Introduction
Platelet activation and aggregation play a
significant role in the pathogenesis of arterial thrombosis and are
directly correlated with ischemic events, such as acute coronary
syndromes (ACS), stroke, transient ischemic attack (TIA) and
peripheral arterial diseases (PAD). Antiplatelet therapy in the
form of low-dose aspirin and clopidogrel are the most commonly used
drugs and the core and cornerstone of the management of CVD.
However, the association of aspirin with gastrointestinal (GI)
adverse effects (ranging from peptic ulcer to fatal bleeding or
perforation) is well known (1).
The CAPRIE (2)
(Clopidogrel versus Aspirin in Patients at Risk of Ischaemic
Events) study showed that fewer GI adverse events were found in the
clopidogrel group than in the aspirin group. Based on the CAPRIE
study, the American College of Cardiology/American Heart
Association (ACC/AHA) issued the recommendation that clopidogrel
was the optimal choice for patients with CVD who had major GI
intolerance to aspirin (primarily those with recent ulcer bleeding)
(3). However, further studies found
that 12% of patients who took clopidogrel with a history of ulcer
and 15% of those who took aspirin underwent recurrent GI bleeding
within one year (4,5). For patients at high GI risk who have
previous major upper gastrointestinal (UGI) complications of peptic
ulcer, UGI bleeding or perforation and who are under treatment of
ongoing antiplatelet therapy, the optimal treatment strategy
remains uncertain.
The conclusion that proton-pump inhibitor (PPI)
reduces the rate of recurrent GI bleeding in high GI risk patients
who receive aspirin has been confirmed by randomized controlled
trials (5). As revealed by Chan
et al (6) and Lai et
al (7), aspirin in combination
with esomeprazole was superior to clopidogrel for preventing ulcer
complications in patients who had previous aspirin-related peptic
ulcer bleeding. However, in a recent observational study, Tsai
et al (8) suggested that
clopidogrel alone and clopidogrel plus PPIs were both related to
lower risk of GI events than aspirin plus PPIs. Apart from the risk
of CV events, clopidogrel alone was superior to aspirin plus PPIs,
while clopidogrel plus PPIs was associated with a significantly
higher risk than aspirin plus PPIs.
Hsu et al (9)
showed that clopidogrel plus esomeprazole was superior to
clopidogrel alone in the prevention of recurrent peptic ulcer in
patients with previous peptic ulcer, while there were no
differences in CV events between the two groups. In addition, it
was reported that aspirin plus PPIs was related to a lower risk of
recurrent hospitalization for major GI complications albeit this
benefit was not evident in the clopidogrel plus PPIs group
(10).
The abovementioned controversial results and data
need confirmation through a meta-analysis by comparing the
recurrent UGI and CV events of three antiplatelet therapies:
clopidogrel alone, clopidogrel plus PPIs and aspirin plus PPIs.
Materials and methods
Data sources and search strategy
Studies published in the EMbase, PubMed and Cochrane
Central Register of Controlled Trials electronic databases were
reviewed. To identify relevant studies, the references of relevant
articles were also searched. All relevant articles published from
January, 1974 to February, 2018 were chosen. Keywords used for the
searches were ‘antiplatelet or aspirin or clopidogrel’, ‘recurrent
or recurrence or relapse’, ‘gastrointestinal hemorrhage or
gastrointestinal bleeding’, ‘ulcer or perforation’ and ‘proton pump
inhibitors or proton pump inhibitor or omeprazole or pantoprazole
or rabeprazole or esomeprazole or lansoprazole’ in different
combinations. There was no limit on sample size, sex or the
location of the original study. Only English articles were
chosen.
Inclusion criteria
Studies were included if they were randomized
controlled trials or observational studies comparing three
antiplatelet therapies - clopidogrel, clopidogrel plus PPIs and
aspirin plus PPIs - for the secondary prevention of CVD in patients
with a history of major UGI complications of peptic ulcer, UGI
bleeding or perforation. Studies were included if they reported
adverse outcomes (at least recurrent UGI events) as the clinical
endpoints, or involved relevant data that could be used in this
analysis.
Exclusion criteria
Studies were excluded if they were systematic
reviews, meta-analyses, case studies or letters to the editor; if
they did not include patients with CVD; if they included patients
who used a combination of aspirin and clopidogrel; if they included
patients who used a combination of nonsteroidal anti-inflammatory
drugs (NSAIDs), anticoagulant agents, cyclooxygenase-2 inhibitors,
other antiplatelet drugs or corticosteroids; if they did not report
the previously mentioned clinical outcomes; or if they were
associated with the same trial or cohort or they were duplicates of
the same study.
Types of participants
Patients were identified as having a record of the
following indications: coronary heart disease (CHD), PAD, ischemic
stroke or TIA and a history of major UGI complications of peptic
ulcer, UGI bleeding (including patients with UGI bleeding while
receiving low-dose aspirin) or perforation. The participants
initiated single antiplatelet therapy by aspirin or clopidogrel for
the secondary prevention of cardiovascular events.
