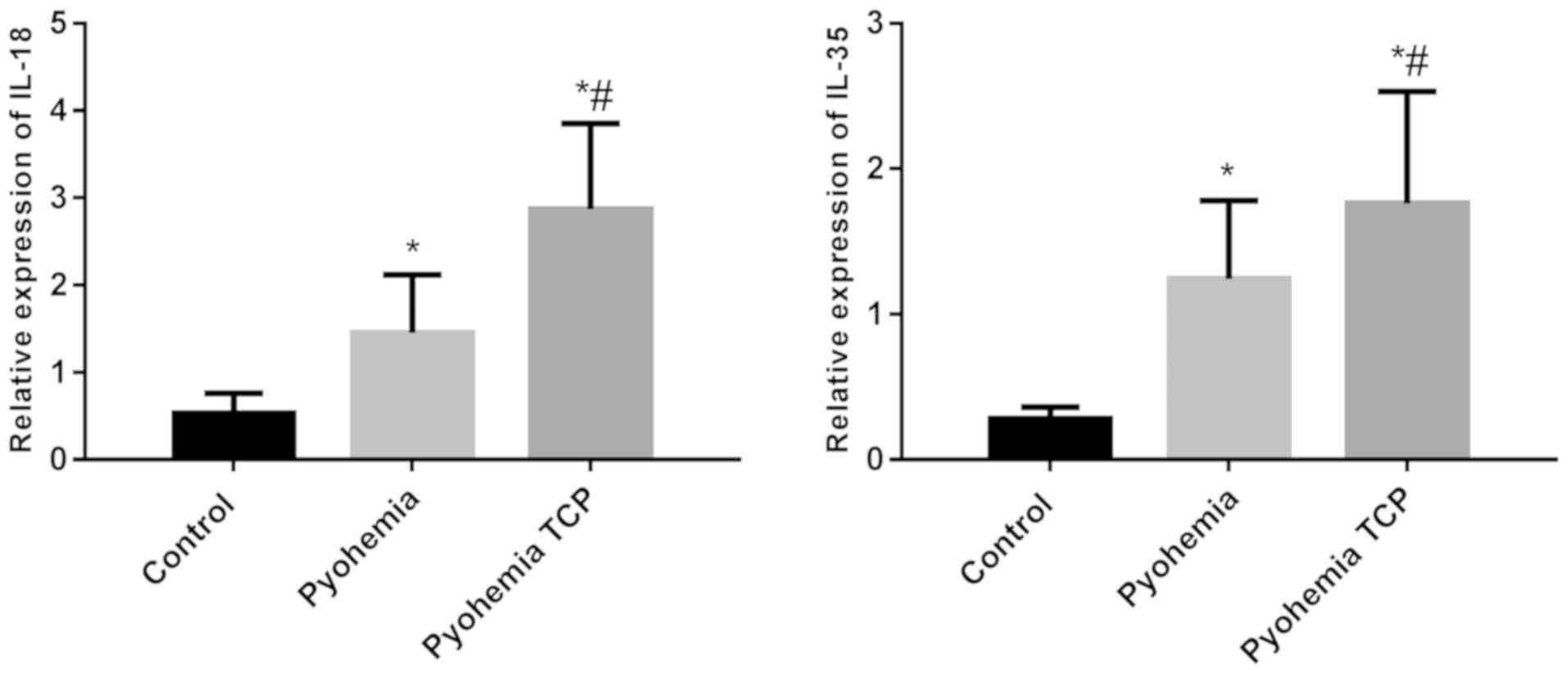
Expression of mRNA of IL-18 and IL-35
in karyocytes in peripheral blood in the three groups
As shown in Fig. 1,
the expression of mRNA of IL-18 in karyocytes in peripheral blood
in the sepsis group and the sepsis TCP group was higher than that
in the control group (P<0.05), and the expression of mRNA of
IL-18 in the sepsis TCP group was higher than that in the sepsis
group (P<0.05). Expression of mRNA of IL-35 in a karyocyte in
peripheral blood in the sepsis group and the sepsis TCP group was
higher than that in the control group (P<0.05), the expression
of mRNA of IL-35 in the sepsis TCP group was higher than that in
the sepsis group (P<0.05).
