Introduction
Several clinical cases of spinal cord injury
following seizure have been reported (1–3),
including those in primary (1) and
secondary epilepsy (3). In grand mal
epilepsy, impairments of the face and head commonly occur due to
mechanical injury (4). Spinal cord
injury is also considered to be caused by trauma or strong muscular
contractions (2,3), especially in severe refractory epilepsy
(2,3). The theory of mechanical damage is also
supported by a high incidence of cervical-spinal cord injury in
walking adults with severe refractory epilepsy accompanied by falls
and other injuries, especially head injury (3). Thoracic and lumbar injury have also
been reported and are considered to be caused by strong muscular
contractions during seizures (2,3).
However, there are some limitations to the mechanical injury
hypothesis, as not all patients with spinal cord injury show
evidence of trauma or other underlying lesions (1). Furthermore, the nature of the
pathological changes following epilepsy-associated spinal cord
injury are not fully understood. A previous study demonstrated
degeneration of the spinal cord in myoclonus epilepsy (5). Another previous study showed that after
seizure, the expression levels of serum C-response protein,
cytokines and inflammatory factors are markedly increased, thus
indicating a systemic seizure-associated inflammatory reaction
(6). The brain and spinal cord share
a common origin, which is supported by the co-expression of
specific neurotransmitters in both locations (7,8).
Therefore, there may be a close connection between spinal cord
injury and the abnormal discharge of brain neurons (7,8).
Previous studies related to spinal cord injury after
epileptic seizure are relatively limited, and whether spinal cord
injury commonly occurs following epileptic seizures is unclear. In
addition, the mechanism underlying epilepsies-mediated injury
remains to be investigated. Our previous study investigated severe
neuronal injury, damage to the blood-brain barrier (BBB) and
neuronal uptake of serum albumin in the brain parenchyma of a
kainic acid (KA)-induced rat model of temporal lobe epilepsy (TLE)
(7,8). The present study aimed to investigate
the pathological changes and underlying mechanisms of spinal cord
injury in TLE. Sprague-Dawley rats were intraperitoneally injected
with pilocarpine (PL; PL group) or stereotaxically administered KA
(KA group) to rule out the possibility of spinal cord injury caused
by cerebrospinal leakage of KA via hippocampal directional
injection. These groups were used to investigate the correlation
between spinal cord injury and epileptic seizures in two different
models.
Materials and methods
Ethical approval and animal
preparation
The present study was approved by The Ethics
Committee of The First People's Hospital of Shanghai Jiaotong
University. All experimental procedures were performed according to
the Laboratory Animal Care standards of The Ethics Committee, and
all efforts were made to minimize the number of animals used and
the level of suffering to those involved. Male Sprague-Dawley rats
(total n=90; weight, 250–300 g; age, 8–9 weeks old; Shanghai
SIPPR-Bk Lab Animal Co., Ltd.) were independently raised in plastic
cages with a 12-h light/dark cycle at 20–25°C with relative
humidity 50–65% and ventilation 8–12 times/h, and free access to
food and water. The rats were divided into three groups: i) The
control group that had a stereotactic (n=15) or intraperitoneal
injection (n=15) of saline, total n=30; ii) the KA group that had a
stereotactic injection of KA, n=30; and iii) the PL group that had
an intraperitoneal injection of PL, n=30.
Model of TLE and electrode
implantation
The rats were anesthetized with an intraperitoneal
injection of 350 mg/kg 10% chloral hydrate (Sigma-Aldrich; Merck
KGaA). Local disinfection using an alcohol cotton ball and aseptic
surgery were conducted to avoid infection. There were no
significant signs of peritonitis, such as fever, abdominal muscle
tension or abdominal tenderness. Following anesthesia, the rats
were fixed onto stereotaxic apparatus (Model UMC4; World Precision
Instruments, Inc.). The fonticulus anterior, sagittal suture and
fonticulus posterior were exposed and adjusted to the same
horizontal position. A microchannel (coordinates with the Bregma:
Anteroposterior, −3.6 mm; mediolateral, −3.4 mm; and dorsoventral,
−3.8 mm) was established in the skull for the intra-hippocampal
administration of 0.5 mM ×1 µl, 0.05 µl/min KA. Preliminary tests
were performed to determine the optimal dosage of KA for triggering
an epileptic seizure; 0.5 mM ×1 µl, 0.05 µl/min, as previously
described (7,8). Rats in the control group were injected
with 1 µl sterile saline. The rats in the PL group received an
intraperitoneal injection of 350 mg/kg PL (0.5 µl), and the control
group received an equal volume of saline. Deep-recording electrodes
(Electroencephalograph Analysis system; version 1.70; Cadwell
Industries, Inc.) were implanted into the bilateral hippocampal
Cornu Ammonis 3 region using the aforementioned coordinates. A
monopolar electrode was implanted into the bone above the
cerebellum. All electrodes were fixed to the skull using screws and
embedded with dental cement (Paladur; Kulzer GmbH) (7,8). To
generate a clear electroencephalograph (EEG) image, a
high-resolution recorder (EEG-1200C; Nihon Kohden Corporation) was
connected. Immediately after surgery, both EEG recording and
behavioral video monitoring were conducted for 12 h. Behavioral
characteristics were evaluated by an independent researcher who was
blinded to the experiment using video recording according to
Racine's V-point Scale (9), as
follows: i) I, movement of the mouth, lips, tongue and vibrissae;
ii) II, head clonus; iii) III, forelimb clonus, frequent and rhythm
head and neck shaking; iv) IV, clonic rearing; and v) V,
uncontrolled jumping and clonic rearing with loss of postural
control.