Outcomes and follow-up
The primary outcomes were recurrent UGI events which
were defined as a hospitalization with the primary diagnosis of UGI
bleeding or ulcer (gastric ulcer, duodenal ulcer or peptic ulcer)
or perforation and the secondary outcomes were at least one of the
following variables: CV events, overall mortality or vascular
death. CV events were defined as either a hospitalization due to
any of the following: CHD, myocardial infarction (MI), unstable
angina (UA), congestive heart failure (CHF), PAD, cerebrovascular
insufficiency (CI), ischemic stroke or TIA. The follow-up time
extended until the first occurrence of outcomes or for those who
did not experience any outcome until the end of the study. All
analyses were based on the previously published studies. Therefore,
there was no need for ethics approval and patient consent. These
outcomes and follow-up periods are summarized in Table I.
 | Table IReported end points and follow-up
periods. |
Table I
Reported end points and follow-up
periods.
| Studies
(Refs.) | Reported end
points | Follow-up
period | Types of
participants |
|---|
| Chan 2005(6) | Recurrent ulcer
bleeding, MI, UA, CI, overall mortality, vascular death | 12 months | Aspirin-induced
upper GI bleeding |
| Lai 2006(7) | Recurrent ulcer
bleeding, perforation, obstruction, MI, CHF, IS, overall mortality,
vascular death | 52 weeks | Aspirin-induced
upper GI bleeding |
| Hsiao 2009(10) | Recurrent ulcer,
ulcer bleeding, perforation | 2 years | Previous peptic
ulcer or GI bleeding or perforation |
| Tsai 2011(8) | Recurrent ulcer,
ulcer bleeding, perforation, CHD, MI, PVD, IS, TIA | 1 year | Previous peptic
ulcer or GI bleeding |
| Hsu 2011(9) | Recurrent ulcer,
ulcer bleeding, UA, MI, IS, overall mortality | 6 months | Previous peptic
ulcer |
Data extraction and quality
assessment
Information and data, including the names of the
authors, country or region of the study, year of article
publication, period of patients' enrollment, number of patients in
each group (clopidogrel, clopidogrel plus PPIs and aspirin plus
PPIs), type of study (RCT or observational study), baseline
characteristics of the patients, the outcomes reported, the
follow-up periods, the medications used by the patients and the
number of events reported for clopidogrel, clopidogrel plus PPIs
and aspirin plus PPIs were carefully extracted by two of the
authors. Any disagreement was discussed and resolved by consulting
a third author.
Since this was a meta-analysis, we followed the
PRISMA guideline statement (11).
The Cochrane Risk of Bias Tool for Non-Randomized Studies of
Interventions (ACROBAT-NRSI) was used to evaluate the quality and
the bias risk of the included trials by two independent reviewers
(12). All the trials were rated as
having a low risk of bias.
Statistical analysis
The statistical analysis was carried out using
Revman 5.3 software with odds ratio (OR) and 95% confidence
intervals (CI) as the analytical parameters. Heterogeneity across
the studies was assessed by the Q statistic test and the
I2 test (The lower the I2 value, the lower
the heterogeneity, whereas heterogeneity would increase with an