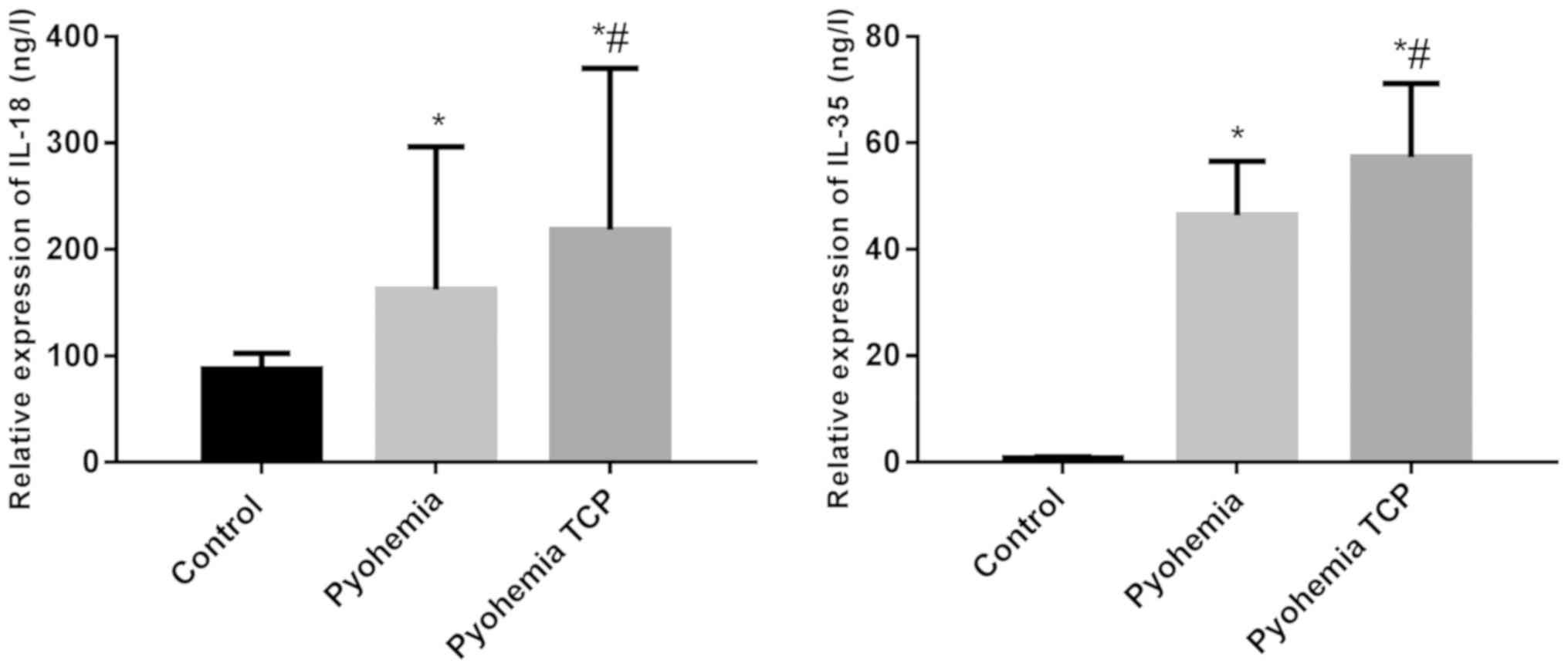
Concentration of IL-18 and IL-35 in
the serum in the three groups
The concentration of IL-18 and IL-35 in the serum in
the three groups was detected and the results showed that the
concentration of IL-18 in the serum in the sepsis group and the
sepsis TCP group was higher than that in the control group
(P<0.05); the concentration of IL-18 in the sepsis TCP group was
higher than that in the sepsis group (P<0.05). The concentration
of IL-35 in the serum in the sepsis group and the sepsis TCP group
were higher than that in the control group (P<0.05); the
concentration of IL-35 in the sepsis TCP group was higher than that
in the sepsis group (P<0.05) (Fig.
2).