Sampling
According to our previous study, pathological
changes in the brain were most significant between days 3 and 7
post-injection of KA (10,11); therefore, all rats were sacrificed on
the 3rd day after TLE induction. The rats were individually
anesthetized with an intraperitoneal injection of 350 mg/kg 10%
chloral hydrate, and sacrificed via cardiac perfusion with 70 ml
warmed saline and 40 ml 4% paraformaldehyde (PFA; Sigma-Aldrich;
Merck KGaA; 4°C; pH 7.4; 0.1 M; 100 ml/each) when in a state of
deep anesthesia. Death was confirmed when respiration and a
heartbeat were undetectable, and the pupils were completely
dilated. The intact brain and spinal cord, including the cervical
cord, thoracic cord and lumbar cord, were excised and submerged in
4% PFA at 4°C for 18 h. The brain and spinal cord were then
dehydrated with 30% sucrose in 0.9% normal saline (pH 7.4) at 4°C
for 3 days; the sucrose was replaced once a day to ensure efficient
dehydration. Samples were dried and embedded in Optimum Cutting
Temperature compound (OCT, cat. no. 4583; Leica Microsystems GmbH),
and stored at −80°C prior to immunoassay analysis. For transmission
electron microscopy (TEM), the brain and spinal cord samples were
fixed with 1.3% glutaraldehyde at 4°C for 3 h immediately after
excision.
Nissl staining for the detection of
neuronal injury
Nissl staining was performed to detect neuronal
injury. After fixed with 4% PFA at 4°C for 18 h and embedded using
OCT at −80°C, sections of the brain and spinal cord were cut into
35 µm slices using a sliding microtome (CM1950; Leica Microsystems
GmbH). After washing with PBS at pH 7.4, the tissues were dried at
55°C for 3 h and immersed in 0.9% crystal violet at 37°C
(Sigma-Aldrich; Merck KGaA) for 2 h. The tissues were dehydrated
with 70, 80, 90 and 100% ethanol for 5 min, and mounted with
neutral balsam. Observations were performed using a fluorescent
microscope (100 µm; Eclipse 80i; Nikon Corporation). ImageJ
software (version 1.42q; National Institutes of Health) was used to
conduct densitometric analysis and to determine the mean analysis
area of Nissl+ cells. An average value was obtained by
observing six random fields from each of the ten slices per
rat.
TEM
The brains, primarily the hippocampus and the
cortex, and the cervical, thoracic and lumbar spinal cord from all
groups were removed and fixed with 1.3% glutaraldehyde at 4°C for 3
h before submission. After embedded with epoxy resin at 60°C for 36
h, samples were cut into 0.06 µm sections and stained with 0.5%
uranyl acetate at 4°C for 1 h. Structural damage of the neuronal
tissue and BBB was observed using a transmission electron
microscope (Scale bars 0.5 µm, 1 µm; JEM-1230; JEOL, Ltd.).
Measurement of brain and spinal cord
edema
The degree of brain and spinal cord edema in TLE was
determined using the wet-dry weight method (12). The wet weight of the tissues was
obtained immediately after dissection and the dry weight was
measured after drying at 55°C for 24 h. The water content and
degree of tissue swelling was calculated as follows: Water content
(%)=(wet weight-dry weight) ×100/wet weight.
Double immunofluorescence staining of
albumin and neuronal cells
To determine the integrity of the BBB and the
distribution of serum albumin, double-staining of albumin and
neuronal cells was conducted. The rats were injected with 4 ml/kg
2% Evans blue (EB), which directly binds to serum albumin, via the
tail vein 4 h before transcardiac perfusion. EB administration was
performed on the 3rd day post-seizure. After fixed with 4% PFA at
4°C for 18 h and embedded using OCT at −80°C, samples from the
brain and spinal cord were cut into 35 µm sections, washed in cold
PBS at pH 7.4, blocked with 10% BSA at 4°C in the dark overnight,
and incubated with a primary mouse anti-neuronal nuclei (NeuN)
antibody (1:2,000; cat. no. A2050; Sigma-Aldrich; Merck KGaA) at
37°C overnight. The sections were washed three times in PBS for 15
min each time and incubated with Alexa Fluor 488-conjugated
secondary antibody (1:1,000; cat. no. A-11001; Invitrogen; Thermo
Fisher Scientific, Inc.) fofr 3 h at room temperature. The sections
were washed three times with PBS for 5 min each time and mounted
using 75% glycerin. Images were captured using a fluorescent
microscope (Scale bars, 500 µm, 100 µm; Eclipse 80i; Nikon
Corporation). ImageJ software (version 1.42q) (National Institutes
of Health) was used to quantify the albumin+ cells in
the spinal cord, which allows for the evaluation of the degree of
spinal cord injury (10).