increasing I2 value) (13). A P-value of ≤0.05 was considered
statistically significant. If the I2 value was >50%,
a random effects model was used for the analysis, whereas for an
I2 value <50%, a fixed effects model was used. Forest
plot was used to graphically display the results. A funnel plot was
used to visually assess the potential publication bias.
Results
Search results
Fig. 1 shows the
selection process for the eligible studies. In total, 173 records
were identified using the abovementioned search terms in electronic
databases. After removing 143 duplicate records and 11 case-only
studies, comments, review articles, animal models or case reports,
19 records remained for assessment. Fourteen studies were excluded
due to the use of a combination of aspirin and clopidogrel or the
inclusion of subjects with unhealed peptic ulcer. Finally, 3
randomized controlled trials (7,8,10) and 2 observational studies (9,11) were
included with a total number of 7,399 patients (3,688 patients were
treated with clopidogrel alone, 1,725 patients were treated with
clopidogrel plus PPIs and 1,986 were treated with aspirin plus
PPIs). We found that the regions of the included studies comprised
only Hong Kong and Taiwan. The general and baseline features of the
included studies are listed in Table
II.
 | Table IIGeneral and baseline features of the
studies included. |
Table II
General and baseline features of the
studies included.
| Studies
(refs.) | N | Mean age
(years) | Males (%) | Cc (%) | Cs (%) | Hp | Type of study | Region | Type of CVD | Antiplatelet
therapy | Type of PPIs |
|---|
| Chan 2005 | 159 | 72.9±9.5 | 64.8 | 5.0 | 8.2 | - | RCT | Hong Kong | CHD, CI, PVD | Aspirin, 80
mg/day | Esomeprazole, 20
mg/twice |
| (6) | 161 | 72.1±10.2 | 67.1 | 8.1 | 13.0 | - | | | | Clopidogrel, 75
mg/day | Alone |
| Lai 2006 | 86 | 75.5±7.8 | 59.3 | 16.3 | 4.7 | - | RCT | Hong Kong | CHD, IS, TIA,
PVD | Aspirin, 100
mg/day | Esomeprazole, 20
mg/day |
| (7) | 84 | 75.8±7.8 | 60.7 | 22.6 | 3.6 | - | | | | Clopidogrel, 75
mg/day | Alone |
| Hsiao 2009 | 538 | 70.79±11.2 | 57.81 | 0.19 | 1.12 | - | Observational | Taiwan | MI, IS | Aspirin, <325
mg/day | Unclear |
| (10) | 11463 | 70.98±11.45 | 60.2 | 0.44 | 0.93 | - | | | | Aspirin, <325
mg/day | Alone |
| | 590 | 70.98±11.45 | 57.46 | 0 | 0 | - | | | | Clopidogrel, 75
mg/day | Unclear |
| | 2036 | 71.50±10.63 | 60.27 | 0.20 | 0.44 | - | | | | Clopidogrel, 75
mg/day | Alone |
| Tsai 2011 | 1203 | 70.53±10.97 | 63.84 | 0.33 | 0.50 | - | Observational | Taiwan | MI, IS | Aspirin, <325
mg/day | Unclear |
| (8) | 1052 | 70.97±10.62 | 61.50 | 0.10 | 0 | - | | | | Clopidogrel, 75
mg/day | Unclear |
| | 1325 | 70.95±10.73 | 60.91 | 0.15 | 0.38 | - | | | | Clopidogrel, 75
mg/day | Alone |
| Hsu 2011 | 83 | 70.60±11.50 | 78.3 | 4.8 | 12.0 | - | RCT | Taiwan | CHD, IS | Clopidogrel, 75
mg/day | Esomeprazole, 20
mg/day |
| (9) | 82 | 73.3±10.70 | 72.0 | 3.7 | 6.1 | - | | | | Clopidogrel, 75
mg/day | Alone |
Aspirin plus PPIs versus
clopidogrel
Recurrent UGI events
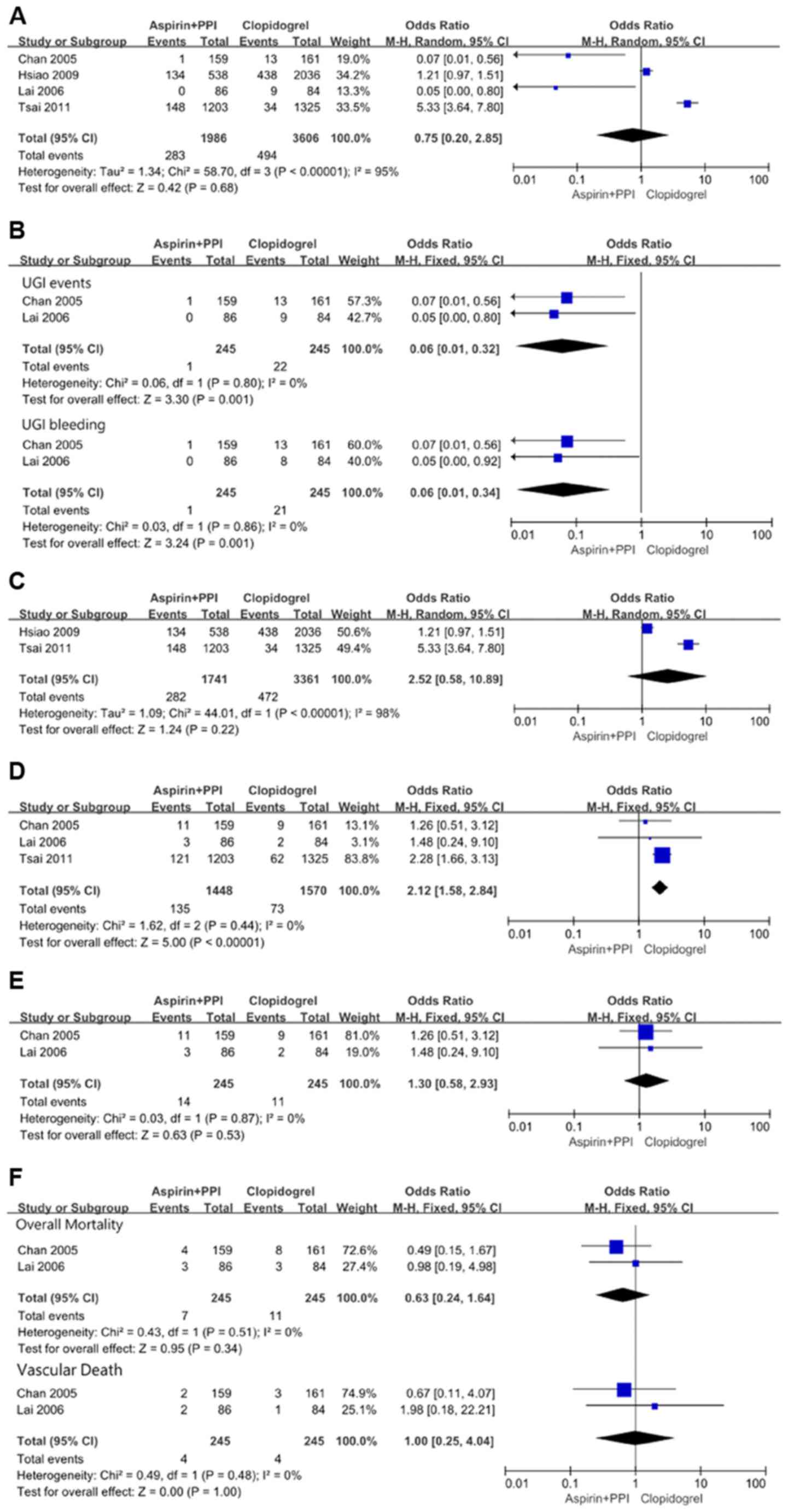
In the overall meta-analysis, there was significant