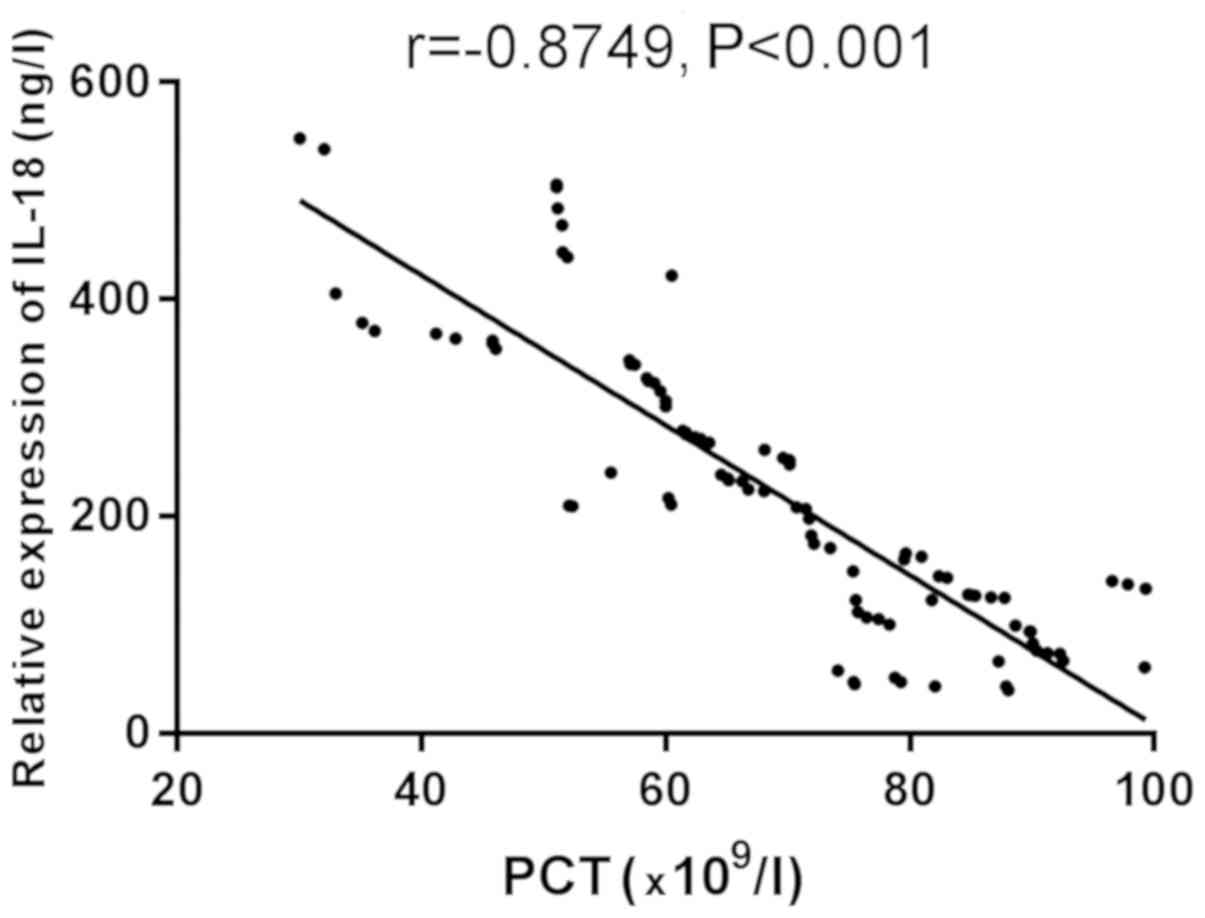
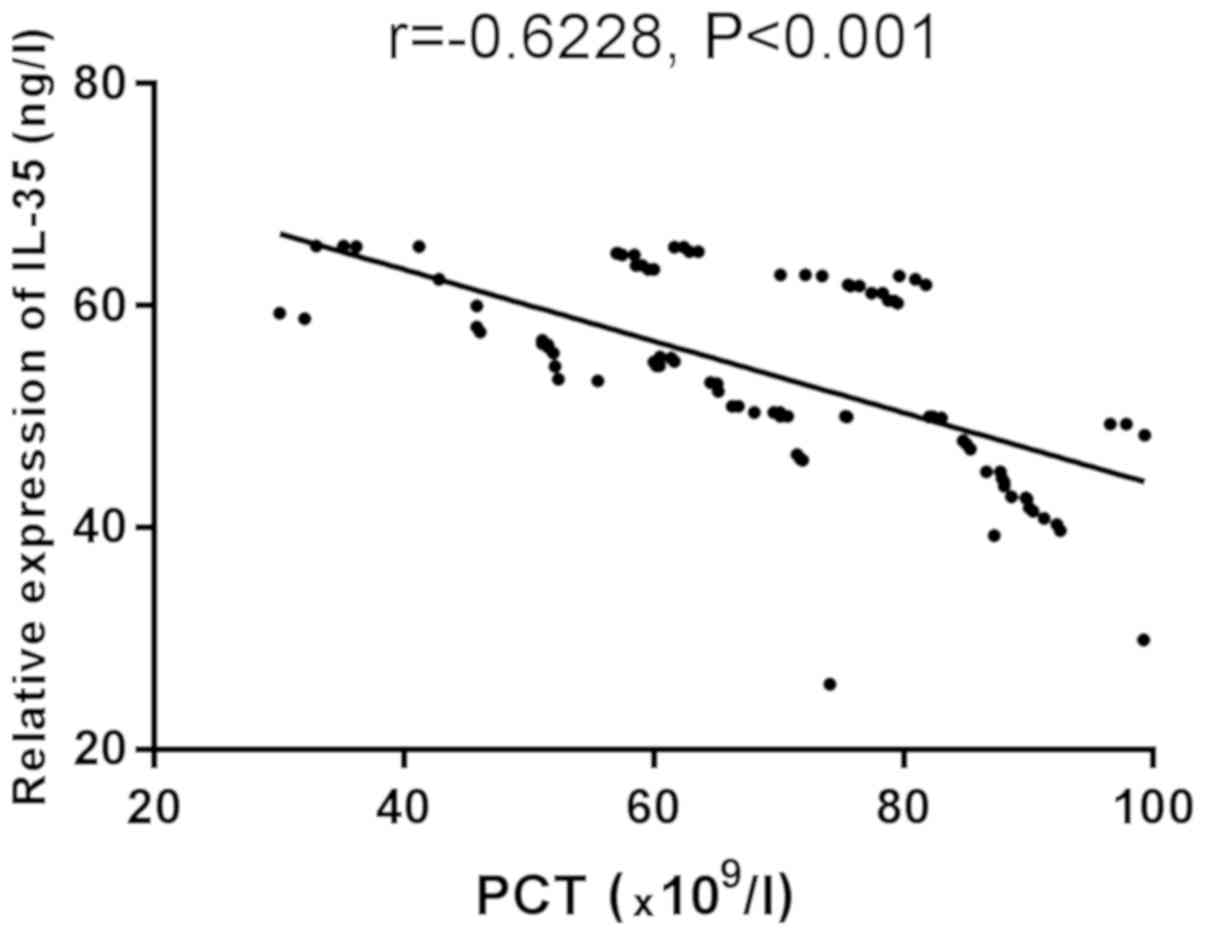
Correlation analysis between platelets
and IL-18 and IL-35
Correlation between platelets and IL-18 and IL-35
was studied. Correlation analysis of the protein concentrations of