Overlap coefficient analysis
To further investigate the relationship between
neurons and albumin extravasation, overlap coefficient analysis was
conducted using Image-Pro Plus 6.0 (Media Cybernetics, Inc.). Then,
two-dimensional curve graphs were constructed to analyze the
overlap degree of two fluorescent outputs.
Immunohistochemistry and
immunofluorescence analyses
To determine whether leukocyte infiltration occurred
in the spinal cord after TLE, immunohistochemistry was conducted to
detect the leukocyte adhesion molecule CD11b. In our previous
study, leukocyte infiltration was detected in the brain (7). In the present study, immunofluorescence
analysis of caspase-3 was conducted to detect apoptosis in the
spinal cord following TLE. After samples were fixed with 4% PFA at
4°C for 18 h and embedded by OCT at −80°C, sections of the brain
and spinal cord were cut into 35 µm sections. Slices of spinal cord
were blocked with 10% BSA at 4°C overnight, and incubated with
mouse anti-rat CD11b (1:1,000; cat. no. MCA215G; Bio-Rad
Laboratories, Inc.) and rabbit anti-rat caspase-3 (1:2,000; cat.
no. 9661l; Cell Signaling Technology, Inc.) antibodies overnight at
4°C. For immunohistochemistry, the sections were incubated with a
biotinylated-secondary antibody (1:3,000; cat. no. BA9500; Vector
Laboratories, Inc.) for 3 h at room temperature after washing with
PBS. Each section was then immersed in color reagent avidin-biotin
complex (1:200 each; ABC staining system; cat. no. PK-6100, Vector
Laboratories) diluted in PBS for 3 min in the dark, and then
mounted with neutral balsam. For immunofluorescence, the sections
were incubated with Alexa Fluor 488-conjugated secondary donkey
anti-mouse IgG (1:2,000; cat. no. A21202; Invitrogen; Thermo Fisher
Scientific, Inc.) overnight at 4°C, and washed with PBS before
mounting with 75% glycerin. Images were captured using a
fluorescent microscope (500 and 100 µm; Eclipse 80i; Nikon
Corporation).
Western blot analysis
Western blotting was performed to detect the
expression levels of intercellular adhesion molecule 1 (ICAM-1),
CD11b and the inflammatory factors tumor necrosis factor (TNF)-α
and interleukin (IL)-6 in the spinal cord. The spinal cord was
separated and crushed by an ultrasonic crusher (H150; OUHOR). Then,
600 µl tissue protein lysis buffer (cat. no. R0020; Solarbio Co.,
Ltd.) was added into 400 µl tissue homogenate and placed in ice for
30 min. Supernatant was extracted after the lysates were
centrifuged at 8,049 × g at 4°C (Eppendorf centrifuge; 5417C) for
20 min. Protein quantitative analysis was conducted according to
the bicinchoninic acid Protein assay kit (cat. no. 23227; Thermo
Fisher Scientific, Inc.). In total, 0.1 ml of each unknown sample
and BSA standard was placed into separately labeled test tubes and
0.2 ml working reagent (solution A and B at 50:1) was added. After
mixing, each tube was covered and incubated at 37°C for 30 min, and
then cooled to room temperature. Measurement of the absorbance of
all samples was conducted with the spectrophotometer set to 562 nm
for 10 min. A standard curve was generated by plotting the average
blank-corrected 562 nm measurement for each BSA standard vs. its
concentration in µg/ml. Protein concentration of each sample was
determined using the standard curve. A total of 50 µg protein/lane
was separated by 10% SDS-PAGE and transferred to a PVDF membrane.
The membrane was incubated with goat anti-rat ICAM-1 (1:500; cat.
no. AF583; R&D systems, Inc.), mouse anti-rat CD11b (1:800;
cat. no. MCA275R; Bio-Rad Laboratories, Inc.), mouse anti-rat TNF-α
(1:1,000; cat. no. MAB510; R&D Systems, Inc.) and goat anti-rat
IL-6 (cat. no. AF506; 1:1,500; R&D Systems, Inc.) antibodies
overnight at 4°C. Mouse monoclonal anti-rat GAPDH (1:1,000; cat.
no. sc-365062; Santa Cruz Biotechnology, Inc.) was used to
standardize the amount of cytosolic protein. The membrane was then
incubated with horseradish peroxidase-conjugated secondary antibody
(1:3,000; cat. no. sc-74088; Santa Cruz Biotechnology, Inc.) for 3
h at room temperature. Diaminobenzidine coloration (DAB staining
kit; cat. no. PW017; Sangon Biotech Co., Ltd.) was conducted at
37°C in the dark for 1 min, and the Tanon 4200 imaging system
(Tanon Science and Technology Co., Ltd.) was used to observe the
results. Analysis was conducted using ImageJ software (version
1.42q; National Institutes of Health).