heterogeneity (χ2=58.70, df=3 and P<0.00001;
I2=95%) among the four studies. In the random effects
model, however, although recurrent UGI events favored aspirin plus
PPIs with OR: 0.75, 95% CI: 0.20-2.85; z=0.42 and P=0.68, the
result was not statistically significant (Fig. 2A). Due to the presence of significant
heterogeneity among the included studies, the above result may be
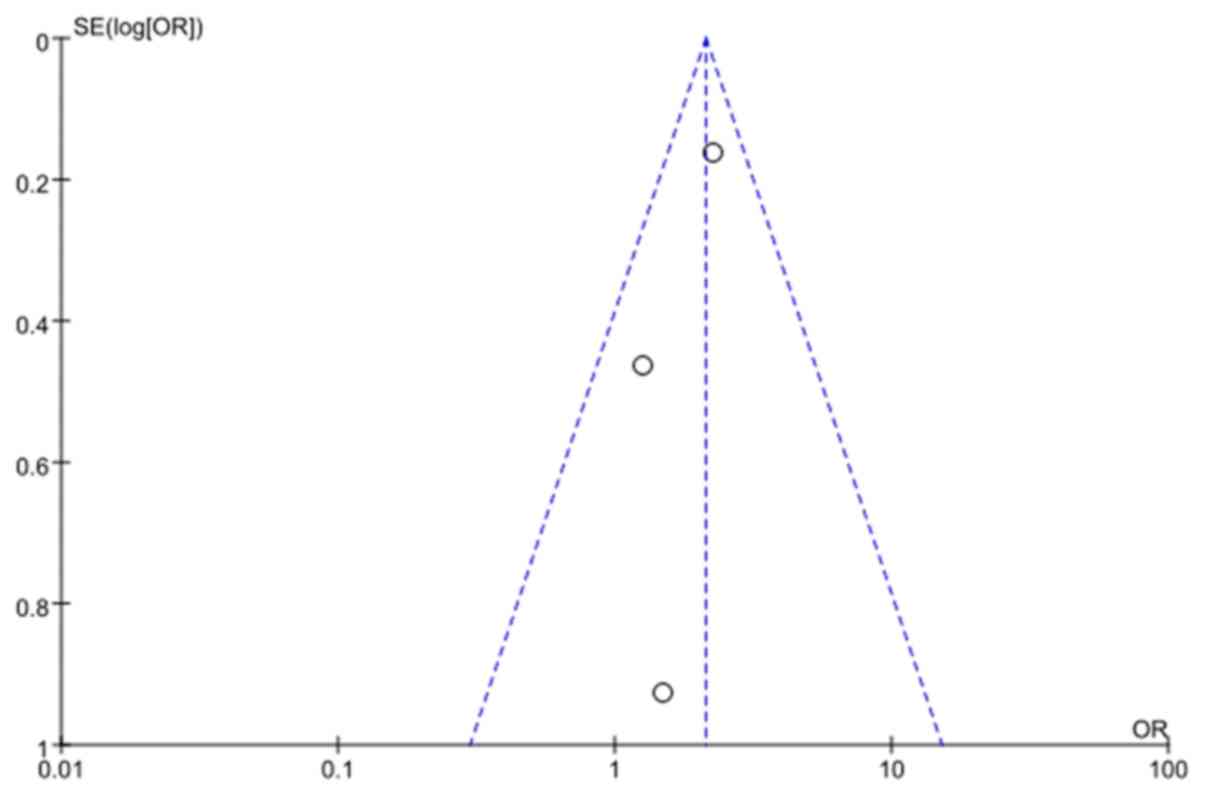
considered inadequate and biased. The funnel plot showed the
minimal publication bias (Fig. 3).
The possible reasons might be the included study type
(observational) and the selection of only English publications for
this analysis.
When randomized data were analyzed separately, in
the study by Chan et al (6),
1 case of recurrent duodenal ulcer bleeding and 13 cases of
recurrent ulcer bleeding (6 gastric ulcers, 5 duodenal ulcers and 2
both gastric and duodenal ulcers) were observed in the
aspirin-plus-esomeprazole group and the clopidogrel group,
respectively. Lai et al (7)
found that no recurrent ulcer complications occurred in the aspirin
plus PPIs group, and 9 cases (8 gastric and/or duodenal ulcers
bleeding and 1 perforated duodenal ulcer) occurred in the
clopidogrel group. No significant heterogeneity
(χ2=0.06, df=1 and P=0.80; I2=0%) was found
between those two studies. In the fixed effects model, recurrent
UGI events were significantly lower with aspirin plus PPIs with OR:
0.06, 95% CI: 0.01-0.32; z=3.30 and P=0.001. This analysis also
showed that recurrent UGI bleeding still significantly favored
aspirin plus PPIs with OR: 0.06, 95% CI: 0.01-0.34; z=3.24 and
P=0.001 (Fig. 2B).
When observational data were analyzed separately,
there was significant heterogeneity (χ2=44.01, df=1 and
P<0.00001; I2=98%) between the two studies. In the
random effects model, recurrent UGI events favored clopidogrel with
OR: 2.52, 95% CI: 0.58-10.89; z=1.24 and P=0.22, but the result was
not statistically significant (Fig.
2C).
Recurrent CV events
In the overall meta-analysis, no significant
heterogeneity (χ2=1.62, df=2 and P=0.44;
I2=0%) was found among the three studies. In the fixed
effects model, the rate of recurrent CV events was significantly
higher with aspirin plus PPIs with OR: 2.12, 95% CI: 1.58-2.84;
z=5.00 and P<0.00001 (Fig. 2D).
There was no significant publication bias as indicated by the
funnel plot (Fig. 4).
When randomized data were separately analyzed in the
study by Chan et al (6),
recurrent ischemic CV events occurred in 11 patients in the aspirin
plus PPIs group (1 MI, 7 UA and 3 CI) and in 9 patients in the
clopidogrel group (1 MI, 6 UA and 2 CI). Lai et al (7) found that 1 patient developed CHF, 1
patient developed MI, and 1 patient developed recurrent stroke in
the aspirin plus PPIs group, while 1 patient developed MI, and 1
patient developed CHF in the clopidogrel group. No significant
heterogeneity (χ2=0.03, df=1, and P=0.87;
I2=0%) was found between the two studies. The recurrent
CV events were similar between the aspirin plus PPIs and
clopidogrel groups with OR: 1.30, 95% CI: 0.58-2.93; z=0.63 and
P=0.53 by the fixed effects model (Fig.
2E).