IL-18 and IL-35 in the serum in the sepsis TCP group and the
platelet count of the patients was performed. Figs. 3 and 4
show that IL-18 and IL-35 are negatively correlated with platelets
(r=−0.8749, −0.6228, P<0.001).
Correlation between serum IL-18 and
IL-35
Correlation between IL-18 and IL-35 in serum was
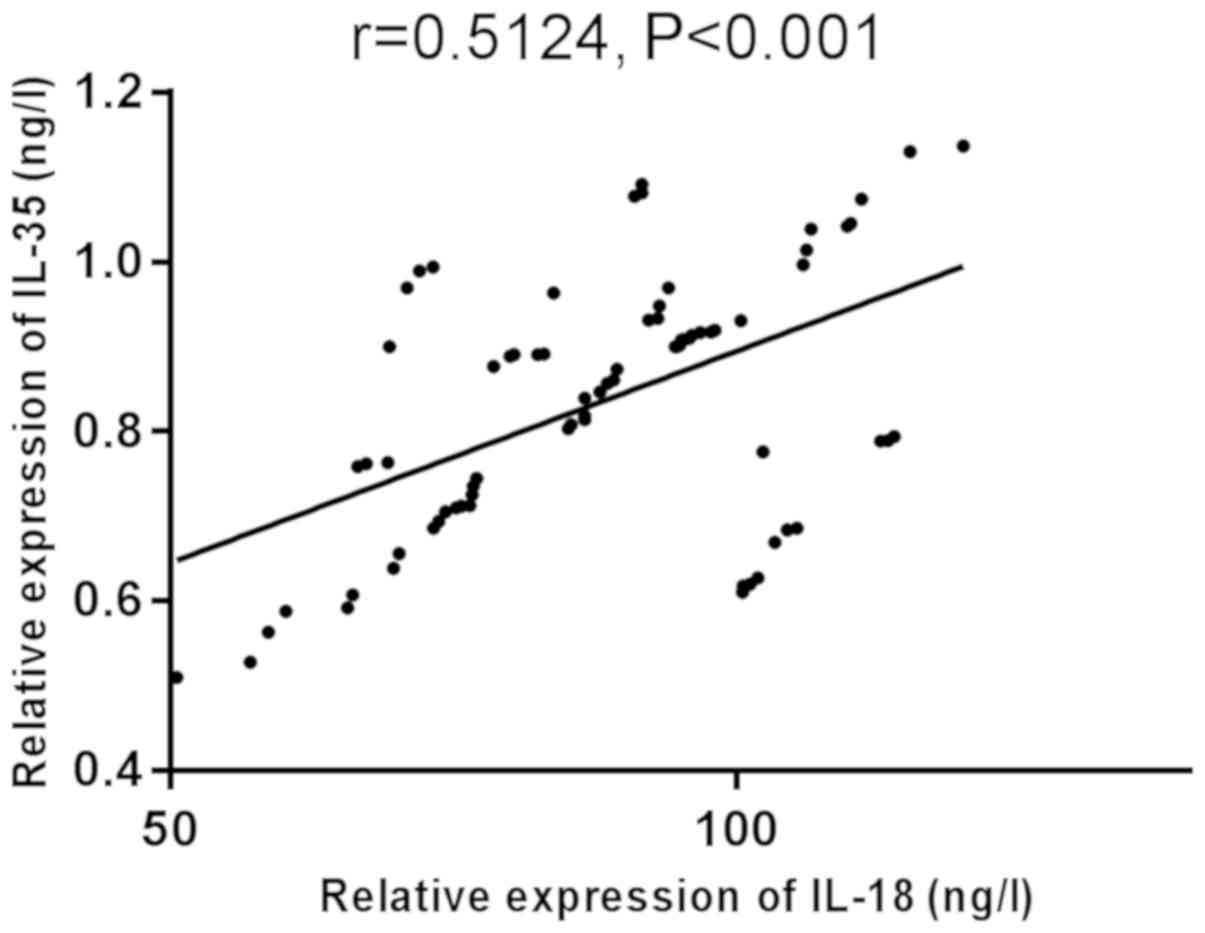
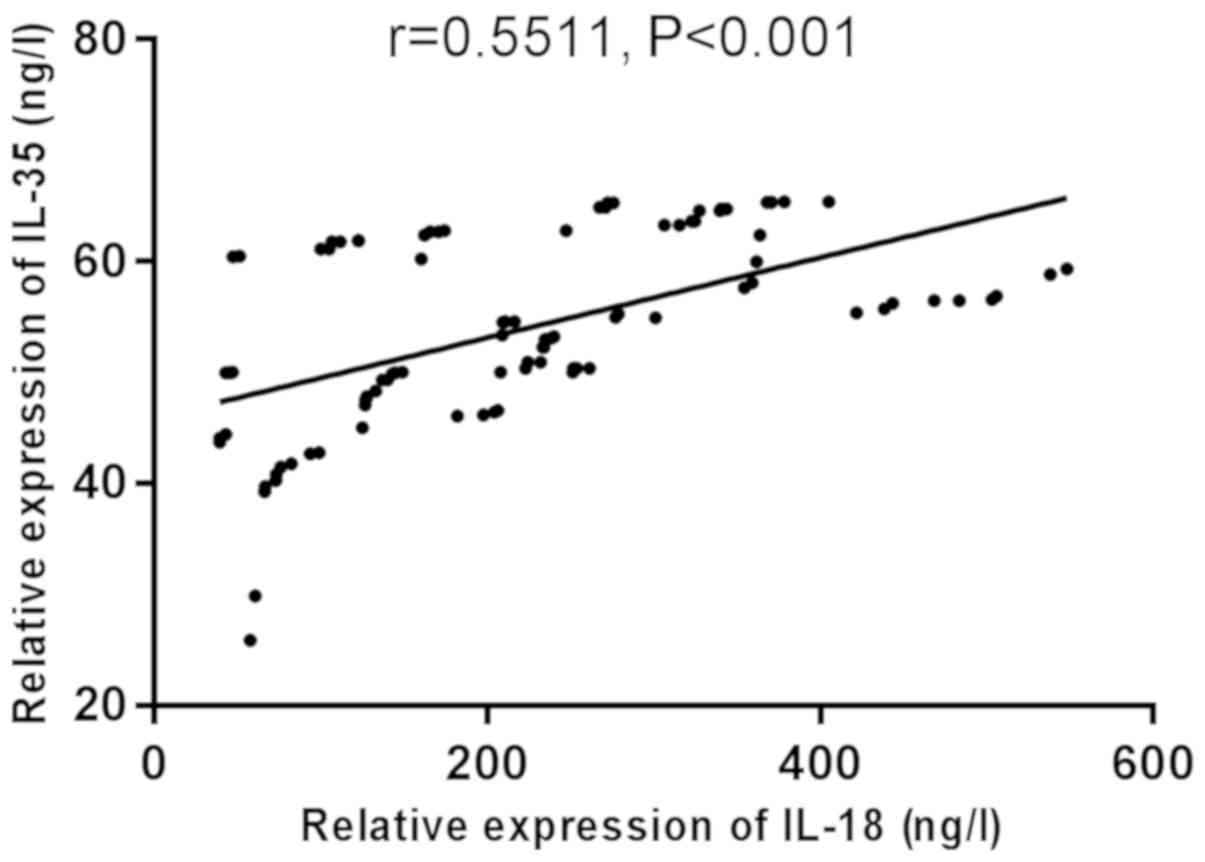
analyzed using Pearson's correlation coefficient (Figs. 5–7).