Fluoro-Jade C (FJC) staining
After washing with PBS at a pH 7.4, the brain and
spinal cord sections (35 µm) were incubated in 0.3 M Triton X-100
and 0.1 M DAPI solution at room temperature for 2 h. The sections
were washed with PBS and dried at 50°C for 1 h, before buffering in
99.9% alcohol and then 70% alcohol at room temperature for 5 min
each. Sections were washed twice by immersion in distilled water
for 10 min each. The sections were then submerged in 0.06%
potassium permanganate solution at room temperature for 10 min and
gently agitated. After rinsing three times in distilled water, the
sections were placed in 0.01% FJC working solution (EMD Millipore)
at 4°C in the dark for 20 min, washed in distilled water and dried
in an oven at 50°C for 10 min. All sections were mounted with 60%
neutral balsam and left to air-dry for ≥1 min.
Statistical analysis
Data were analyzed using SPSS 24.0 (IBM Corp.) and
are presented as the mean ± SEM. ANOVA test was used to compare the
mean values. Dunnett's test was used for comparison between
experimental group and control group. Tukey's test was used in
pairwise comparison among three groups. Rank-sum test was used to
conduct behavioral analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline analysis
The body weights of the rats did not differ
significantly between groups (Table
I; P=0.725). All rats were raised in the same conditions and no
drugs were administration prior to experimentation.
 | Table I.Baseline weights of male
Sprague-Dawley rats in all groups. No significant difference was
observed between groups at the baseline level. |
Table I.
Baseline weights of male
Sprague-Dawley rats in all groups. No significant difference was
observed between groups at the baseline level.
| Group | Weight, g |
|---|
| Control group
(total, n=30) | 270.58±2.12 |
| PL group
(n=30) | 273.02±2.53 |
| KA group
(n=30) | 269.33±2.05 |
| P-value (total,
ANOVA) | 0.725 |
Behavioral analysis and EEG
recording
Behavioral analysis results identified grade II–V
seizures in both the KA and the PL group (Table II). The present results suggested
that rats in the control group exhibited normal brain activity
(Fig. 1A), while sharp and slow
waves were recorded in the PL group during upper limb convulsion
(Fig. 1B). In addition, long-lasting
multiple-spike waves were observed in the KA group during
tonic-clonic seizures (Fig. 1C).
 | Table II.Behavioral analysis of rats in all