Overall mortality and vascular
death
Of the 12 patients who died in the study reported by
Chan et al (6), 4 were in the
aspirin plus PPIs group (1 patient died from MI, 1 CI, 1 renal
failure and 1 uncertain causes), and 8 were in the clopidogrel
group (1 patient died from MI, 1 from an intracranial hemorrhage, 1
from heart failure, 3 from sepsis and 2 from uncertain causes). In
the study by Lai et al (7), 3
patients in the aspirin plus PPIs group died of pneumonia, MI and
recurrent stroke. In the clopidogrel group, 3 patients died of
chronic obstructive airway disease, CHF and chronic renal failure.
No significant heterogeneity was found between the two studies. In
the fixed effects model, overall mortality and vascular death
showed no statistical difference between the aspirin plus PPIs
group and the clopidogrel group with OR: 0.63, 95% CI: 0.24-1.64;
z=0.95 and P=0.34 and OR: 1.00, 95% CI: 0.25-4.04; z=0.00 and
P=1.00, respectively (Fig. 2F).
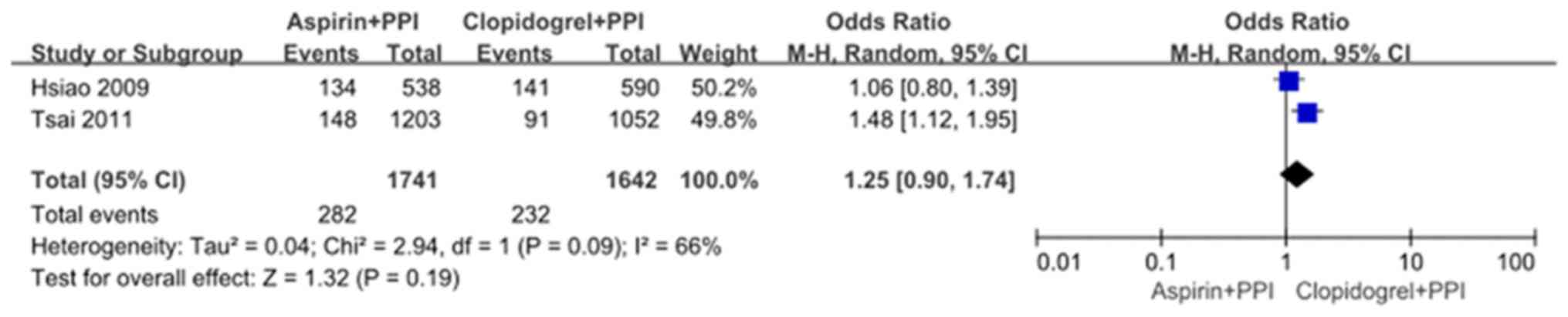
Aspirin plus PPIs versus clopidogrel
plus PPIs
Two observational studies by Hsiao et al
(10) and Tsai et al
(8) compared recurrent UGI events
between the aspirin plus PPIs group and the clopidogrel plus PPIs
group. Significant heterogeneity was found between the two studies
(χ2=2.94, df=1, and P=0.09; I2=66%). In the
random effects model, recurrent UGI events were not significantly
different with OR: 1.25, 95% CI: 0.90-1.74; z=1.32 and P=0.19
(Fig. 5). There were several causes
of heterogeneity, including co-medications and comorbidities
(Fig. 5). There was no significant
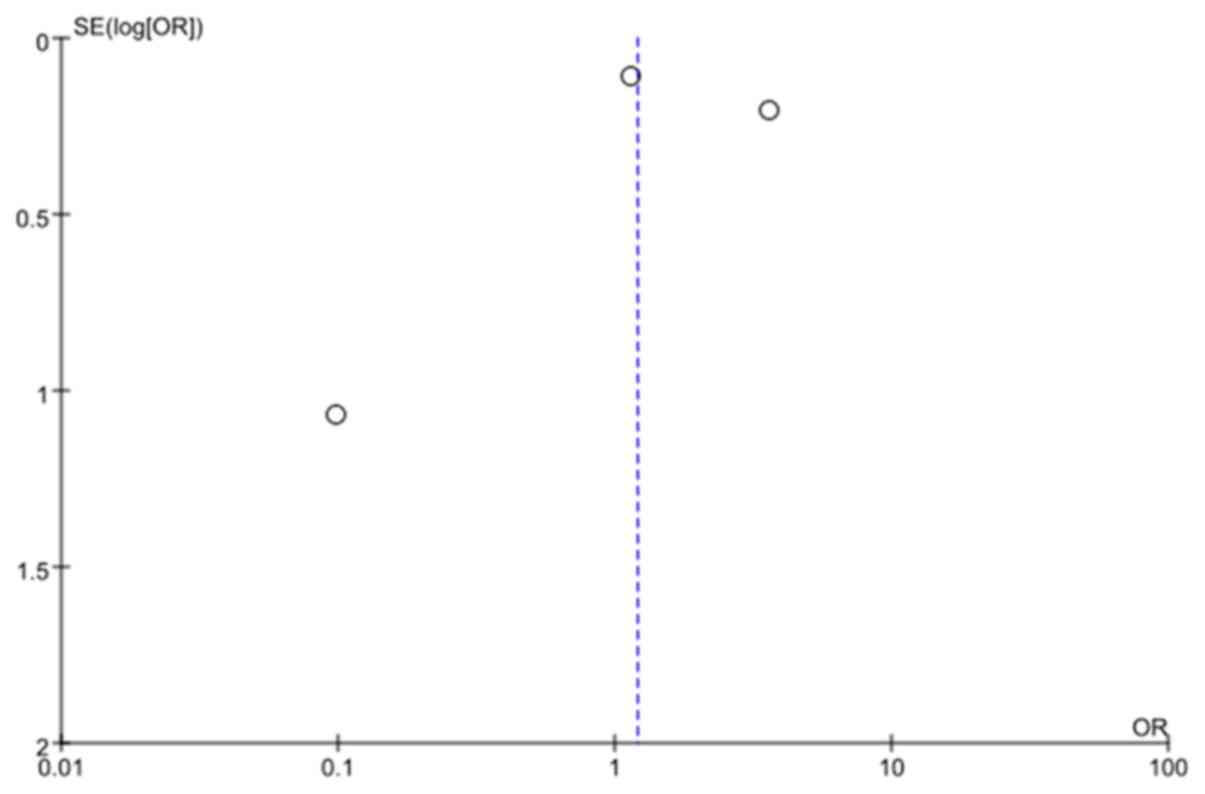
publication bias as indicated by the funnel plot (Fig. 6).