There was a significant positive correlation between serum IL-18
and IL-35 in the control group, sepsis group, and sepsis TCP group
(r=0.5124, 0.5718, 0.5511, P<0.001).
Discussion
Sepsis is one of the serious complications of acute
and critically ill patients with shock, burns and severe trauma in
clinic, often leading to septic shock and multiple organ
dysfunction syndrome, which poses a serious threat to human health
(17). Bone marrow is the most
vulnerable. Inflammatory factors and bacterial toxins in the body
inhibit hematopoietic cells in bone marrow (including
megakaryocytes), causing thrombocytopenia by inhibiting
megakaryocytes from producing platelets (18). Moreover, many ways facilitate
platelet activation, thus activating the coagulation pathway,
forming local microthrombus and increasing platelet consumption,
which can cause thrombocytopenia; the severity of sepsis is closely
related to the above processes (19,20).
IL-18, a gene multi-dominant polypeptide regulator,
is involved in growth and differentiation of cells and regulates
the body's immune response (21).
Study by Cui et al (22)
reported that the expression level of IL-18 increases, and the
expression of miR-130a decreases in plasma and miRNA of patients
with severe sepsis and thrombocytopenia, suggesting that IL-18 and
miR-130a may be involved in the pathophysiological process of
severe sepsis accompanied by thrombocytopenia. As a member of the
family of IL-12, IL-35 belongs to heterodimeric proteins and is
made up of Ebi3 (β-chain) and p35 (α-chain) (23). Studies have found that IL-35 can
facilitate the secretion of IL-10 in rheumatoid arthritis and can
inhibit the production of IL-17 and IFN-γ, thereby effectively
inhibiting the inflammation in antigen-specificity immune response
state; IL-35 plays an immunomodulatory role in a variety of
inflammatory diseases (24). A study
by Du et al (25) suggests
that IL-35 can be used as a novel candidate biomarker for the
diagnosis of early neonatal sepsis, and it is superior to PCT in
the diagnosis.
This study found that when hemoglobin, albumin,
creatinine, total bilirubin, platelet count, whole blood leukocyte
count, fibrinogen and CRP were compared between the three groups,
the differences were statistically significant (P<0.05). There
was a significant difference in albumin, creatinine, total
bilirubin and platelet count between the sepsis group and the
sepsis TCP group. There was no difference in APACHE II score and
SOFA score between the sepsis group and the sepsis TCP group; there
was no significant difference in the source of infection, the
infection site, the number of organ damage and the type of organ
damage between the two groups (P>0.05); the number of patients
with shock, ICU mortality and 28-day mortality in the sepsis group
were lower than those in the sepsis TCP group. Oberholzer et
al (26) found that the level of
IL-18 in patients with sepsis was significantly higher than that in
healthy people; the levels of IL-18 in patients with septic shock
and patients with sepsis who died were higher than those in
patients without shock and patients with sepsis who survived. Sun
and Zhang (27) detected the level
of IL-18 in patients with ICU sepsis in the first 3 days, and it
was found that the severity and prognosis of patients with sepsis
were closely related to the increase of level of IL-18. It is
reported that the high expression of IL-18 in the serum may be an
early predictive factor of death (28). IL-35 in plasma is associated with the
severity of sepsis and can also be used as a warning factor of
sepsis to predict illness condition (29). This study showed that the expression
levels of mRNA of IL-18 and IL-35 in karyocytes in peripheral blood
in the sepsis group and the sepsis TCP group were higher than those
in the control group, and the expression levels of mRNA of IL-18
and IL-35 in the sepsis TCP group were higher than those in the
sepsis group (P<0.05). By detecting the concentrations of IL-18
and IL-35 in the serum in the three groups, the results showed that
the concentrations of IL-18 and IL-35 in the serum in the sepsis
group and the sepsis TCP group were higher than those in the
control group, and the concentrations of IL-18 and IL-35 in the
sepsis TCP group were higher than those in the sepsis group.