groups. |
Table II.
Behavioral analysis of rats in all
groups.
|
| Grade |
|
|---|
|
|
|
|
|---|
| Groups | 0 | I | II | III | IV | V | Death |
|---|
| Control (total,
n=30) | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| KA (n=30) | 0 | 0 | 3 | 5 | 14 | 8 | 0 |
| PL (n=30) | 0 | 0 | 1 | 8 | 11 | 10 | 0 |
Tissue damage in the brain and spinal
cord following seizure
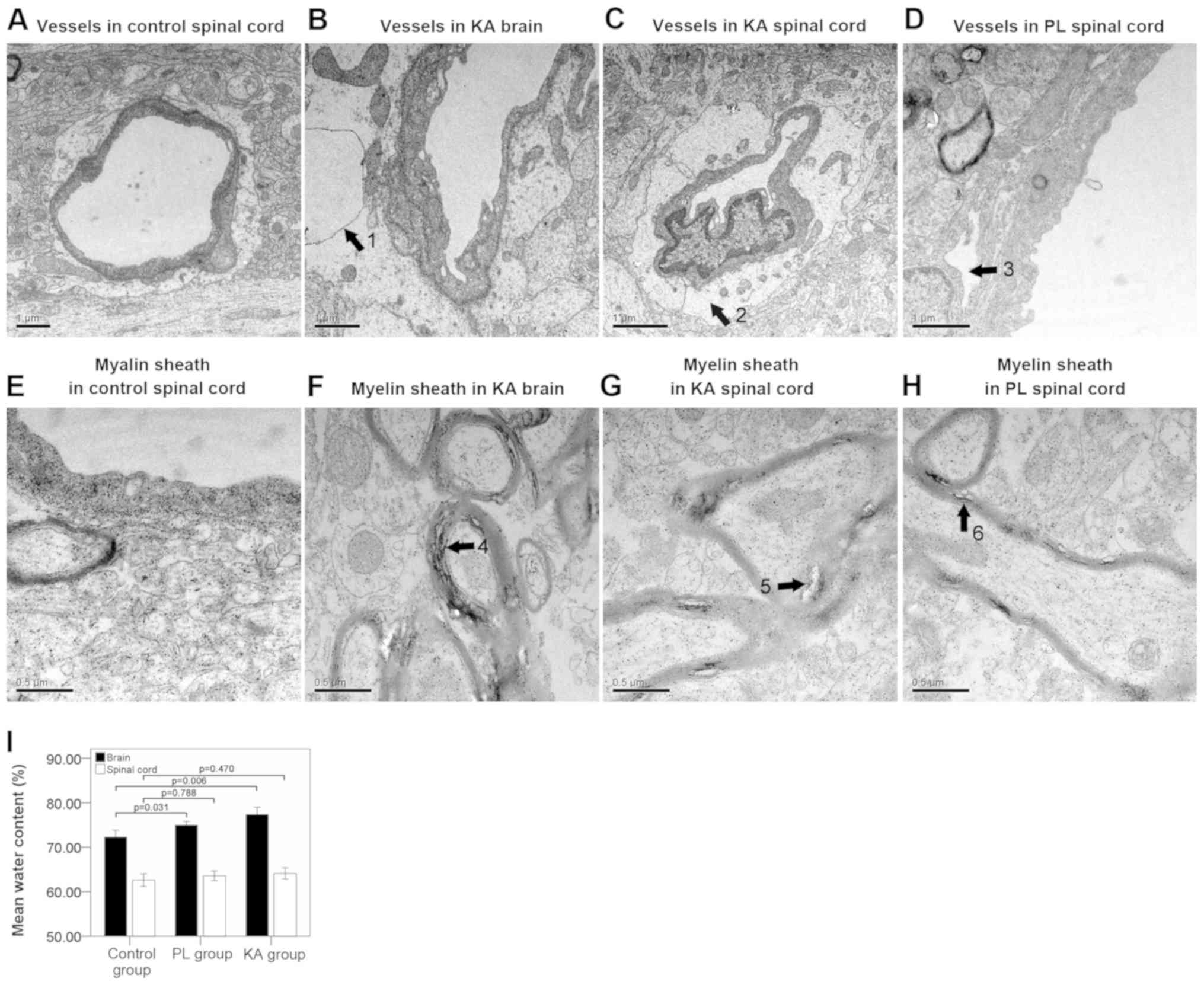
TEM results suggested that the structure of the
microvessels (Fig. 2A) and myelin
sheath (Fig. 2E) were normal in the
control group. However, significant edema and vacuolization of the
BBB basement membrane were observed in the brain (Fig. 2B) and spinal cords (Fig. 2C) of rats in the KA group. Separation
of the basement membrane was detected in the spinal cords of the PL
group (Fig. 2D). The present study
identified significant separation of the myelin sheath in the brain
(Fig. 2F) and spinal cords (Fig. 2G) of the KA group, while a lesser
degree of separation was observed in the spinal cords of the PL
group rats (Fig. 2H). Brain-water
content analysis results suggested a significant brain edema in
both the KA (P=0.006) and the PL group (P=0.031) compared with the
control group. However, the degree of spinal cord edema compared
between control group and TLE groups (both KA and PL groups) was
not significant (Fig. 2I). The
present results suggested that basal lamina separation of the BBB
and structural looseness of the myelin sheath were predominantly
observed in the spinal cords of rats with grade IV (8/25)-V (12/18)
seizure attacks.
Neuronal cell damages in the brain and
spinal cord following seizure
Nissl staining results indicated severe structural
damage and decreased neuronal cell numbers in the brains of the PL
group (Fig. 3B and D; P<0.001)
and the KA group (Fig. 3C and D;
P<0.001), compared with normal staining in the control group
(Fig. 3A). Semi-quantitative
analysis results suggested a significant decrease in the gray value
of the Nissl bodies in the spinal cords of both the PL and KA group
(Fig. 3D; P<0.001). The mean area
of Nissl+ cells in the spinal cord was significantly
decreased in the KA group (Fig. 3G and
H; P=0.041) compared with control group (Fig. 3E and H). The mean area of
Nissl+ cells was slightly decreased in the PL group
(Fig. 3F) compared with control
group (Fig. 3E), but the difference
was not statistically significant (Fig.
3H; P=0.773).