Clopidogrel plus PPIs versus
clopidogrel
Recurrent UGI events
In the overall meta-analysis, significant
heterogeneity (χ2=30.42, df=2, and P<0.01;
I2=93%) was found in the three studies. In the random
effects model, recurrent UGI events were not significantly
different with OR: 1.20, 95% CI: 0.40-3.65; z=0.33 and P=0.74
(Fig. 7A). There was no significant
publication bias, as indicated by the funnel plot (Fig. 8).
When observational data were separately analyzed,
there was significant heterogeneity (χ2=24.12, df=1, and
P<0.01; I2=96%) between the two studies. In the
random effects model, recurrent UGI events still showed significant
difference with OR: 2.00, 95% CI: 0.65-6.16; z=1.21 and P=0.23
(Fig. 7B).
Recurrent CV events
No significant heterogeneity (χ2=0.74,
df=1, and P=0.39; I2=0%) was found between the two
studies. In the fixed effects model, recurrent CV events were
significantly higher with clopidogrel plus PPIs with OR: 2.57, 95%
CI: 1.89-3.51; z=5.97 and P<0.01 (Fig. 7C).
Discussion
The use of antiplatelet drugs is limited by
potential adverse GI complications including peptic ulcer, GI
bleeding or perforation, especially in patients with previous GI
events. By this analysis, we compared the incidence of recurrent
UGI and CV events in patients at high risk of UGI bleeding who were
prescribed one of the three antiplatelet therapies - clopidogrel
alone, clopidogrel plus PPIs or aspirin plus PPIs - for the
secondary prevention of CVD.
Current results revealed no significant difference
in the overall rates of recurrent UGI events between aspirin plus
PPIs and clopidogrel alone (Fig.
2A). However, aspirin plus PPIs was associated with a lower
risk of recurrent UGI events and UGI bleeding when compared to
clopidogrel in subgroups from two randomized studies by Chan et
al and by Lai et al (6,7), in
which the patients had previous aspirin-induced ulcer bleeding
(Fig. 2B). Chan et al
(6) studied patients who took
aspirin for vascular disease prevention but presented with ulcer
bleeding.
After the healing of ulcers, patients who were
negative for Helicobacter pylori (Hp) were randomly assigned
to receive either 75 mg of clopidogrel daily plus esomeprazole
placebo twice daily or 80 mg of aspirin daily plus 20 mg of
esomeprazole twice daily for 12 months. A total of 320 patients
were enrolled. Recurrent ulcer bleeding occurred in 13 patients
receiving clopidogrel and 1 receiving aspirin plus esomeprazole.
The cumulative incidence of recurrent bleeding during the 12-month
period was 8.6 and 0.7%, respectively (P=0.001). Another 170
patients who developed ulcer bleeding after the use of low-dose
aspirin were enrolled by Lai et al (7). After the healing of ulcers and the
eradication of Hp, the patients were randomly assigned to receive
esomeprazole 20 mg/day and aspirin 100 mg/day or clopidogrel 75
mg/day. During a median follow-up period of 52 weeks, no patient in
the esomeprazole group developed recurrent ulcer complications,
while 9 patients in the clopidogrel group did. The cumulative
incidences of recurrent ulcer complications were 0% and 13.6%,
respectively (P=0.0019). Therefore, we conclude that among patients
with previous aspirin-induced ulcer bleeding, aspirin plus PPIs was
superior to clopidogrel alone in preventing recurrent ulcer
bleeding. A recent large retrospective cohort study by Hsiao et
al (10) reported that the mean
drug cost per person/year was several times higher in clopidogrel
users than in users of aspirin plus a PPI. Our findings show that
clopidogrel is not an ideal substitute for aspirin in patients with
previous UGI bleeding, especially aspirin-induced bleeding.
As stated above, 12% of patients with a previous
ulcer who took clopidogrel had recurrent GI bleeding within one
year (4). The unclear mechanisms by
which clopidogrel led to recurrent ulcer bleeding prompted further
studies. Animal studies have shown that platelet
adenosine-diphosphate (ADP) receptor antagonists hinder the healing
of gastric ulcers by suppressing the releasing of platelet-derived
growth factors (14). Therefore, for
patients with high GI bleeding risk, clopidogrel alone may not be
safe enough and concomitant PPIs prophylaxis is necessary. Whether
the concomitant use of clopidogrel and PPIs was superior to aspirin
plus PPIs or clopidogrel alone in high GI bleeding risk patients
for recurrences of UGI adverse effects is not clear. Our analysis
revealed no significant difference in recurrent UGI events when
clopidogrel plus PPIs were compared to aspirin plus PPIs and
clopidogrel alone (Figs. 5, and
7A and B). Our results were similar to those of the
study by Ng et al (15).
Therefore, clopidogrel plus PPIs was not superior to either aspirin
plus PPIs or clopidogrel alone for reducing recurrent UGI events in
high GI bleeding risk patients.