Related studies have found that the concentration of IL-35 in the
serum of patients with sepsis is significantly higher than that in
healthy people, suggesting that the expression level of IL-35
increases in patients with sepsis, and the concentration of IL-35
in patients with sepsis who die is significantly higher than that
in survivors (30). Thrombocytopenia
is one of the most common abnormalities in patients with severe
sepsis (24). In this study, it is
found that the concentration of IL-35 in patients with sepsis and
thrombocytopenia is higher than that in patients with sepsis, which
provides reference for future clinical research. Related literature
reports that platelet counts in children with immune
thrombocytopenic purpura have a negative correlation with serum
IL-18 (31). Little is known about
the correlation between IL-35 and platelet count. In the present
study, the correlation analysis was on the concentrations of IL-18
and IL-35 in the serum in the sepsis TCP group and the platelets of
patients. It was found that IL-18 and IL-35 are negatively
correlated with platelets (r=−0.8749, −0.6228, P<0.001). This
was possibly caused by the death ligand in platelets being highly
expressed due to inflammatory factors, which enhance the killing
effect of lymphocytes and cause apoptosis of platelets, thereby
causing a decrease in platelet count. The specific mechanism
remains to be further investigated. It has been reported that
elevated serum IL-35 levels in patients with sepsis are associated
with logistic organ dysfunction (LOD) or simplified acute
physiology score (SAPS II) and are associated with inflammatory
markers (32). In this study, the
correlation between serum IL-18 and IL-35 was analyzed, and serum
IL-18 was found to be significantly positively correlated with
IL-35. This indicates that IL-35 may be involved in the regulation
of IL-18. However, there is currently no research in this
direction, and more investigations are needed.
This study investigated the expression and clinical
significance of IL-18 and IL-35 in the serum of patients with
sepsis TCP, and confirmed that there is a correlation between the
expression of IL-18 and IL-35 and platelets in the serum of
patients with sepsis TCP, which has certain clinical significance.
However, since this is a retrospective study, all confounding
factors that may exist in critically ill patients in different time
cannot be controlled.
In conclusion, IL-18 and IL-35 are negatively
correlated with the degree of thrombocytopenia in patients with
sepsis, which indicates that these two factors play an important
role in the pathogenetic process of sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ conceived the study and drafted the manuscript.
MZ and XR detected CRP and NPY concentrations. ML and SW performed
some of the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jinan Central Hospital Affiliated to Shandong University (Jinan,
China). Patients who participated in this research had complete
clinical data. Patients and their family signed an informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dewitte A, Lepreux S, Villeneuve J,
Rigothier C, Combe C, Ouattara A and Ripoche J: Blood platelets and
sepsis pathophysiology: A new therapeutic prospect in critically
[corrected] ill patients? Ann Intensive Care. 7:1152017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K;
International Forum of Acute Care Trialists, : Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Larkin CM, Santos-Martinez MJ, Ryan T and
Radomski MW: Sepsis-associated thrombocytopenia. Thromb Res.
141:11–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaguchi A, Lobo FL, Vincent JL and Pradier
O: Platelet function in sepsis. J Thromb Haemost. 2:2096–2102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandijck DM, Blot SI, De Waele JJ, Hoste
EA, Vandewoude KH and Decruyenaere JM: Thrombocytopenia and outcome
in critically ill patients with bloodstream infection. Heart Lung.
39:21–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu R, Lin F, Bao C, Huang H, Ji C, Wang S,
Jin L, Sun L, Li K, Zhang Z, et al: Complement 5a receptor-mediated
neutrophil dysfunction is associated with a poor outcome in sepsis.
Cell Mol Immunol. 13:103–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li MF, Li XL, Fan KL, Yu YY, Gong J, Geng
SY, Liang YF, Huang L, Qiu JH, Tian XH, et al: Platelet
desialylation is a novel mechanism and a therapeutic target in
thrombocytopenia during sepsis: An open-label, multicenter,
randomized controlled trial. J Hematol Oncol. 10:1042017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeaman MR and Bayer AS: Staphylococcus
aureus, platelets, and the heart. Curr Infect Dis Rep.
2:281–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Claushuis TA, van Vught LA, Scicluna BP,
Wiewel MA, Klein Klouwenberg PM, Hoogendijk AJ, Ong DS, Cremer OL,
Horn J, Franitza M, et al Molecular Diagnosis and Risk
Stratification of Sepsis Consortium, : Thrombocytopenia is
associated with a dysregulated host response in critically ill
sepsis patients. Blood. 127:3062–3072. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwak A, Lee Y, Kim H and Kim S:
Intracellular interleukin (IL)-1 family cytokine processing enzyme.
Arch Pharm Res. 39:1556–1564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eidt MV, Nunes FB, Pedrazza L, Caeran G,
Pellegrin G, Melo DA, Possuelo L, Jost RT, Dias HB, Donadio MV, et
al: Biochemical and inflammatory aspects in patients with severe
sepsis and septic shock: The predictive role of IL-18 in mortality.
Clin Chim Acta. 453:100–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okuhara Y, Yokoe S, Iwasaku T, Eguchi A,
Nishimura K, Li W, Oboshi M, Naito Y, Mano T, Asahi M, et al:
Interleukin-18 gene deletion protects against sepsis-induced
cardiac dysfunction by inhibiting PP2A activity. Int J Cardiol.