Albumin extravasation in the brain and
spinal cord following seizure
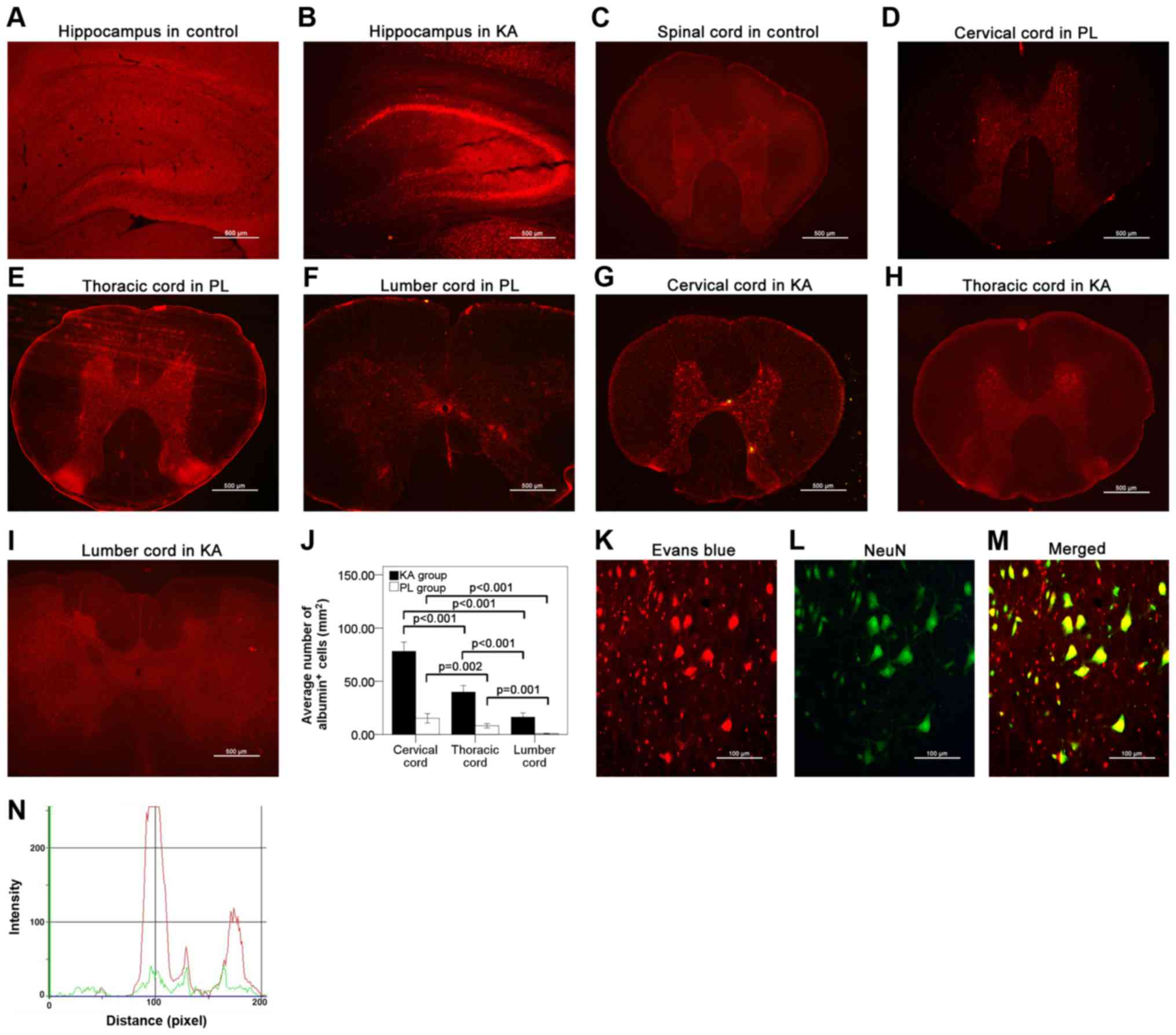
Albumin extravasation was not detected in the brain
(Fig. 4A) or the spinal cords
(Fig. 4C) of the control group.
However, significant albumin extravasation was observed in the
brain (Fig. 4B) and the spinal cord
(Fig. 4D and I) in both the PL and
KA groups, particularly in the hippocampus (Fig. 4B), cervical cord (Fig. 4D, G and J) and thoracic cord
(Fig. 4E, H and J). A lesser degree
of albumin extravasation was observed in the lumbar cord (Fig. 4F, I and J). The present results
suggested that in the spinal cord albumin extravasation was
primarily observed in rats with grade IV–V seizures. Additionally,
double-staining results identified the presence of albumin in the
neuronal cells (Fig. 4K-M). This
colocalization effect of albumin in neuronal cells was also
identified by overlap analysis results (Fig. 4N), which is in line with results from
previous studies (7).
Neuronal degeneration in the brain,
but not the spinal cord following seizure
The present results suggested that FJC+
staining was not detected in the brain (Fig. 5A) or spinal cords (Fig. 5C) of the control group. The present
results suggested that a small degree of FJC+ staining
was detected in the cortex and hippocampus (Fig. 5B), but not in the spinal cords
(Fig. 5D) of the TLE group.
Increases in ICAM-1 expression level
in the spinal cord after TLE
To investigate the involvement of BBB injury in the
spinal cord after TLE, the expression level of the vascular
adhesion molecule ICAM-1 was assessed using immunofluorescence and
western blotting. The present results suggested that following TLE,
the expression levels of ICAM-1 were significantly increased in the
injured spinal cords in both the PL group (Fig. 6B, D and E; P=0.007) and the KA group
(Fig. 6C-E; P=0.002), compared with
the control group (Fig. 6A).