Nevertheless, aspirin plus PPIs was associated with
a significantly higher risk of recurrent CV events when compared to
clopidogrel alone (Fig. 2F). This
result might be due to the fact that aspirin inhibits platelet
aggregation to a relatively weak extent. Aspirin inhibits
thromboxane A2 production by irreversible acetylation of the
platelet cyclooxygenase enzyme. Clopidogrel selectively and
irreversibly binds to the P2Y12 receptor and inhibits platelet
aggregation, thereby blocking the ADP-dependent pathway of platelet
activation. Comparative clinical trials suggested that blockade of
this pathway may be more powerful and effective than thromboxane A2
inhibition (16,17). Similarly, the CAPRIE (2) trial showed that compared with aspirin,
clopidogrel reduced the combined risk of ischaemic stroke, MI or
vascular death in high CV risk patients (by 8.7%).
However, our analysis showed no significant
difference in recurrent CV events, overall mortality and vascular
death in the aspirin-induced ulcer bleeding subgroups when aspirin
plus PPIs was compared to clopidogrel alone (Fig. 2E and F).
Furthermore, our analysis showed that clopidogrel
plus PPIs was associated with a significantly higher risk of
recurrent CV events when compared to clopidogrel alone (Fig. 7C). Clopidogrel is a prodrug that is
converted to an active metabolite in the liver, with the
bioactivation mediated by hepatic cytochrome P450 2C19(18). It was reported that there was a
potential cytochrome P450 2C19-dependent drug-drug interaction
between clopidogrel and PPIs (19,20). As
the competitive inhibitors of cytochrome P450 2C19, PPIs may alter
the pharmacokinetics of clopidogrel and potentially lead to a
higher risk for recurrent adverse CV events (21,22).
Tsai et al studied a total number of 3,580 patients in the
population-based database from Taiwan's National Health Insurance
(8). It was included that PPI
prophylaxis was associated with a higher risk of CV events in
patients who received clopidogrel [with PPI vs. without PPI; HR
2.15 (1.48-3.11)]. Nevertheless, some studies did not show an
interaction between clopidogrel and PPIs (22,23). Hsu
et al (9) noted that there
was no evidence of an interaction between esomeprazole and
clopidogrel. As in this study, esomeprazole was administered before
breakfast and clopidogrel was given at bedtime. The very short
half-lives of PPIs and clopidogrel made this approximately 14-16 h
drug administration separation minimize any potential interactions.
These findings may provide new ideas for reducing drug
interactions.
This analysis is new in several ways. As far as we
know, this is the first meta-analysis to compare recurrent UGI and
CV events of three antiplatelet therapies - clopidogrel alone,
clopidogrel plus PPIs and aspirin plus PPIs - for the secondary
prevention of CVD in patients at high GI bleeding risk. Second,
this interesting idea is very important clinically. Third, the
randomized and observational studies were combined and were also
analyzed separately.
There are several limitations to our meta-analysis.
First, there are inherent limitations to an observational study
design. As with any non-randomized design, significant differences
in comorbidities between different treatment groups could have
affected the findings. Second, there was a small sample size of
randomized studies included in our meta-analysis when compared to
observational studies, and the results might not be very accurate.
Third, only 5 studies were included in our meta-analysis, and an
assessment of publication bias by a funnel plot may not provide
sufficient power to reveal asymmetry. Fourth, the studies included
in this meta-analysis are solely from Asia, so the conclusion only
applied to the Asia population. A recent meta-analysis suggested
the therapeutic effect of PPIs to reduce recurrent bleeding rates
after ulcer bleeding was more efficacious in Asia than elsewhere.
This may be the result of an enhanced pharmacodynamic effect of
PPIs in Asian patients (24). Fifth,
we planned to differentiate the risks of the dosage of PPIs, the
different PPIs types and the CYP2C19 genotypes for the weakening of
clopidogrel efficacy, however, the included studies did not
sufficiently report this aspect or did not study this aspect.
Therefore, it was impractical to perform an analysis of these
variables. Finally, our analysis only discussed single antiplatelet
therapy and did not include dual antiplatelet therapy for patients
who had experienced prior UGI complications.
In summary, our analysis suggests that in patients
at high UGI bleeding risk, whether aspirin-induced or not, who
require ongoing single antiplatelet therapy for the secondary
prevention of CVD, aspirin plus PPIs may be a more cost-effective
option and should be considered as the first choice rather than
clopidogrel alone or clopidogrel plus PPIs for UGI protection.
However, in terms of CV protection, clopidogrel alone appears to be
superior for reducing CV risk while clopidogrel plus PPIs may be
associated with a higher CV risk due to potential drug-drug
interaction in this subset of patients. Further studies are still
needed to confirm our results due to the limitations of our
meta-analysis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Ethics approval and consent to
participate
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and JL contributed to the study design. ZJ
contributed to data extraction and quality assessment. HY was
responsible for the analysis and discussion of the data. All
authors read and approved the final manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Farrell B, Godwin J, Richards S and Warlow
C: UK-TIA Study Group: The United Kingdom transient ischaemic
attack (UK-TIA) aspirin trial: Final results. J Neurol Neurosurg
Psychiatry. 54:1044–1054. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
CAPRIE Steering Committee: A randomised,
blinded, trial of clopidogrel versus aspirin in patients at risk of
ischaemic events (CAPRIE). Lancet 348: 1329-1339, 1996.