243:396–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Eckl J, Geiger C, Schendel DJ and
Pohla H: A novel and effective method to generate human
porcine-specific regulatory T cells with high expression of IL-10,
TGF-β1 and IL-35. Sci Rep. 7:39742017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao P, Su Z, Lv X and Zhang J:
Interleukin-35 in asthma and its potential as an effective
therapeutic agent. Mediators Inflamm. 2017:59318652017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sha X, Meng S, Li X, Xi H, Maddaloni M,
Pascual DW, Shan H, Jiang X, Wang H and Yang XF: Interleukin-35
inhibits endothelial cell activation by suppressing MAPK-AP-1
pathway. J Biol Chem. 290:19307–19318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G;
SCCM/ESICM/ACCP/ATS/SIS, : 2001 SCCM/ESICM/ACCP/ATS/SIS
International Sepsis Definitions Conference. Crit Care Med.
31:1250–1256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vardas K, Apostolou K, Briassouli E,
Goukos D, Psarra K, Botoula E, Tsagarakis S, Magira E, Routsi C,
Nanas S, et al: Early response roles for prolactin cortisol and
circulating and cellular levels of heat shock proteins 72 and 90α
in severe sepsis and SIRS. BioMed Res Int. 2014:8035612014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang G, Wang XM, Meng JX, Luan CL, Chen
JF, Wu YQ, Zhang XN and He ZY: Efficacy of recombinant human
thrombopoietin and recombinant human interleukin 11 for treatment
of chemotherapy induced thrombocytopenia in acute myeloid leukaemia
patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 26:234–238. 2018.(In
Chinese). PubMed/NCBI
|
|
19
|
Semple JW and Freedman J: Platelets and
innate immunity. Cell Mol Life Sci. 67:499–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeaman MR: Platelets in defense against
bacterial pathogens. Cell Mol Life Sci. 67:525–544. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Y, Jiang W, Liu L, Wang X, Ding C,
Tian Z and Zhou R: Dopamine controls systemic inflammation through
inhibition of NLRP3 inflammasome. Cell. 160:62–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui YL, Wang B, Gao HM, Xing YH, Li J, Li
HJ, Lin Z and Wang YQ: Interleukin-18 and miR-130a in severe sepsis
patients with thrombocytopenia. Patient Prefer Adherence.
10:313–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vignali DA and Kuchroo VK: IL-12 family
cytokines: Immunological playmakers. Nat Immunol. 13:722–728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kochetkova I, Golden S, Holderness K,
Callis G and Pascual DW: IL-35 stimulation of CD39+ regulatory T
cells confers protection against collagen II-induced arthritis via
the production of IL-10. J Immunol. 184:7144–7153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du WX, He Y, Jiang HY, Ai Q and Yu JL:
Interleukin 35: A novel candidate biomarker to diagnose early onset
sepsis in neonates. Clin Chim Acta. 462:90–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oberholzer A, Steckholzer U, Kurimoto M,
Trentz O and Ertel W: Interleukin-18 plasma levels are increased in
patients with sepsis compared to severely injured patients. Shock.
16:411–414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun RQ and Zhang SL: The value of serum
interleukin-18 and 10 in the evaluation of severity and prognosis
in the early stage of sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi
Xue. 23:299–301. 2011.(In Chinese). PubMed/NCBI
|
|
28
|
Emmanuilidis K, Weighardt H, Matevossian
E, Heidecke CD, Ulm K, Bartels H, Siewert JR and Holzmann B:
Differential regulation of systemic IL-18 and IL-12 release during
postoperative sepsis: High serum IL-18 as an early predictive
indicator of lethal outcome. Shock. 18:301–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lally KP, Cruz E and Xue H: The role of
anti-tumor necrosis factor-alpha and interleukin-10 in protecting
murine neonates from Escherichia coli sepsis. J Pediatr
Surg. 35:852–855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao HQ, Li WM, Lu ZQ, Sheng ZY and Yao
YM: The growing spectrum of anti-inflammatory interleukins and
their potential roles in the development of sepsis. J Interferon
Cytokine Res. 35:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaheen IA, Botros SKA and Morgan DS:
Detection of expression of IL-18 and its binding protein in
Egyptian pediatric immune thrombocytopenic purpura. Platelets.
25:193–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao J, Xu F, Lin S, Tao X, Xiang Y, Lai X
and Zhang L: IL-35 is elevated in clinical and experimental sepsis
and mediates inflammation. Clin Immunol. 161:89–95. 2015.
View Article : Google Scholar : PubMed/NCBI
|