Increases in the expression levels of
inflammatory factors in the spinal cord after TLE
To investigate whether an inflammatory reaction
contributed to spinal cord injury following TLE, the expression
levels of the leukocyte adhesion molecule CD11b, which binds to the
receptor ICAM-1, and TNF-α and IL-6 were detected using
immunohistochemistry and western blotting. The present results
suggested that a small number of CD11b+ leukocytes had
infiltrated into the spinal cord in the PL group (Fig. 7B and D) and the KA group (Fig. 7C and D) compared with the control
group (Fig. 7A). Furthermore,
western blot analysis results suggested a significant increase in
TNF-α and IL-6 expression levels in both the KA and PL groups
(Fig. 7E-H) compared with the
control group.
Apoptosis in the spinal cord after
TLE
To investigate the role of apoptosis in spinal cord
injury following TLE, the expression level of caspase-3 was
detected using immunofluorescence analysis. Compared with the
control group (Fig. 8A), a
significant increase in the number of caspase-3+ cells
was detected in the spinal cord after TLE in the PL (Fig. 8B and D) and KA groups (Fig. 8C and D). Multiple-staining of
caspase-3 (Fig. 8E), albumin
(Fig. 8F) and nucleus (Fig. 8G) revealed caspase-3+
staining in albumin+ neurons (Fig. 8H).
Discussion
Spinal cord injury following epileptic seizure has
been reported in a number of clinical cases (13). However, whether spinal cord injury
frequently occurs following seizure and its associated pathological
characteristics have not, to the best of our knowledge, previously
been investigated.
The present results suggested that following TLE,
the number and volume of Nissl bodies was significantly decreased,
thus indicating neuronal damage. Structural looseness and
separation of the myelin sheath were also identified in the present
study, which are also suggestive of neuronal axon injury. The
present results suggested that pathological changes were observed
in both the brain and the spinal cord, but were more significant in
the hippocampus, cervical cord and thoracic cord. To further
investigate the possible mechanism of spinal cord injury, the
integrity of the BBB and indicators of the inflammatory response
were assessed; our previous study indicated that significant BBB
damage was associated with neuronal dysfunction (10). The present TEM results suggested a
significant vacuolization and edema of the BBB basement membrane.
In addition, significant serum albumin extravasation into the brain
and the spinal cord was also detected, further indicating
structural and functional damage to the BBB as a result of spinal
cord injury. Double-staining results suggested that albumin was
localized to the neurons, indicating that neuronal damage may be
associated with albumin extravasation and excessive albumin
absorption, which is in line with results from a previous study
(10). In the present study,
neuronal degeneration was not detected in the spinal cord, but a
small number of albumin+ neurons stained positive for
caspase-3, which is an indicator of apoptosis (14). Therefore, the present results require
further investigation.
To investigate the involvement of the inflammatory
response in spinal cord injury, leukocyte infiltration and the
expression levels of inflammatory mediators were assessed in the
present study. Western blot analysis results suggested a
significant elevation in the expression levels of the leukocyte
adhesion molecule CD11b and the receptor for CD11b ICAM-1, which
mediates leukocyte infiltration into the brain (15). The present immunohistochemistry
results also indicated a small number of CD11b+
leukocytes in the spinal cord. Moreover, western blotting results
suggested a significant increase in the expression levels of TNF-α
and IL-6, which was indicative of an inflammatory reaction during
seizure-associated spinal cord injury.
Case reports have supported the hypothesis that
spinal cord injury is primarily caused by trauma, as head injury,
spinal canal stenosis and vertebral fracture are observed in a
number of patients, particularly walking adults with a high
incidence of fall accidents (2,3).
However, not all patients with spinal cord injury present with
evidence of trauma or other potential injuries (1), indicating that there are other
mechanisms of spinal cord injury after seizure. The present results
suggested that pathological changes were primarily detected in rats
that had experienced grade IV and V seizures. Furthermore, the
greatest degree of spinal cord injury was observed in the cervical
and thoracic cord, which is consistent with previous clinical
reports (2,3). However, trauma-associated spinal cord
injury is not uniformly distributed within the spinal cross-section
and most of the injuries are accompanied by edema (16). In addition, the extent of spinal cord
damage is dependent on the degree of vascular injury (16). The present results suggested that the
pathological change in the spinal cross-section had bilateral
symmetry and macroscopic structural damage was not observed. The
present results suggested that neuronal damage was predominantly
distributed in the anterior horn of the spinal cord, and grade IV
seizure was not accompanied by trauma or falls. The present results
do not support the view that spinal cord injury is caused by
trauma, thus further studies are required for clarification of
these results.