|
|
3
|
Braunwald E, Antman EM, Beasley JW, Califf
RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J,
Levin TN, Pepine CJ, Schaeffer JW, Smith EE III, Steward DE and
Theroux P: ACC/AHA 2002 guideline update for the management of
patients with unstable angina and non-ST-segment elevation
myocardial infarction: summary article: a report of the American
College of Cardiology/American Heart Association Task Force on
Practice Guidelines (Committee on the Management of Patients With
Uns Table Angina). Circulation. 106:1893–1900. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ng FH, Wong SY, Chang CM, Chen WH, Kng C,
Lanas AI and Wong BC: High incidence of clopidogrel-associated
gastrointestinal bleeding in patients with previous peptic ulcer
disease. Aliment Pharmacol Ther. 18:443–449. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lai KC, Lam SK, Chu KM, Wong BC, Hui WM,
Hu WH, Lau GK, Wong WM, Yuen MF, Chan AO, et al: Lansoprazole for
the prevention of recurrences of ulcer complications from long-term
low-dose aspirin use. N Engl J Med. 346:2033–2038. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chan FK, Ching JY, Hung LC, Wong VW, Leung
VK, Kung NN, Hui AJ, Wu JC, Leung WK, Lee VW, et al: Clopidogrel
versus aspirin and esomeprazole to prevent recurrent ulcer
bleeding. N Engl J Med. 352:238–244. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lai KC, Chu KM, Hui WM, Wong BC, Hung WK,
Loo CK, Hu WH, Chan AO, Kwok KF, Fung TT, et al: Esomeprazole with
aspirin versus clopidogrel for prevention of recurrent
gastrointestinal ulcer complications. Clin Gastroenterol Hepatol.
4:860–865. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsai YW, Wen YW, Huang WF, Chen PF, Kuo KN
and Hsiao FY: Cardiovascular and gastrointestinal events of three
antiplatelet therapies: Clopidogrel, clopidogrel plus proton-pump
inhibitors, and aspirin plus proton-pump inhibitors in patients
with previous gastrointestinal bleeding. J Gastroenterol. 46:39–45.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hsu PI, Lai KH and Liu CP: Esomeprazole
with clopidogrel reduces peptic ulcer recurrence, compared with
clopidogrel alone, in patients with atherosclerosis.
Gastroenterology. 140:791–798. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hsiao FY, Tsai YW, Huang WF, Wen YW, Chen
PF, Chang PY and Kuo KN: A comparison of aspirin and clopidogrel
with or without proton pump inhibitors for the secondary prevention
of cardiovascular events in patients at high risk for
gastrointestinal bleeding. Clin Ther. 31:2038–2047. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339 (jul21
1)(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sterne JAC, Higgins JPT and Reeves BC: A
Cochrane Risk of Bias Assessment Tool: for Non-Randomized Studies
of Interventions (ACROBAT-NRSI). Version 1.0.0, September 24, 2014.
Retrieved from http://www.riskofbias.info.
Accessed: May 21, 2015.
|
|
13
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma L, Elliott SN, Cirino G, Buret A,
Ignarro LJ and Wallace JL: Platelets modulate gastric ulcer
healing: Role of endostatin and vascular endothelial growth factor
release. Proc Natl Acad Sci USA. 98:6470–6475. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ng FH, Wong BC, Wong SY, Chen WH and Chang
CM: Clopidogrel plus omeprazole compared with aspirin plus
omeprazole for aspirin-induced symptomatic peptic ulcers/erosions
with low to moderate bleeding/re-bleeding risk - a single-blind,
randomized controlled study. Aliment Pharmacol Ther. 19:359–365.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hass WK, Easton JD, Adams HP Jr,
Pryse-Phillips W, Molony BA, Anderson S and Kamm B: Ticlopidine
Aspirin Stroke Study Group: A randomized trial comparing
ticlopidine hydrochloride with aspirin for the prevention of stroke
in high-risk patients. N Engl J Med. 321:501–507. 1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Savi P, Bernat A, Dumas A, Aït-Chek L and
Herbert JM: Effect of aspirin and clopidogrel on platelet-dependent
tissue factor expression in endothelial cells. Thromb Res.
73:117–124. 1994.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim KA, Park PW, Hong SJ and Park JY: The
effect of CYP2C19 polymorphism on the pharmacokinetics and
pharmacodynamics of clopidogrel: A possible mechanism for
clopidogrel resistance. Clin Pharmacol Ther. 84:236–242.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sibbing D, Morath T, Stegherr J, Braun S,
Vogt W, Hadamitzky M, Schömig A, Kastrati A and von Beckerath N:
Impact of proton pump inhibitors on the antiplatelet effects of
clopidogrel. Thromb Haemost. 101:714–719. 2009.PubMed/NCBI
|
|
20
|
Gilard M, Arnaud B, Cornily JC, Le Gal G,
Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF and
Boschat J: Influence of omeprazole on the antiplatelet action of
clopidogrel associated with aspirin: The randomized, double-blind
OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol.
51:256–260. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Juurlink DN, Gomes T, Ko DT, Szmitko PE,
Austin PC, Tu JV, Henry DA, Kopp A and Mamdani MM: A
population-based study of the drug interaction between proton pump
inhibitors and clopidogrel. CMAJ. 180:713–718. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ho PM, Maddox TM, Wang L, Fihn SD, Jesse
RL, Peterson ED and Rumsfeld JS: Risk of adverse outcomes
associated with concomitant use of clopidogrel and proton pump
inhibitors following acute coronary syndrome. JAMA. 301:937–944.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Depta JP and Bhatt DL: Omeprazole and
clopidogrel: Should clinicians be worried? Cleve Clin J Med.
77:113–116. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Leontiadis GI, Sharma VK and Howden CW:
Systematic review and meta-analysis: Enhanced efficacy of
proton-pump inhibitor therapy for peptic ulcer bleeding in Asia - a
post hoc analysis from the Cochrane Collaboration. Aliment
Pharmacol Ther. 21:1055–1061. 2005.PubMed/NCBI View Article : Google Scholar
|