Systemic inflammatory responses occur during
epileptogenesis (6). The present
study identified an inflammatory reaction in the PL and KA groups,
however pathological changes in the spinal cord were largely
located in the cervical and the thoracic cord, which does not
support the previously reported mechanism of systemic inflammation
(6). In the current study, upper
limb convulsions were indicated to be a frequent behavioral
manifestation of rats during seizure attacks. Injured neurons are
primarily distributed in the anterior horn in the spinal cord. If
spinal cord injury was associated with hyperactivity of the spinal
neurons remains to be determined. Moreover, the brain and spinal
cord are closely associated, possessing similar neurotransmitter
expression profiles (8) and the
brain is theorized to originate from the spinal cord (7). The principal cause and potential
mechanism of spinal cord injury following seizure remains to be
elucidated, and the potential sequela and reversible nature of
these injuries requires further investigation.
In conclusion, the present results suggested that
spinal cord injury may commonly occur in rats following severe
seizure attacks. The present results suggested that seizures caused
damage to the BBB, albumin extravasation, inflammation and
apoptosis, which was associated with spinal cord injury and its
pathological changes.
Acknowledgements
The authors would like to thank Professor YuQiang
Ding and his colleagues affiliated to the Department of
neuropathophysiology, Tongji University, Shanghai, for their
technical and theoretical support.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81271441).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
JL, SL and YZ designed the main content and protocol
of this study. The experiments were conducted by JL and SL.
Supplementary experiments were conducted by ZL. Data collection and
statistical analysis were conducted by GL and KG. HZ edited the
figures. HZ, HW ZL and LZ participated and gave important advice in
the design and redesign of the study, and also aided in performing
the study, including improvement of test procedure, image analysis
and revision of the article.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First People's Hospital of Shanghai Jiaotong
University (approval no. 2012-DF-50).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee S, Lee JE, Yang S and Chang H: A case
of central cord syndrome related status epilepticus-a case report.
Ann Rehabil Med. 35:574–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roohi F and Fox A: Burst fracture of the
first lumbar vertebra and conus-cauda syndrome complicating a
single convulsive seizure: A challenge of diagnosis in the
emergency department. J Emerg Med. 31:381–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kruitbosch JM, Schouten EJ, Tan IY,
Veendrick-Meekes MJ and de Vocht JW: Cervical spinal cord injuries
in patients with refractory epilepsy. Seizure. 15:633–636. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zwimpfer TJ, Brown J, Sullivan I and
Moulton RJ: Head injuries due to falls caused by seizures: A group
at high risk for traumatic intracranial hematomas. J Neurosurg.
86:433–437. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukuhara N: Fukuhara disease. Brain Nerve.
60:53–58. 2008.(In Japanese). PubMed/NCBI
|
|
6
|
Gouveia TL, Vieira de Sousa PV, de Almeida
SS, Nejm MB, Vieira de Brito JM, Cysneiros RM, de Brito MV, Salu
BR, Oliva ML, Scorza FA and Naffah-Mazzacoratti Mda G: High serum
levels of proinflammatory markers during epileptogenesis. Can
omega-3 fatty acid administration reduce this process? Epilepsy
Behav. 51:300–305. 2015.PubMed/NCBI
|
|
7
|
Riva MA, Bellani I, Tremolizzo L, Lorusso
L, Ferrarese C and Cesana G: The neurologist in dante's inferno.
Eur Neurol. 73:278–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen HY, van Vliet EA, Bright KA, Hanthorn
M, Lytle NK, Gorter J, Aronica E and Boison D: Glycine transporter
1 is a target for the treatment of epilepsy. Neuropharmacology.
99:554–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Liu J, Wang S, Liu S and Zhao Y:
Neuronal uptake of serum albumin is associated with neuron damage
during the development of epilepsy. Exp Ther Med. 12:695–701. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Wang S, Liu J, Wang F, Liu Y and
Zhao Y: Leukocyte infiltration triggers seizure recurrence in a rat
model of temporal lobe epilepsy. Exp Ther Med. 12:695–701. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jungner M, Grände PO, Mattiasson G and
Bentzer P: Effects on brain edema of crystalloid and albumin fluid
resuscitation after brain trauma and hemorrhage in the rat.
Anesthesiology. 112:1194–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cottrell P, Ahmed S, James C, Hodson J,
McDonnell PJ, Rauz S and Williams GP: Neuron J is a rapid and
reliable open source tool for evaluating corneal nerve density in
herpes simplex keratitis. Invest Ophthalmol Vis Sci. 55:7312–7320.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brentnall M, Rodriguez-Menacol L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahman A and Fazal F: Hug tightly and say
goodbye: Role of endothelial ICAM-1 in leukocyte transmigration.
Antioxid Redox Signal. 11:823–839. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perovitch M, Perl S and Wang H: Current
advances in magnetic resonance imaging (MRI) in spinal cord trauma:
Review article. Paraplegia. 30:305–316. 1992.PubMed/NCBI
|